 Vol.3, No.1, 81-87 (2012) Journ al of Biophysical Chemistry http://dx.doi.org/10.4236/jbpc.2012.31010 Laccases stabilization with phosphatidylcholine liposomes Meritxell Martí1*, Andrea Zille2, Artur Cavaco-Paulo3, José Luís Parra1, Luisa Coderch1 1Institute of Advanced Chemistry of Catalonia (IQAC-CSIC), Barcelona, Spain; *Corresponding Author: meritxell.marti@iqac.csic.es 2IBMC—Institute for Molecular and Cell Biology, Porto, Portugal 3Textile Engineering Department, University of Minho, Guimarães, Portugal Received 13 October 2011; revised 24 November 2011; accepted 8 December 2011 ABSTRACT In recent years, there has been an upsurge of interest in enzyme treatment of textile fibres. Enzymes are globular proteins whose catalytic function is due to their three dimensional struc- ture. For this reason, stability strategies make use of compounds that avoid dismantling or distorting protein 3D structures. This study is concerned with the use of microencapsulation techniques to optimize enzyme stabilization. La- ccases were embedded in phophatidylcholine liposomes and their encapsulation capacity was assessed. Their enzymatic activity and stability were analyzed, comparing free-enzymes, enzy- mes in liposomes, and the lipid fraction sepa- rated from the aqueous fraction. An increase in their encapsulation efficiency was found at higher lipid/laccase ratios. Relative activity of enzyme-containing vesicles has also been shown to be ret ained muc h more than that o f free nativ e enzymes. The loss of activity of laccases en- trapped in the vesicles in the total stability pro- cess is lower than 10% compared with 40% to 60% of loss of free-laccases after heating the samples for 3 days. Laccase stabilization could be of interest to future textile or cosmetic appli- cations because of their potential for environ- mentally friendly oxidation tech nologies. Keywords: MLV Liposome; E n zymes; Laccases; Encapsulation; Stability 1. INTRODUCTION Enzymes have been used in different industries inclu- ding textiles for washing, scouring, dyeing, etc. Protein enzymes can interact with all products used in the process, and their large 3D structure enables their interaction with different chemical products in solution due to the variety of side chains of the amino acids. Enzyme stabilization has assumed considerable importance owing to the in- creasing number of enzyme applications and to the need for realizing their full potential as catalysts 1. Liposomes are defined as a structure composed of lipid vesicle bilayers enclosing an aqueous volume. These structures have long been used as carrier systems for the delivery of vaccines, therapeutic drugs and hor- mones because of easy preparation, good biocompatibility, low toxicity and commercial availability 2,3. Efficient functioning of enzymes inside liposomes opens up new possibilities of applications in biocatalysis and bioanaly- tical tools 4-6. It has been observed that enzymes are considerably stabilized within the nano-environment of liposomes since they are protected from unfolding and proteolysis. Liposomes can effectively protect enzymes from aggression of external agents such as proteases 7. In addition, enzymes entrapped in liposomes are stabi- lized against unfolding forces owing to hydrophobic interactions between the enzyme and th e liposome mem- brane 8. Moreover, enzymes encapsulated inside lipo- somes retain their activity even at very low concentra- tions 9. Since liposomes are optically translucent, they can be used as optical sensor elements 10. Novel lip- osome-based nano-sized biosensor systems have been prepared by encapsulating an enzyme using porin em- bedded in the lipid membrane. As a result, the enzyme activity within the liposome can be monitored using pyranine as a fluorescent pH indicator 11 . Furthermore, some enzymes such us horseradish peroxidase encapsu- lated in liposomes have been directly detected without lysis using Luminol chemiluminiscence 12. There are two main areas of application of enzyme- containing lipid vesicles: biomedicine (enzyme-replace- ment therapy) and the food industry (cheese ripening process). In both cases the lipid vesicles are carriers that protect the enzymes from contact with blood or milk, respectively 6. The stability effect of enzyme encapsu- Copyright © 2012 SciRes. OPEN ACCESS  M. Martí et al. / Journal of Biophysical Chemistry 3 (2012) 81-87 82 lation in liposomes has been studied for many enzymes in these two fields 6. However, few studies have been performed with laccases that are used in textile and cosmetic industries 13-15. Laccases are of special interest because of their ability to oxidise both phenolic and non-phenolic lignin related compounds as well as other environmental pollutants, which makes them very useful for biotechnologies. The use of laccases in textiles is currently enjoying rapid growth; they are used for decolorizing textile effluents 16,17, bleaching 18, dyeing 19, synthesizing dyes 20 and for modifying the surface of fabrics 21,22. In the cosmetic field, laccases can replace H2O2 as an oxidizing agent in dye formulation 23. Enzymes are globular proteins whose catalytic function is due to their three-dimensional confo rmation. For this reason, stability strategies make use of compounds that avoid dismantling or distorting protein 3D structures. Therefore, microen- capsulation, particularly with liposomes, can be envi- saged as a good strategy for stabilizing laccases. Of the different existing oxidant enzymes, laccases have been the subject of intensive research in the last decades due to their low substrate specificity in the textile and cosmetic fields as stated above. Therefore, new strategies are needed for stabilizing and maintaining their enzyma- tic activity. Liposomes have also aroused a great deal of interest in the textile industry 24,25 and in the cosmetic industry 26,27. This work seeks to shed light on the behaviour of laccases microencapsulated in liposomes. Enzyme stabilization was determined by the evaluation of the thermostability of the free and entrapped laccases. Effi- cient functioning of laccases inside liposomes would open up new avenues for textile or cosmetic applications. 2. MATERIALS AND METHODS 2.1. Liposomes Formation and Enzime Encapsulation Liposome suspension of 2 wt% of phosphatidylcholine (PC) and 10 wt% laccases solution (weight ratio of lipid to laccase 1/5 lipid/laccases (LpLc)) and also another suspension of 10 wt% of PC and 5 wt% of laccases solu- tion (weight ratio of lipid to laccase 1/0.5 lipid/laccases (LpLc)) were prepared using the film hydration method. To obtain these liposome suspensions, 0.1g or 0.5g, re- spectively of Lipoid S-100 (Lipoid GmbH, Germany) were prepared in solution in 30 ml of chloroform. The chloroform was then removed by a rotary evaporator under reduced pressure. A thin film of lipid was observed after the solvent were removed. The lipid film was hy- drated with 5 ml buffer solution (pH 5) containing the laccase solution, 5.86 mg/ml, commercial Trametes vil- losa of Novozymes Spain S.A., (0.5 ml or 0.25 ml, re- spectively) and, after 10 minutes sonication in a water bath multilamellar vesicles (MLV) were obtained. Lipo- somes suspensions of 2 wt% and 10 wt% of PC (Lp) without enzymes were also prepared following the same methodology but using only a buffer solution without laccases. The amount of protein was determined for the laccase (Lc) solution and also for the lipid alone to ev aluate pos- sible interferences. To quantify the laccases entrapped in liposomes, LpLc was precipitated and separated from the supernatant by centrifugation at 14000 RPM for 15 min- utes using a Centrifuge 5415-Eppendorf (Germany). Af- ter separation, the supernatant was retained. The precipi- tate was filled at 1ml with buffer and agitated vigorously and centrifuged again. This process was repeated two more times and all supernatants were kept together (Sn LpLc). Finally, the eppendorf with the precipitate lipo- somes (V LpLc), the supernatant (Sn LpLc) and the full LpLc solution, were filled with a 0.125% Triton X-100 solution, (octylphenol ethoxylated with 10 units of eth- ylene oxide and active matter of 100%) supplied by Tenneco S.A. (Spain), and agitated vigorously to solubi- lise the lipid bilayer. The amount of protein was deter- mined in all samples following the Bradford method in order to obtain the amount of encapsulated laccase. The formulations evaluated are summarized in Figure 1. The Bradford method was used to quantify the protein in these samples. It is based on the formation of a com- plex between the dye, Brilliant Blue G, Sigma (USA), and the proteins in solution, which produces an increase Figure 1. Diagram of the protein quantification and of stability assays. Copyright © 2012 SciRes. OPEN ACCESS 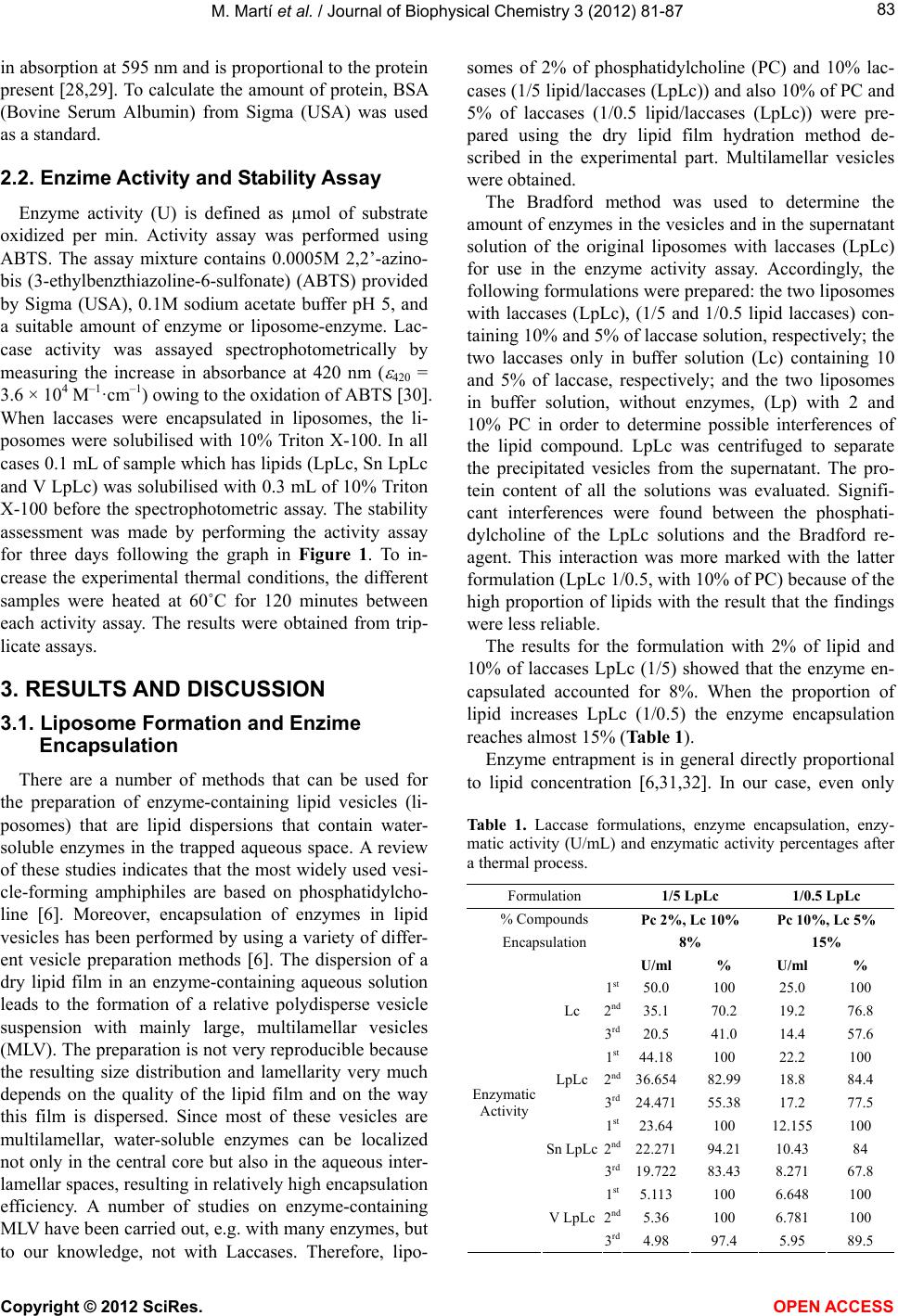 M. Martí et al. / Journal of Biophysical Chemistry 3 (2012) 81-87 83 in absorption at 595 nm and is proportional to the protein present 28,29. To calculate the amount of protein, BSA (Bovine Serum Albumin) from Sigma (USA) was used as a standard. 2.2. Enzime Activity and Stability Assay Enzyme activity (U) is defined as µmol of substrate oxidized per min. Activity assay was performed using ABTS. The assay mixture contains 0.0005M 2,2’-azino- bis (3-ethylbenzthiazoline-6-sulfonate) (ABTS) provided by Sigma (USA), 0.1M sodium acetate buffer pH 5, and a suitable amount of enzyme or liposome-enzyme. Lac- case activity was assayed spectrophotometrically by measuring the increase in absorbance at 420 nm ( 420 = 3.6 × 104 M–1·cm–1) owing to the oxidation of ABTS 30. When laccases were encapsulated in liposomes, the li- posomes were solubilised with 10% Triton X-100. In all cases 0.1 mL of sample which has lipids (LpLc, Sn LpLc and V LpLc) was solubilised with 0.3 mL of 10% Triton X-100 before the spectrophotometric assay. The stability assessment was made by performing the activity assay for three days following the graph in Figure 1. To in- crease the experimental thermal conditions, the different samples were heated at 60˚C for 120 minutes between each activity assay. The results were obtained from trip- licate assays. 3. RESULTS AND DISCUSSION 3.1. Liposome Formation and Enzime Encapsulation There are a number of methods that can be used for the preparation of enzyme-containing lipid vesicles (li- posomes) that are lipid dispersions that contain water- soluble enzymes in the trapped aqueous space. A review of these studies indicates that the most widely used vesi- cle-forming amphiphiles are based on phosphatidylcho- line 6. Moreover, encapsulation of enzymes in lipid vesicles has been performed by using a variety of differ- ent vesicle preparation methods 6. The dispersion of a dry lipid film in an enzyme-containing aqueous solution leads to the formation of a relative polydisperse vesicle suspension with mainly large, multilamellar vesicles (MLV). The preparation is not very reproducible because the resulting size distribution and lamellarity very much depends on the quality of the lipid film and on the way this film is dispersed. Since most of these vesicles are multilamellar, water-soluble enzymes can be localized not only in the cen tral core but also in the aqueous inter- lamellar spaces, resulting in relatively high encapsulation efficiency. A number of studies on enzyme-containing MLV have been carried out, e.g. with many enzymes, but to our knowledge, not with Laccases. Therefore, lipo- somes of 2% of phosphatidylcholine (PC) and 10% lac- cases (1/5 lipid/laccases (LpLc)) and also 10% of PC and 5% of laccases (1/0.5 lipid/laccases (LpLc)) were pre- pared using the dry lipid film hydration method de- scribed in the experimental part. Multilamellar vesicles were obtained. The Bradford method was used to determine the amount of enzymes in the vesicles and in the supernatant solution of the original liposomes with laccases (LpLc) for use in the enzyme activity assay. Accordingly, the following formulations were prepared: the two liposomes with laccases (LpLc), (1/5 and 1/0.5 lipid laccases) con- taining 10% and 5% of laccase solution, respectively; the two laccases only in buffer solution (Lc) containing 10 and 5% of laccase, respectively; and the two liposomes in buffer solution, without enzymes, (Lp) with 2 and 10% PC in order to determine possible interferences of the lipid compound. LpLc was centrifuged to separate the precipitated vesicles from the supernatant. The pro- tein content of all the solutions was evaluated. Signifi- cant interferences were found between the phosphati- dylcholine of the LpLc solutions and the Bradford re- agent. This interaction was more marked with the latter formulation (LpLc 1/0.5, with 10% of PC) because of the high proportion of lipids with the result that the findings were less reliable. The results for the formulation with 2% of lipid and 10% of laccases LpLc (1/5) showed that the enzyme en- capsulated accounted for 8%. When the proportion of lipid increases LpLc (1/0.5) the enzyme encapsulation reaches almost 15% (Table 1). Enzyme entrapment is in general directly proportional to lipid concentration 6,31,32. In our case, even only Table 1. Laccase formulations, enzyme encapsulation, enzy- matic activity (U/mL) and enzymatic activity percentages after a thermal process. Formulation 1/5 LpLc 1/0.5 LpLc % Compounds Pc 2%, Lc 10% Pc 10%, Lc 5% Encapsulation 8% 15% U/ml % U/ml % 1st 50.0 100 25.0 100 2nd 35.1 70.2 19.2 76.8 Lc 3rd 20.5 41.0 14.4 57.6 1st 44.18 100 22.2 100 2nd 36.654 82.99 18.8 84.4 LpLc 3rd 24.471 55.38 17.2 77.5 1st 23.64 100 12.155100 2nd 22.271 94.21 10.43 84 Sn LpLc 3rd 19.722 83.43 8.271 67.8 1st 5.113 100 6.648 100 2nd 5.36 100 6.781 100 Enzymatic Activity V LpLc 3rd 4.98 97.4 5.95 89.5 Copyright © 2012 SciRes. OPEN ACCESS  M. Martí et al. / Journal of Biophysical Chemistry 3 (2012) 81-87 84 two lipid/laccases ratios were studied, but a clear in- crease on encapsulation percentage with the increase on lipid concentration was obtained. The encapsulation effi- ciency of protein has been reported to depend on interac- tion between the protein and the lipid bilayer 32. The enzyme entrapment can be inc re ased b y ma nip ul at ion of the liposomal lipid composition or by increasing the lipid concentration, in order to favour electrostatic in- teractions 32. Sometimes the entrapment efficiency decreases with increasing the lipid content 31. The ad- dition of more lipid increases the lamelarity of the vesi- cle population rather than producing more vesicles of the same lamelarity 31. Therefore, modification of lipid composition and lipid/enzyme ratios would be the base of further work with the aim to improve encapsulation efficiency and to deep inside the protein-lipid interac- tions. Similar results have been reported for enzyme entrap- ment efficiency of other enzymes in MLV. These varied in most cases from below 5% 33-35 to about 15% 36. Thus, although a smaller amount of enzymes and a lower activity could be found in the liposome formulation of 10% of lipid and 5% of laccases LpLc (1/0.5), the higher encapsulation obtained could demonstrate more clearly the effect of the liposome on the stability of the encapsu- lated laccases. 3.2. Enzime Activity and Stability Assay Laccase activity was evaluated by the ABTS method as detailed in the experimental part. The same amount of laccases in liposomes and laccases in the buffer solution of 5% and 10% were assessed. As expected, the enzyme activity of the solution containing 10% of laccases (50.0 U/ml, 10% Lc) is approximately double that of the solu- tion containing 5% laccases (24.9 U/ml, 5% Lc) since the enzyme concentration is also the double. In both cases, the presence of liposomes leads to a decrease in the en- zyme activity of about 11.6% for 1/5 LpLc (44.2 U/ml, 10% LpLc) and 11.2 % for 1/0.5 LpLc (22.2 U/ml, 5% LpLc). These decreases in laccase activities when for- mulated with PC liposomes were expected because the barrier of the lipid membrane diminished the activity of the enzyme entrapped in the liposomes 37. It should be borne in mind that formulations with a high amount of enzymes are more prone to lose activity in the thermal or proteolytical processes. Therefore, the similar percentage of lost activity found for the two formulations could be due to the high PC content in the 1/0.5 LpLc (10%Pc) and to the high amount of Laccases in 1/5 LpLc formula- tion (10% Laccases). Even though en zyme activity is decreased in the initial LpLc formulation when lipids were present, the stabili- ties of the two formulations were assayed. The main aim of this work was to study a possible increase in enzyme stability in its formulation entrapped in liposomes. The influence of enzyme entrapment on enzyme stability was also investigated. To assess stability, a protocol was de- signed to compare laccase stability in buffer solution (Lc) and laccase stability formulated with liposomes (LpLc) (Figure 1). In addition to evalu atin g th e to tal formulation of laccases in liposomes, aliquots of the formulation were centrifuged to separate the supernatant (Sn LpLc) from the pellet (V LpLc) (in which laccases are encapsu- lated) and the activities of the two samples were also determined. To toughen the conditions, samples were heated for 2 hours at 60˚C and the activity was measured the following day. This process was continued for three successive days. The enzyme activity graph of the different samples ob- tained during the three days of the experiment is shown in Figure 2. The first day of the experiment during which the samples were not heated, the laccases in the liposome solution presented a lower activity than the laccases in buffer solution, as discussed above. However, after 3 days, the LpLc formulation maintained higher activity values than the free laccases Lc. The graph clearly shows the stabilization obtained when laccases are bound to liposome vesicles. Relative activity of other enzyme- containing vesicles has also been demonstrated to retain activity much longer than native enzyme 31 as in our case. The stabilization of the supernatant should also to be noted. However, higher stabilization was obtained for the enzyme entrapped in the vesicles. The two formula- tions show similar activity (5 - 7 U/ml) for the Laccases entrapped in the vesicles. This occurs because the en- zyme entrapment is double in this formulation despite the presence of only 50% of the Laccase concentration in the 1/0.5 LpLc. The influence of the liposomes on the stability of lac- case activity is clearly demonstrated by the percentage of laccase activity of each sample during the whole stability process (Table 1). The greater stabilization of the lac- cases that are exclusively entrapped in the vesicles should be noted. The activity loss of laccases entrapped in the vesicles in the total stability process is lower than 10% when compared with 40% to 60% of activity of free-laccases after heating the samples for 3 days. These results confirm that the increase in the stability under- gone by laccases in the liposome solution is mainly due to the encapsulation degree of the enzymes. In general, encapsulation of enzymes in liposomes has demonstrated the enhancement of their stability versus denaturizing 6,9. For example, other enzymes such as amylogucosidase entrapped in multilamellar vesicles (MLVs) composed of other phospholipids such as di- palmitoylphosphatidylcholine (PPC) have been much ore stable than free enzymes 38. The rate of hydroly- m Copyright © 2012 SciRes. OPEN ACCESS 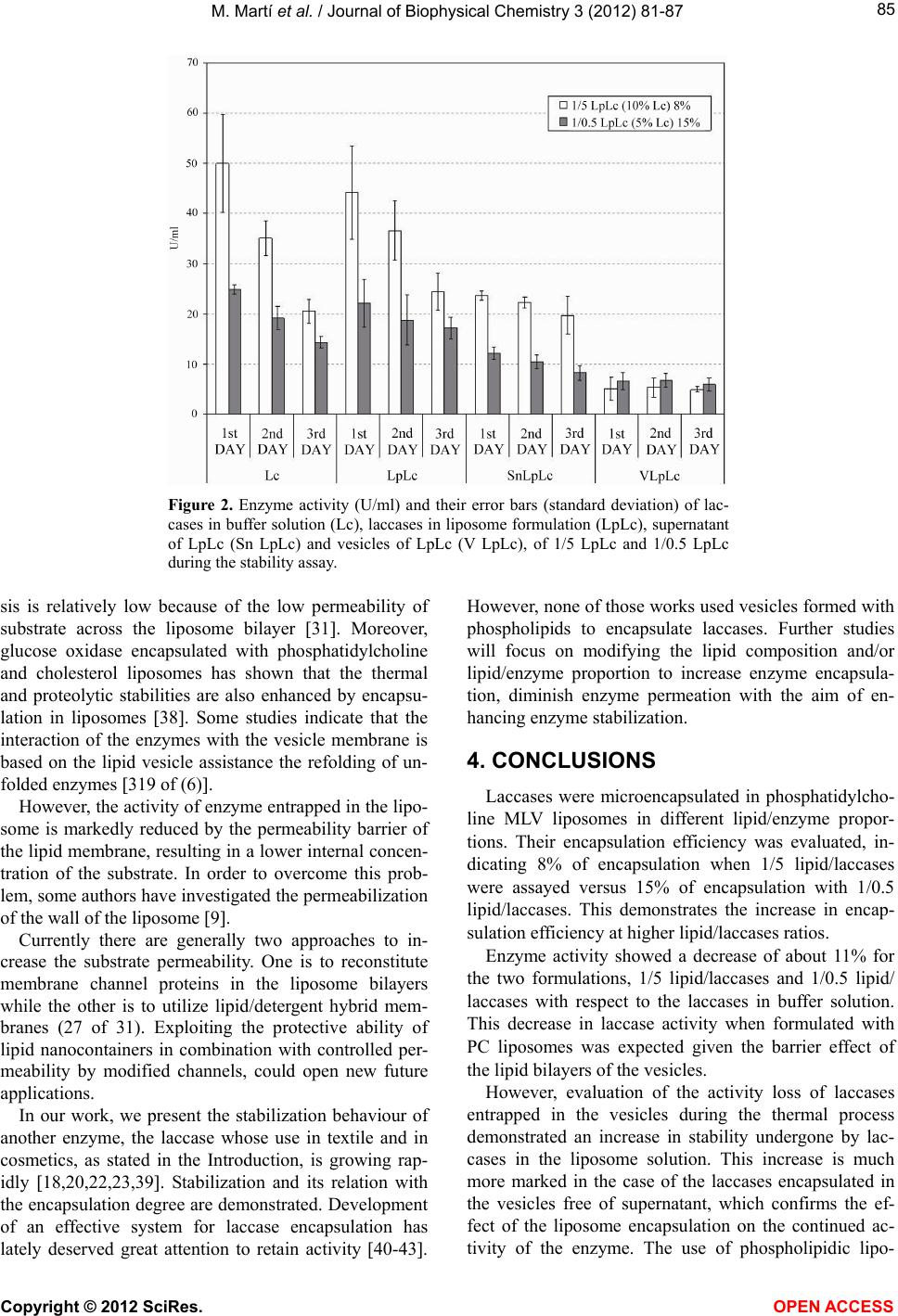 M. Martí et al. / Journal of Biophysical Chemistry 3 (2012) 81-87 Copyright © 2012 SciRes. 85 Figure 2. Enzyme activity (U/ml) and their error bars (standard deviation) of lac- cases in buffer solution (Lc), laccases in liposome formulation (LpLc), supernatant of LpLc (Sn LpLc) and vesicles of LpLc (V LpLc), of 1/5 LpLc and 1/0.5 LpLc during the stability a ssay. sis is relatively low because of the low permeability of substrate across the liposome bilayer 31. Moreover, glucose oxidase encapsulated with phosphatidylcholine and cholesterol liposomes has shown that the thermal and proteolytic stabilities are also enhanced by encapsu- lation in liposomes 38. Some studies indicate that the interaction of the enzymes with the vesicle membrane is based on the lipid vesicle assistance the refolding of un- folded enzymes 319 of (6). OPEN ACCESS However, the activity of enzyme entrapp ed in the lipo- some is markedly reduced by the permeability barrier of the lipid membrane, resulting in a lower internal concen- tration of the substrate. In order to overcome this prob- lem, some authors have investigated th e permeabilization of the wall of the liposome 9. Currently there are generally two approaches to in- crease the substrate permeability. One is to reconstitute membrane channel proteins in the liposome bilayers while the other is to utilize lipid/detergent hybrid mem- branes (27 of 31). Exploiting the protective ability of lipid nanocontainers in combination with controlled per- meability by modified channels, could open new future applications. In our work, we present the stabilization behaviour of another enzyme, the laccase whose use in textile and in cosmetics, as stated in the Introduction, is growing rap- idly 18,20,22,23,39. Stabilization and its relation with the encapsulation degree are demonstrated. Development of an effective system for laccase encapsulation has lately deserved great attention to retain activity 40-43. However, none of those works used vesicles formed with phospholipids to encapsulate laccases. Further studies will focus on modifying the lipid composition and/or lipid/enzyme proportion to increase enzyme encapsula- tion, diminish enzyme permeation with the aim of en- hancing enzyme stabilization. 4. CONCLUSIONS Laccases were microencapsulated in phosphatidylcho- line MLV liposomes in different lipid/enzyme propor- tions. Their encapsulation efficiency was evaluated, in- dicating 8% of encapsulation when 1/5 lipid/laccases were assayed versus 15% of encapsulation with 1/0.5 lipid/laccases. This demonstrates the increase in encap- sulation efficiency at higher lipid/laccases ratios. Enzyme activity showed a decrease of about 11% for the two formulations, 1/5 lipid/laccases and 1/0.5 lipid/ laccases with respect to the laccases in buffer solution. This decrease in laccase activity when formulated with PC liposomes was expected given the barrier effect of the lipid bilayers of the vesicles. However, evaluation of the activity loss of laccases entrapped in the vesicles during the thermal process demonstrated an increase in stability undergone by lac- cases in the liposome solution. This increase is much more marked in the case of the laccases encapsulated in the vesicles free of supernatant, which confirms the ef- fect of the liposome encapsulation on the continued ac- tivity of the enzyme. The use of phospholipidic lipo-  M. Martí et al. / Journal of Biophysical Chemistry 3 (2012) 81-87 86 somes in the laccases encapsulation warrants further studies that modify the lipid composition and/or lipid/ enzyme proportion to increase enzyme encapsulation and consequently enzyme stabilization. The study of other lipid/laccases ratios would allow us to determine their possible linearity with encapsulation efficiency and with enzyme activity as it happen with other enzymes and phospholipids [31,32]. Laccase stabilization could be of considerable interest to future textile or cosmetic appli- cations owing to their potential for environmentally friendly oxidation technologies. 5. ACKNOWLEDGEMENTS The authors are indebted to Ms. I. Yuste for technical support. The authors also wish to thank G. von Knorring for improving the final version of the manuscript. This work was supported by FCT (SFRH/ BPD/37045/2007; PTDC/CTM/100627/2008), QREN, COMPETE— Programa Operacional Factores de Co mpetitividade na sua componente FEDER. REFERENCES [1] Iyer, P.V. and Ananthanarayan, L. (2008) Enzyme stabil- ity and stabilization-aqueous and non-aqueous environ- ment. Process Biochemistry, 43, 1019-103. doi:10.1016/j.procbio.2008.06.004 [2] Gregoriadis, G., Florence, A.T. and Patel, H.M. (1993) Liposomes in drug delivery. Harwood Academic Publish- ers, London. [3] Gregoriadis, G. (1999) DNA vaccines: A role for lipo- somes. Current Opinion in Molecular Therapeutics, 1, 39-42. [4] Rosenberg, M.F., Jones, M.N. and Vadgama, P.M. (1991) A liposomal enzyme electrode for measuring glucose. Biochimica et Biophysica Acta, 1115, 157-165. [5] Walde, P. and Marzetta, B. (1998) Bilayer permeabil- ity-based substrate selectivity of an enzyme in liposomes. Biotechnology and Bioengeering, 57, 216-219. doi:10.1002/(SICI)1097-0290(19980120)57:2<216::AID- BIT10>3.3.CO;2-2 [6] Walde, P. and Ichikawa, S. (2001) Enzymes inside lipid vesicles: Preparation, reactivity and applications, Bio- mololecular Engineering, 18, 143-177. doi:10.1016/S1389-0344(01)00088-0 [7] Winterhalter, M., Hilty, C., Bezrukov, S.M., Nardin, C., et al. (2001) Controlling membrane permeability with bac- terial porins: Application to encapsulated enzymes. Ta- lanta, 55, 965-971. doi:10.1016/S0039-9140(01)00494-5 [8] Han, X., Li, G. and Li, K. (1998) FTIR study of the ther- mal denaturation of a-actinin in its lipid-free and diole- oylphosphatidylglycerol-bound states and the central and N-terminal domains of a-actinin in D2O. Biochemistry, 37, 10730-10737. doi:10.1021/bi9800451 [9] Nasseau, M., Boublik, Y., Meier, W., Winterhalter, M., et al. (2001) Substrate-permeable encapsulation of enzymes maintains effective activity, stabilizes against denatura- tion and protects against proteolytic degradation. Bio- thechnology and Bioengeering, 75, 615-618. doi:10.1002/bit.10074 [10] Kulin, S., Kishore, R., Helmerson, K. and Locascio, L. (2003) Optical manipulation and fusion of liposomes as microreactors. Langmuir, 19, 8206-8210. doi:10.1021/la0344433 [11] Vamvakaki, V., Fournier, D. and Chaniotakis, N.A. (2005) Fluorescence detection of enzymatic activity within a liposome based nanobiosensor. Biosensors and Bioelec- tronics, 21, 384-388. doi:10.1016/j.bios.2004.10.028 [12] Kamidate, T., Komatsu, K., Tani, H. and Ishida, A. (2008) Direct determination of horseradish peroxidase encapsu- lated in liposomes by using luminol chemiluminescence. Analytical Sciences, 24, 477-481. doi:10.2116/analsci.24.477 [13] Minussi, R.C., Pastore, G.M. and Durán, N. (2002) Poten- tial applications of laccase in the food industry. Trends in Food Science & Technology, 13, 205-216. doi:10.1016/S0924-2244(02)00155-3 [14] Kuhad, R.C., Singh, A. and Eriksson, K.E.L. (1997) Ad- vances in biochemical engineering biotechnology. In: Eriksson, K.E.L., Ed., Biotechnology in the Pulp and Paper Industry, Springer Verlag, Berlin. [15] Rodríguez Couto, S. and Toca Herrera, J.L. (2006) Indus- trial and biotechnological applications of laccases: A re- view. Biotechnology Advances, 24, 500-513. doi:10.1016/j.biotechadv.2006.04.003 [16] Zille, A., Tzanov, T., Gübitz, G. and Cavaco-Paulo, A. (2003) Immobilized laccase for decolourization of reac- tive black 5 dyeing. Biotechnology Letters, 25, 1473- 1477. doi:10.1023/A:1025032323517 [17] Zille, A., Górnacka, B., Rehorek, A. and Cavaco-Paulo, A. (2005) Degradation of azo dyes by trametes villosa lac- case over long periods of oxidative conditions. Applied and Environmental Microbiology, 71, 6711-6718. doi:10.1128/AEM.71.11.6711-6718.2005 [18] Vinoid, S. (2001) Enzymatic decolourisation of denims: A novel approach. Colourage, 48, 25-26. [19] Tzanov, T., Silva, C.J., Zille, A., Oliveira, J. and Cavaco- Paulo, A. (2003) Effect of some process parameters in enzymatic dyeing of wool. Applied Biochemistry and Biothecnology, 111, 1-13. doi:10.1385/ABAB:111:1:1 [20] Setti, L., Giuliani, S., Spinozzi, G. and Pifferi, P.G. (1999) Laccase catalyzed-oxidative coupling of 3-methyl 2-ben- zothiazolinone hydrazone and methoxyphenols. Enzyme and Microbial Technology, 25, 285-289. doi:10.1016/S0141-0229(99)00059-9 [21] Silva, C., Silva, C.J., Zille, A., Güebitz, G.M. and Cavaco- Paulo, A. (2007) Laccase immobilization on enzymati- cally functionalized polyamide 6,6 fibres. Enzyme and Micr obial Technology, 41, 867-875. doi:10.1016/j.enzmictec.2007.07.010 [22] Kim, S.Y., Zille, A., Murkovic, M., Güebitz, G. and Cavaco-Paulo, A. (2007) Enzymatic polymerization on the surface of the functionalized cellulose fibres. Enzyme and Microbial Technology, 40, 1782-1787. Copyright © 2012 SciRes. OPEN ACCESS  M. Martí et al. / Journal of Biophysical Chemistry 3 (2012) 81-87 Copyright © 2012 SciRes. OPEN ACCESS 87 doi:10.1016/j.enzmictec.2007.01.001 [23] Roure, M., Delattre, P. and Froger, H. (1992) Composi- tion for an enzymatic coloration of keratin fibres, espe- cially for hair and its use in a dyeing process. European Patent Application EP0504005. [24] Montazer, M., Validi, M. and Toliyat, T. (2006) Influence of temperature on stability of multilamellar liposomes in wool dyeing. Journal of Liposome Research, 16, 81-89. doi:10.1080/08982100500528883 [25] Martí, M., de la Maza, A., Parra, J.L. and Coderch, L. (2001) Dyeing wool at low temperatures: New method using liposomes. Textile Research Journal, 71, 678-682. doi:10.1177/004051750107100805 [26] De Pera, M., Coderch, L., Fonollosa, J., De la Maza, A., et al. (2000) Effect of internal wool lipid liposomes on skin repair. Skin pharmacol. Applied Skin Physiology, 13, 188- 195. doi:10.1159/000029925 [27] Ramírez, R., Martí, M., Cavaco-Paulo, A., Siva, R., De la Maza, A., Parra, J.L. and Coderch, L. (2009) Liposome formation with wool lipid extracts rich in ceramides. Journal of Liposome Research, 19, 77-83. doi:10.1080/08982100802538838 [28] Bradford, M.M. (1976) A rapid and sensitive method for the quantization of microgram quantities of protein util- izing the principle of protein-dye binding. Analytical Bio- chemistry, 72, 248-254. doi:10.1016/0003-2697(76)90527-3 [29] Technical Bulletin of Bradford Reagent, Sigma-Aldrich (USA). [30] Childs, R.E. and Bardsley, W.G. (1975) The steady-state kinetics of peroxidase with 2, 2’-azino-di-(3-ethyl-benz- thiazoline-6-sulphonic acid) as chromogen. Biochemical Journal, 145, 93-103. [31] Li , M., Handford, M.J., Kim, J. W. and Peeples, T.L. (2007 ) Amyloglucosidase enzymatic reactivity inside lipid vesi- cles. Journal of Biological Engineering, 1, 4. doi:10.1186/1754-1611-1-4 [32] Colletier, J-P., Chaize, B., Winterhalter, M. and Fournier, D. (2002) Protein encapsulation in liposomes: Efficiency depends on interactions between protein and phosphol- ipid bilayer. BMC Biotechnology, 2, 9. doi:10.1186/1472-6750-2-9 [33] Kirby, C.J., Brooker, B.E. and Law, B.A. (1987) Acceler- ated ripening of cheese using liposome-encapsulated en- zymes. International Journal of Food Science and Tech- nology, 22, 355-375. doi:10.1111/j.1365-2621.1987.tb00499.x [34] Naoi, M., Naoi, M., Shimizu, T., Malviya, A. and Yagi, K. (1977) Permeability of amino acids into liposomes. Bio- chimica et Biophysica Acta, 471, 305-310. doi:10.1016/0005-2736(77)90258-9 [35] Law, B.A. and King, J.S. (1985) Use of liposomes for Proteinase addition to Cheddar cheese. Journal of Dairy Research, 52, 183-188. doi:10.1017/S0022029900024006 [36] Alkhalaf, W., Piard, J-C., El Soda, M., Gripon, J-C., Desmazeaud, M. and Vassal, L. (1988) Liposomes as proteinase carriers for the accelerated ripening of saint- paulin type cheese. Journal Food Science, 53, 1674-1679. doi:10.1111/j.1365-2621.1988.tb07813.x [37] Chaize, B., Colletier, J.P., Winterhalter, M. and Fournier, D. (2004) Encapsulation of enzymes in liposomes. High encapsulation efficiency and control of substrate perme- ability. Artificial cell blood subtitutes and biomed. Bio- technology, 32, 67-75. [38] Rodriguez-Nogales, J.M. (2004) Kinetic Behavior and stability of glucose oxidase entrapped in liposomes. Jour- nal of Chemical Technology and Biotechnology, 79, 72- 78. doi:10.1002/jctb.944 [39] Kunamneni, A., Ghazi, I., Camarero, S., Ballesteros, A. and Plou, F.J. (2008) Decolorization of synthetic dyes by laccase immobilized on epoxy-activated carriers. Process Biochemistry, 43, 169-178. doi:10.1016/j.procbio.2007.11.009 [40] Khani, Z., Jolivalt, C., Cretin, M., Tingry, S. and Innocent, C. (2006) Alginate/carbon composite beads for laccasse and glucose oxidase encapsulation: Application in biofuel cell technology. Biotechnology Letters, 28, 1779-1786. doi:10.1007/s10529-006-9160-1 [41] Lloret, L., Eibe s, G., Feij o, G., Moreira, M. T., Lema, J.M. and Hollman, F. (2011) Immobilization of laccase by en- capsulation in a sol-gel matrix and its chrarcterization and use for the removal of estrogens. Biotechnology Progress, 27, 1570-1579. doi:10.1002/btpr.694 [42] Niu, J., Yin, L. and Jiang, F. (2011) In situ encapsulation of laccase in nanofibers by electrospinning for develop- ment of enzyme biosensors for chlorophenol monitoring. Analyst, 136, 4802-4808. [43] Mazur, M., Krywko-Cendrowska, A., Krysinski, P. and Rogalski, J. (2009) Encapsulation of laccase in a con- ducting polymer matrix: A simple route towards polypyr- role microcontainers. Synthe tic Metals, 159, 1731-1738. doi:10.1016/j.synthmet.2009.05.018
|