Paper Menu >>
Journal Menu >>
 Vol.2, No.4, 368-372 (2010) Natural Science http://dx.doi.org/10.4236/ns.2010.24044 Copyright © 2010 SciRes. OPEN ACCESS Interfacial control on microstructure, morphology and optics of beta-AgI nanostructures fabricated on sputter-disordered Ag-Sn bilayers D. Bharathi Mohan1, C. S. Sunandana2 1 SEG-CEMUC-Department of Mechanical Engineering, University of Coimbra, Coimbra, Portugal; dhanabharathi@yahoo.com 2 School of Physics, University of Hyderabad, Hyderabad, India; csssp75@yahoo.com Received 26 October 2009; revised 7 December 2009; accepted 10 February 2010. ABSTRACT We report for the first time a non-template based facile growth of hexagonal (β) AgI nanorods and nanoplates easily fabricated by rf magnetron sputtering on Ag/Sn bilayers upon controlled iodination. The structural and morphological evolution of the β-AgI nanostructures is cha- racterized by X-Ray Diffraction, Atomic Force Microscopy and optical spectroscopy. Sput- tering induced disorder in precursor Ag films, high external stress and high defect concentra- tions at the Sn-AgI interface particularly facili- tates the development of layered hexagonal structure of β-AgI nanostructures. Extremely sensitive room temperature optical absorbance involving evolution of W1,2 and W3 exciton tran- sitions and emission spectra involving phonon replica corroborate the formation of β-AgI na- nostructures with high defect concentrations, are aimed at improving the efficiency of photo- graphic process and looking at microelec- trodic and optoelectronic applications. Keywords: Thin Films; Nanostructures; Crystal Structure; Optical Properties 1. INTRODUCTION Motivated by an extreme mesoscopic ionic conductivity and superior photographic prowess, lately, many re- searchers have synthesized Ag/AgI, AgI/γ-Al2O3 and AgI nanostructures in with controlled nano feature sizes and shapes by routes, including electrochemical, tem- plate-chemical, Ultrasonic pyrolysis, W/O microemul- sions and solution methods[1-8]. These works, however, focused on the formation of highly stable β-AgI phase at room temperature [9] and its structural disordering, for- mation of highly conducting interfacial layers (i.e. 7H and 9R polytype of AgI with stacking fault arrange- ments), shape dependent properties and quantum con- finement effects in nanorods. In this work, rf magnetron sputtering is exploited as an innovative technique to fab- ricate β-AgI nanostructures with different shapes-that could lead to miniaturized nanoscale opto-electronic devices [10]. Sputtering introduces structural disorder in Ag films while doping introduces extra disorder and external stress in the host and thus provides localized states for the nucleation of nanoparticles in an effec- tively kinetically controlled process [11]. To test and implement these ideas we fabricated Ag/Sn bilayers by sputtering where an ultra thin layer (~3.5 nm to 14 nm) of Sn serves as capping agent for Ag particles that in- troduces external stress at the Ag/Sn interfaces eventu- ally controlling nanomorphology of silver iodide. Mor- eover, doping could stabilize the crystal structure by strengthening the cation (or anion) sublattice of the iono- covalent semiconductor (CdS or AgI) and introducing a certain number of donors/acceptors in the forbidden gap of the host semiconductor thereby impacting the electri- cal and optical properties of the host semiconductor [12]. Sn -with valences 2 and 4- was chosen because it is a covalent metal and mixes well with Ag and could con- trols the iodization kinetics [13] enabling realization of desired optimized nanostructure even for a single Ag/Sn ratio. 2. EXPERIMENTAL METHODS Ag/Sn bilayers were produced using rf magnetron sput- tering (MagSput-1G2-RF-HOT-UPG) with Sn layer thick- ness varied from 3.5 nm to 14 nm while Ag layer thick- ness was fixed as 90 nm. Silver (99.99% purity) and tin (99.99% purity) targets each ~55 nm diameter and ~3 mm thick was used for sputtering; the base pressure was always maintained as 1E-6 mbar. At first, Ag films were sputtered onto commercial float glass substrates 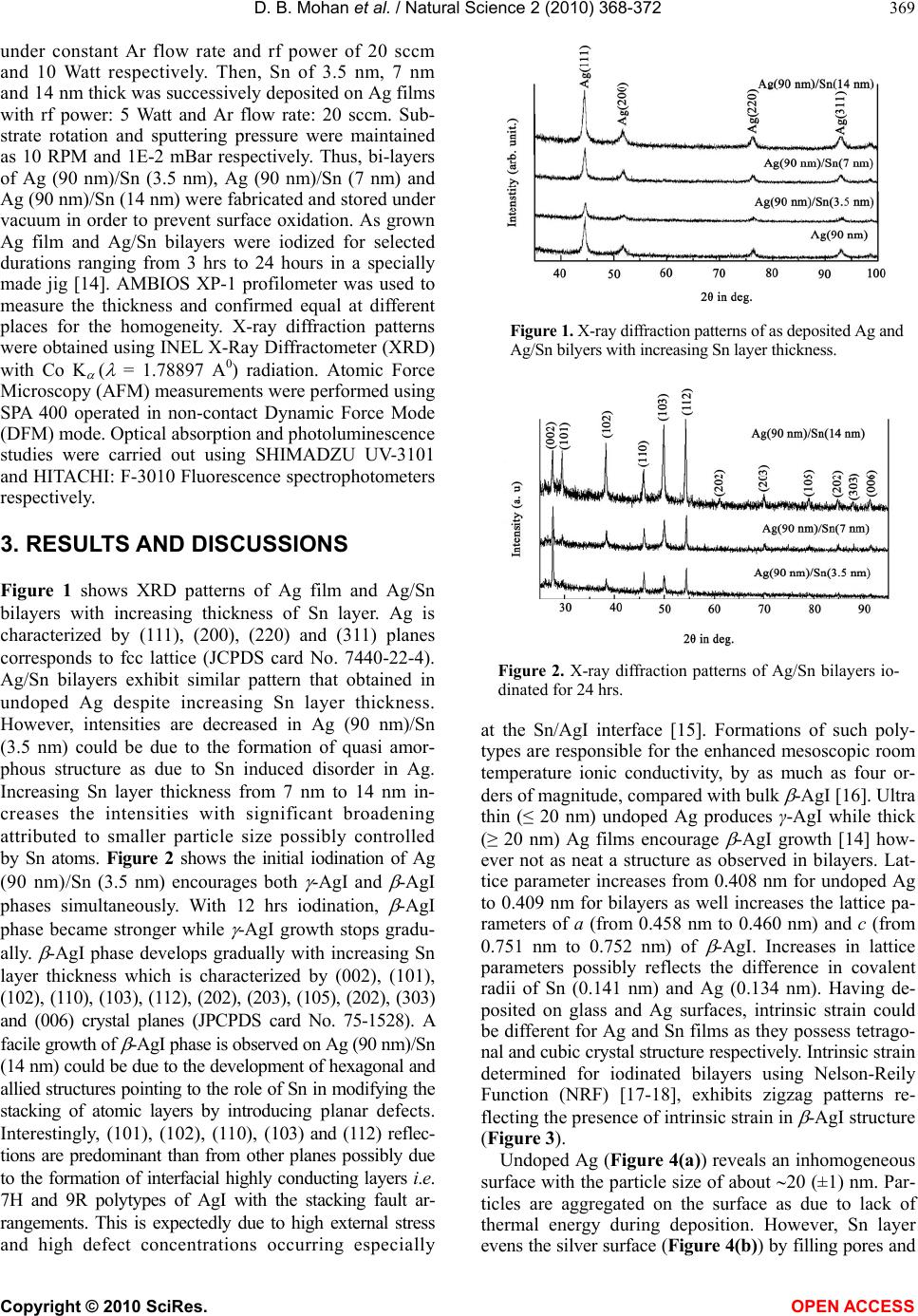 D. B. Mohan et al. / Natural Science 2 (2010) 368-372 Copyright © 2010 SciRes. OPEN ACCESS 369 under constant Ar flow rate and rf power of 20 sccm and 10 Watt respectively. Then, Sn of 3.5 nm, 7 nm and 14 nm thick was successively deposited on Ag films with rf power: 5 Watt and Ar flow rate: 20 sccm. Sub- strate rotation and sputtering pressure were maintained as 10 RPM and 1E-2 mBar respectively. Thus, bi-layers of Ag (90 nm)/Sn (3.5 nm), Ag (90 nm)/Sn (7 nm) and Ag (90 nm)/Sn (14 nm) were fabricated and stored under vacuum in order to prevent surface oxidation. As grown Ag film and Ag/Sn bilayers were iodized for selected durations ranging from 3 hrs to 24 hours in a specially made jig [14]. AMBIOS XP-1 profilometer was used to measure the thickness and confirmed equal at different places for the homogeneity. X-ray diffraction patterns were obtained using INEL X-Ray Diffractometer (XRD) with Co K ( = 1.78897 A0) radiation. Atomic Force Microscopy (AFM) measurements were performed using SPA 400 operated in non-contact Dynamic Force Mode (DFM) mode. Optical absorption and photoluminescence studies were carried out using SHIMADZU UV-3101 and HITACHI: F-3010 Fluorescence spectrophotometers respectively. 3. RESULTS AND DISCUSSIONS Figure 1 shows XRD patterns of Ag film and Ag/Sn bilayers with increasing thickness of Sn layer. Ag is characterized by (111), (200), (220) and (311) planes corresponds to fcc lattice (JCPDS card No. 7440-22-4). Ag/Sn bilayers exhibit similar pattern that obtained in undoped Ag despite increasing Sn layer thickness. However, intensities are decreased in Ag (90 nm)/Sn (3.5 nm) could be due to the formation of quasi amor- phous structure as due to Sn induced disorder in Ag. Increasing Sn layer thickness from 7 nm to 14 nm in- creases the intensities with significant broadening attributed to smaller particle size possibly controlled by Sn atoms. Figure 2 shows the initial iodination of Ag (90 nm)/Sn (3.5 nm) encourages both -AgI and -AgI phases simultaneously. With 12 hrs iodination, -AgI phase became stronger while -AgI growth stops gradu- ally. -AgI phase develops gradually with increasing Sn layer thickness which is characterized by (002), (101), (102), (110), (103), (112), (202), (203), (105), (202), (303) and (006) crystal planes (JPCPDS card No. 75-1528). A facile growth of -AgI phase is observed on Ag (90 nm)/Sn (14 nm) could be due to the development of hexagonal and allied structures pointing to the role of Sn in modifying the stacking of atomic layers by introducing planar defects. Interestingly, (101), (102), (110), (103) and (112) reflec- tions are predominant than from other planes possibly due to the formation of interfacial highly conducting layers i.e. 7H and 9R polytypes of AgI with the stacking fault ar- rangements. This is expectedly due to high external stress and high defect concentrations occurring especially Figure 1. X-ray diffraction patterns of as deposited Ag and Ag/Sn bilyers with increasing Sn layer thickness. Figure 2. X-ray diffraction patterns of Ag/Sn bilayers io- dinated for 24 hrs. at the Sn/AgI interface [15]. Formations of such poly- types are responsible for the enhanced mesoscopic room temperature ionic conductivity, by as much as four or- ders of magnitude, compared with bulk -AgI [16]. Ultra thin (≤ 20 nm) undoped Ag produces γ-AgI while thick (≥ 20 nm) Ag films encourage -AgI growth [14] how- ever not as neat a structure as observed in bilayers. Lat- tice parameter increases from 0.408 nm for undoped Ag to 0.409 nm for bilayers as well increases the lattice pa- rameters of a (from 0.458 nm to 0.460 nm) and c (from 0.751 nm to 0.752 nm) of -AgI. Increases in lattice parameters possibly reflects the difference in covalent radii of Sn (0.141 nm) and Ag (0.134 nm). Having de- posited on glass and Ag surfaces, intrinsic strain could be different for Ag and Sn films as they possess tetrago- nal and cubic crystal structure respectively. Intrinsic strain determined for iodinated bilayers using Nelson-Reily Function (NRF) [17-18], exhibits zigzag patterns re- flecting the presence of intrinsic strain in -AgI structure (Figure 3). Undoped Ag (Figure 4(a)) reveals an inhomogeneous surface with the particle size of about 20 (±1) nm. Par- ticles are aggregated on the surface as due to lack of thermal energy during deposition. However, Sn layer evens the silver surface (Figure 4(b)) by filling pores and 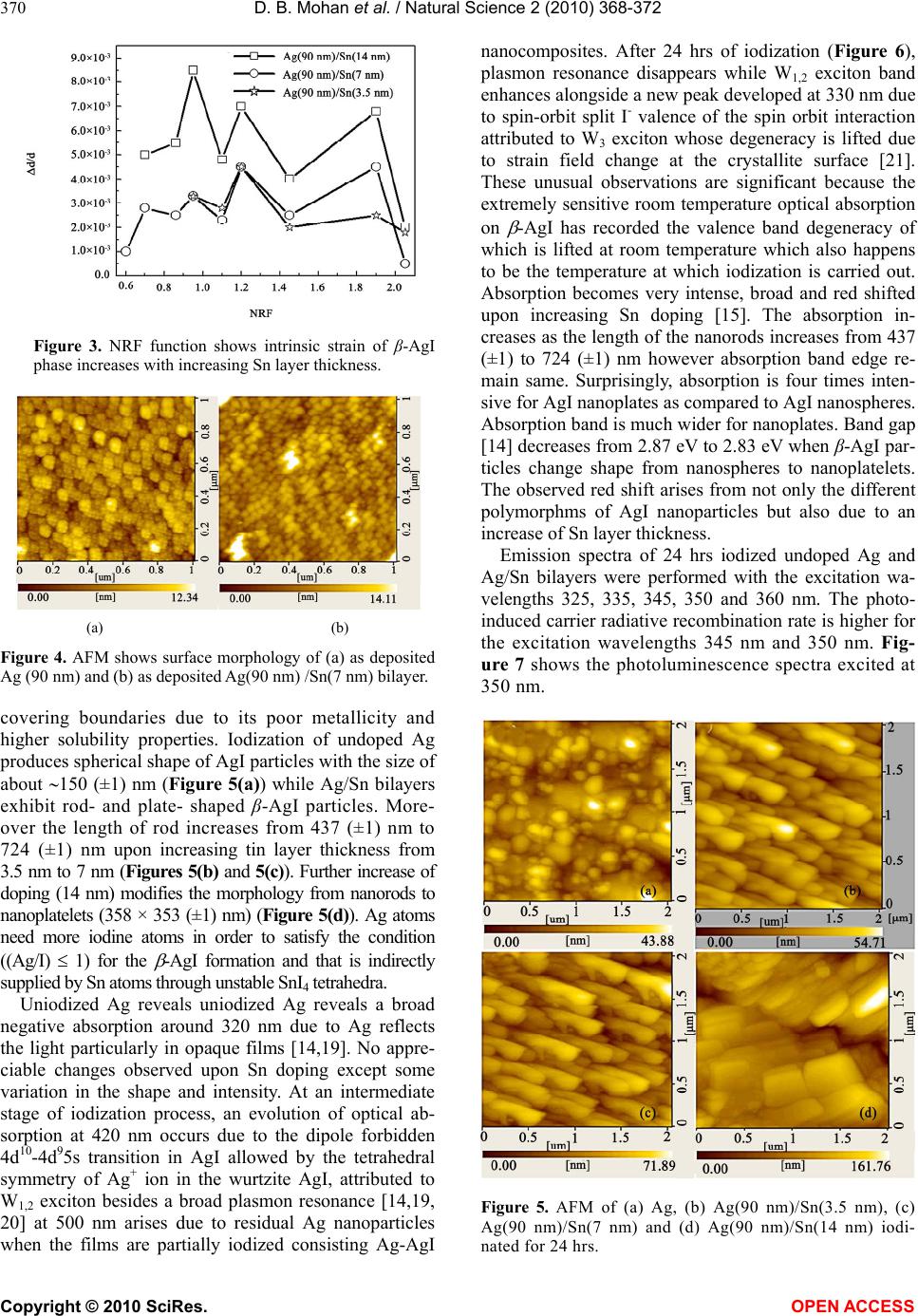 D. B. Mohan et al. / Natural Science 2 (2010) 368-372 Copyright © 2010 SciRes. OPEN ACCESS 370 Figure 3. NRF function shows intrinsic strain of β-AgI phase increases with increasing Sn layer thickness. (a) (b) Figure 4. AFM shows surface morphology of (a) as deposited Ag (90 nm) and (b) as deposited Ag(90 nm) /Sn(7 nm) bilayer. covering boundaries due to its poor metallicity and higher solubility properties. Iodization of undoped Ag produces spherical shape of AgI particles with the size of about 150 (±1) nm (Figure 5(a)) while Ag/Sn bilayers exhibit rod- and plate- shaped β-AgI particles. More- over the length of rod increases from 437 (±1) nm to 724 (±1) nm upon increasing tin layer thickness from 3.5 nm to 7 nm (Figures 5(b) and 5(c)). Further increase of doping (14 nm) modifies the morphology from nanorods to nanoplatelets (358 × 353 (±1) nm) (Figure 5(d)). Ag atoms need more iodine atoms in order to satisfy the condition ((Ag/I) 1) for the -AgI formation and that is indirectly supplied by Sn atoms through unstable SnI4 tetrahedra. Uniodized Ag reveals uniodized Ag reveals a broad negative absorption around 320 nm due to Ag reflects the light particularly in opaque films [14,19]. No appre- ciable changes observed upon Sn doping except some variation in the shape and intensity. At an intermediate stage of iodization process, an evolution of optical ab- sorption at 420 nm occurs due to the dipole forbidden 4d10-4d95s transition in AgI allowed by the tetrahedral symmetry of Ag+ ion in the wurtzite AgI, attributed to W1,2 exciton besides a broad plasmon resonance [14,19, 20] at 500 nm arises due to residual Ag nanoparticles when the films are partially iodized consisting Ag-AgI nanocomposites. After 24 hrs of iodization (Figure 6), plasmon resonance disappears while W1,2 exciton band enhances alongside a new peak developed at 330 nm due to spin-orbit split I- valence of the spin orbit interaction attributed to W3 exciton whose degeneracy is lifted due to strain field change at the crystallite surface [21]. These unusual observations are significant because the extremely sensitive room temperature optical absorption on -AgI has recorded the valence band degeneracy of which is lifted at room temperature which also happens to be the temperature at which iodization is carried out. Absorption becomes very intense, broad and red shifted upon increasing Sn doping [15]. The absorption in- creases as the length of the nanorods increases from 437 (±1) to 724 (±1) nm however absorption band edge re- main same. Surprisingly, absorption is four times inten- sive for AgI nanoplates as compared to AgI nanospheres. Absorption band is much wider for nanoplates. Band gap [14] decreases from 2.87 eV to 2.83 eV when β-AgI par- ticles change shape from nanospheres to nanoplatelets. The observed red shift arises from not only the different polymorphms of AgI nanoparticles but also due to an increase of Sn layer thickness. Emission spectra of 24 hrs iodized undoped Ag and Ag/Sn bilayers were performed with the excitation wa- velengths 325, 335, 345, 350 and 360 nm. The photo- induced carrier radiative recombination rate is higher for the excitation wavelengths 345 nm and 350 nm. Fig- ure 7 shows the photoluminescence spectra excited at 350 nm. Figure 5. AFM of (a) Ag, (b) Ag(90 nm)/Sn(3.5 nm), (c) Ag(90 nm)/Sn(7 nm) and (d) Ag(90 nm)/Sn(14 nm) iodi- nated for 24 hrs. 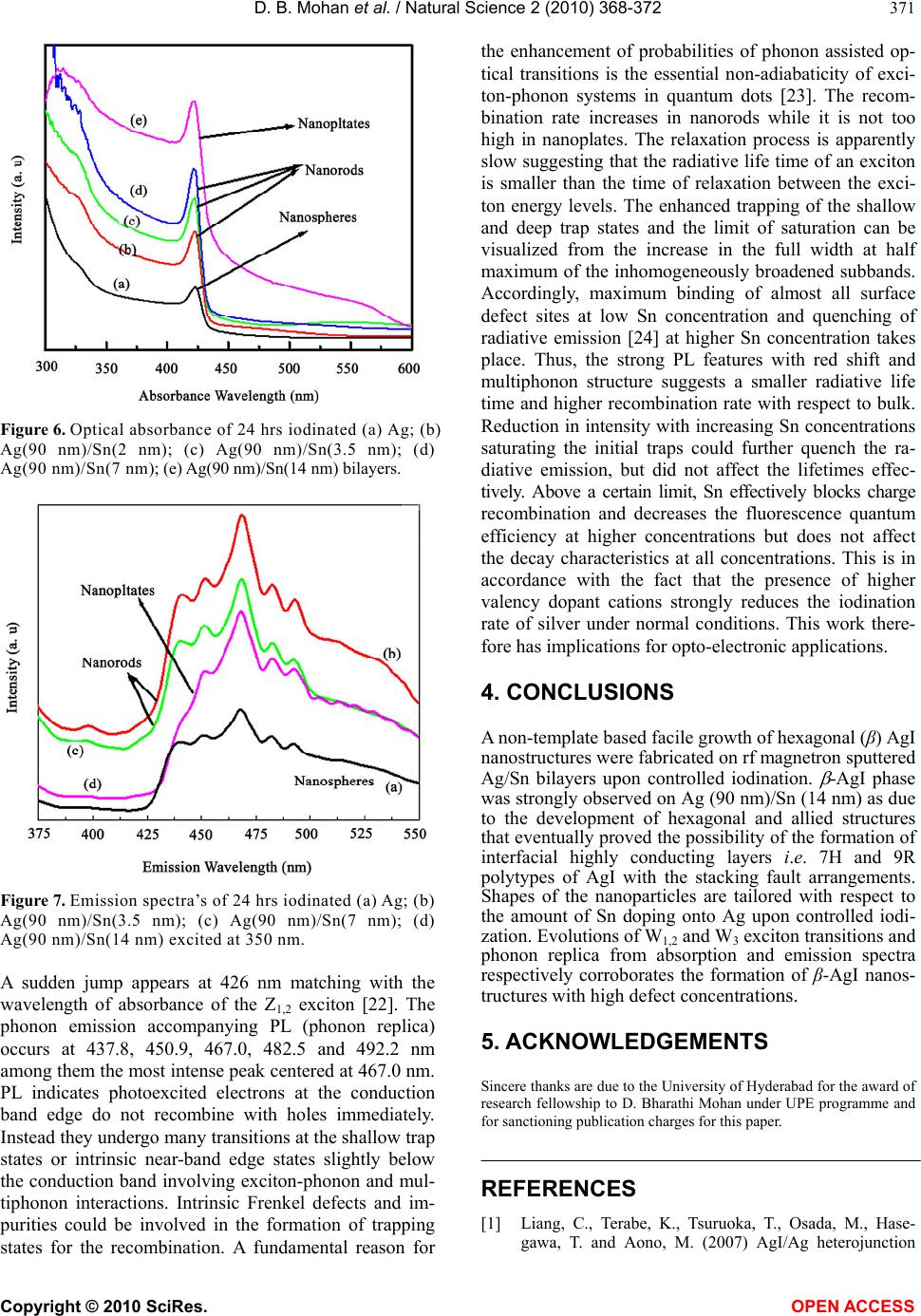 D. B. Mohan et al. / Natural Science 2 (2010) 368-372 Copyright © 2010 SciRes. OPEN ACCESS 371 Figure 6. Optical absorbance of 24 hrs iodinated (a) Ag; (b) Ag(90 nm)/Sn(2 nm); (c) Ag(90 nm)/Sn(3.5 nm); (d) Ag(90 nm)/Sn(7 nm); (e) Ag(90 nm)/Sn(14 nm) bilayers. Figure 7. Emission spectra’s of 24 hrs iodinated (a) Ag; (b) Ag(90 nm)/Sn(3.5 nm); (c) Ag(90 nm)/Sn(7 nm); (d) Ag(90 nm)/Sn(14 nm) excited at 350 nm. A sudden jump appears at 426 nm matching with the wavelength of absorbance of the Z1,2 exciton [22]. The phonon emission accompanying PL (phonon replica) occurs at 437.8, 450.9, 467.0, 482.5 and 492.2 nm among them the most intense peak centered at 467.0 nm. PL indicates photoexcited electrons at the conduction band edge do not recombine with holes immediately. Instead they undergo many transitions at the shallow trap states or intrinsic near-band edge states slightly below the conduction band involving exciton-phonon and mul- tiphonon interactions. Intrinsic Frenkel defects and im- purities could be involved in the formation of trapping states for the recombination. A fundamental reason for the enhancement of probabilities of phonon assisted op- tical transitions is the essential non-adiabaticity of exci- ton-phonon systems in quantum dots [23]. The recom- bination rate increases in nanorods while it is not too high in nanoplates. The relaxation process is apparently slow suggesting that the radiative life time of an exciton is smaller than the time of relaxation between the exci- ton energy levels. The enhanced trapping of the shallow and deep trap states and the limit of saturation can be visualized from the increase in the full width at half maximum of the inhomogeneously broadened subbands. Accordingly, maximum binding of almost all surface defect sites at low Sn concentration and quenching of radiative emission [24] at higher Sn concentration takes place. Thus, the strong PL features with red shift and multiphonon structure suggests a smaller radiative life time and higher recombination rate with respect to bulk. Reduction in intensity with increasing Sn concentrations saturating the initial traps could further quench the ra- diative emission, but did not affect the lifetimes effec- tively. Above a certain limit, Sn effectively blocks charge recombination and decreases the fluorescence quantum efficiency at higher concentrations but does not affect the decay characteristics at all concentrations. This is in accordance with the fact that the presence of higher valency dopant cations strongly reduces the iodination rate of silver under normal conditions. This work there- fore has implications for opto-electronic applications. 4. CONCLUSIONS A non-template based facile growth of hexagonal (β) AgI nanostructures were fabricated on rf magnetron sputtered Ag/Sn bilayers upon controlled iodination. -AgI phase was strongly observed on Ag (90 nm)/Sn (14 nm) as due to the development of hexagonal and allied structures that eventually proved the possibility of the formation of interfacial highly conducting layers i.e. 7H and 9R polytypes of AgI with the stacking fault arrangements. Shapes of the nanoparticles are tailored with respect to the amount of Sn doping onto Ag upon controlled iodi- zation. Evolutions of W1,2 and W3 exciton transitions and phonon replica from absorption and emission spectra respectively corroborates the formation of β-AgI nanos- tructures with high defect concentrations. 5. ACKNOWLEDGEMENTS Sincere thanks are due to the University of Hyderabad for the award of research fellowship to D. Bharathi Mohan under UPE programme and for sanctioning publication charges for this paper. REFERENCES [1] Liang, C., Terabe, K., Tsuruoka, T., Osada, M., Hase- gawa, T. and Aono, M. (2007) AgI/Ag heterojunction  D. B. Mohan et al. / Natural Science 2 (2010) 368-372 Copyright © 2010 SciRes. OPEN ACCESS 372 nanowires: Facile electrochemical synthesis, and en- hanced ionic conductivity. Advanced Functional Materi- als, 17(9), 1466-1472. [2] Lee, W., Yoo, H.-L. and Lee, J.-K. (2001) Template route toward a novel nanostructured superionic conductor film. Chemical Communications, 2001(24), 2530-2531. [3] Guo, Y., Lee, J. and Maier, J. (2006) Preparation and characterization of AgI nanoparticles with controlled size, morphology and crystal structure. Solid State Ionics, 177(26-32), 2467-2471. [4] Guo, Y.-G., Hu, Y.-S., Lee, J.-S. and Maier, J. (2006) High-performance rechargeable all-solid-state silver bat- tery based on superionic AgI nanoplates. Electrochemis- try Communications, 8(7), 1179-1184. [5] El-Kouedi, M., Foss, C.A.Jr., Bodolosky-Bettis, S.A. and Bachman, R.E. (2002) Structural analysis of AgI and Au/AgI nanocomposite films by powder x-ray diffraction: Evidence for preferential orientation. Journal of Physical Chemistry B, 106(29), 7205-7209. [6] Xu, S. and Lee, Y. (2003) Different morphology at dif- ferent reactant molar ratios: Synthesis of silver halide low-dimensional nanomaterials in microemulsions. Journal of Material Chemistry, 13(1), 163-165. [7] Validzic, I.L., Jankovic, I.A., Mitric, M., Bibic, N. and Nedeljkovic, J.M. (2007) Growth and quantum confinement in AgI nanowires. Materials Letters, 61(16), 3522-3525. [8] Validzic, I.L., Jokanovic, V., Uskokovic, D.P. and Ne- deljkovic, J.M. (2008) Influence of solvent on the struc- tural and morphological properties of AgI particles pre- pared using ultrasonic spray pyrolysis. Materials Chem- istry and Physics, 107(1), 28-32. [9] Chandra, S. (1981) Superionic solids: Principles and appli- cations. North-Holland Publishing Company, Amsterdam. [10] Mochizuki, S. and Fujishiro, F. (2003) Shallow and deep excited states of mesocopic structure in AgI-γAl2O3 composites. Physica Status Solidi (c), 0(2), 763-766. [11] Mohan, D.B. and Sunandana, C.S. (2007) AgI nanos- tructure development in sputter-disordered and Al-doped Ag films probed by XRD, SEM, optical absorption and photoluminescence. Applied Physics A, 86(1), 73-82. [12] Mohan, D.B. and Sunandana, C.S. (2004) Nanophases in mechanochemically synthesized AgI-CuI system: Struc- ture, phase stability and phase transition. Journal of Physics and Chemistry of Solids, 65(10), 1669-1677. [13] Kuiry, S.C., Roy, S.K. and Bose, S.K. (1997) Influence of short circuiting on the kinetics and mechanism of io- dide film growth on Ag and Cd-doped Ag. Metallurgical and Materials Transactions B, 28(6), 1189-1198. [14] Mohan, D.B. and Sunandana, C.S. (2006) Iodization of rf sputter induced disordered Ag thin films reveals volume plasmon-exciton “transition”. Journal of Applied Physics, 100(6), 4314. [15] Mohan, D.B. and Sunandana, C.S. (2005) Effect of Sn doping on the growth and optical properties of AgI na- noparticles. Journal Nanoscience and Nanotechnology, 5(9), 1-5. [16] Guo, Y.J., Lee, J.-K. and Maier, J. (2005) AgI nanoplates with mesoscopic superionic conductivity at room tem- perature. Advanced Materials, 17(23), 2815-2819. [17] Vook, R.W. and Witt, F. (1965) Structure and annealing behavior of metal films deposited on substrates near 80°K: I. copper films on glass. Journal of Vacuum Science and Technology, 2(1), 49-57. [18] Dayal, P.B., Mehta, B.R., Aparna, Y. and Shivaprasad, S.M. (2002) Surface-mediated structural transformation in CdTe nanoparticles dispersed in SiO2 thin films. Ap- plied Physics Letters, 81(22), 4254-4256. [19] Mohan, D.B., Sreejith, K. and Sunandana, C.S. (2007) Surface plasmon-exciton transition in ultra-thin silver and silver iodide films. Applied Physics B, 89(1), 59-63. [20] Economou, E.N. (1969) Surface plasmons in thin films. Physical Review, 182(2), 539-554. [21] Cardona, M. (1963) Optical properties of the silver and cuprous halides. Physical Review, 129(1), 69-78. [22] Mochizuki, S. and Fujishiro, F. (2003) Structural, elec- trical and optical studies on AgI-anatase composites. Journal of Physics: Condensed Matter, 15(29), 5057-5072. [23] Kumar, P.S. (2002) Ionic and mesoscopic aspects of cation stabilized silver iodide. Ph.D Thesis, University of Hyderabad, Hyderabad, India. [24] Kumar, P.S. and Sunandana, C.S. (2002) Steady-state photoluminescence characteristics of Sb-doped AgI thin films. Nano Letters, 2(9), 975-978. |

