Journal of Cancer Therapy
Vol.2 No.4(2011), Article ID:7799,9 pages DOI:10.4236/jct.2011.24075
Deuterium Depletion May Delay the Progression of Prostate Cancer
![]()
1Saint John’s Hospital, Budapest, Hungary; 2HYD LLC. for Cancer Research and Drug Development, Budapest, Hungary; 3Planimeter Ltd., Budapest, Hungary; 4University of Pécs, Medical School, Institute of Public Health, Pécs, Hungary.
Email: ad.kovacs@mail.janoskorhaz.hu, gullerimre@yahoo.com, {krempels, isomlyai, *gsomlyai}@hyd.hu, janosi@planimeter.hu, {zoltan.gyongyi, istvan.ember}@aok.pte.hu, bokor28@yahoo.co.uk
Received July 7th, 2011; revised August 10th, 2011; accepted August 19th, 2011.
Keywords: DeuteriumDepletion, DDW, Prostate Cancer, Phase II Clinical Trial, Retrospective Evaluation, Median Survival
ABSTRACT
Deuterium-depleted water (DDW) is a new promising agent in cancer therapy. The efficiency of the method is based on the discovery, that cancer cells are extremely sensitive to depletion of deuterium (D) and might cause necrosis of the tumour. The purpose of this study was to show the efficacy of D-depletion in prostate cancer (PC) patients. In the double blind, four-month-long, randomized Phase II clinical trial the daily water intake was replaced with DDW in 22 PC patients. Other 22 PC patients took normal water while both groups received the same forms of conventional treatment. In the retrospective study, 91 DDW-treated PC patients were evaluated and median survival time (MST) in the subgroups was calculated. The time course of changes in DDW dose and PSA is presented in two cases. In the prospective trial seven patients in the treated group and one patient in the placebo group achieved partial response (p = 0.046). In the treated group, the net decrease in the prostate volume was three times higher (160.3 cm3 vs. 54.0 cm3; p = 0.0019), urination complaints ceased at a higher rate (8 vs. 0 patients, p = 0.0041), and the one-year survival rate was also higher (2 vs. 9 deaths; p = 0.034). The 91 retrospectively evaluated patients achieved an MST of 11.02 years, despite the fact that 46 of them suffered from distant metastasis. In the two monitored patients, drop of PSA level correlated with the DDW intake. In summary, D-depletion prolonged MST in patients with PC. The method proved to be safe thus its integration in the PC cure as an adjuvant or complementary therapy would be considered.
1. Introduction
Hydrogen has a naturally occurring stable isotope, deuterium (D), with a mass number of 2. It is present in surface waters mainly in the form of HDO at a concentration of appr. 16.8 mmol/L. The two isotopes, hydrogen (1H) and deuterium (2D) have the largest mass difference among stable isotopes of the same element, resulting in significantly different chemical and physical properties [1-3].
Although the effect of D at an elevated concentration in biological systems has been investigated in several experiments [4-5], these studies ignored the significance of natural D-concentration. The first paper, suggesting that reduced D-concentration has an impact on living organisms, was published in 1993 [6]. Since then, numerous studies have been conducted on different cell lines using culture media prepared with deuterium-depleted water (DDW) [6-7]. The growth rate of different cancer cell lines significantly decreased in DDW-containing media in vitro. In mice xenotransplanted with MDA-MB-231, MCF-7 human breast adenocarcinomas or PC-3 human prostate tumors, DDW resulted in complete or partial tumour regression [7-8]. The apoptosistriggering effect of DDW was observed both in vitro [7] and in vivo [8]. D-depletion exerts an influence on protooncogenes and tumor suppressor genes, such as c-myc, Ha-ras and p53. Expression of these genes, induced by carcinogen exposure, was significantly lower when the animals received DDW as drinking water [9]. The most striking discovery was that cancer cells proved to be extremely sensitive to D-depletion which might even cause the necrosis of tumours, while non-cancer cells are able to tolerate the decreasing D-concentration. This finding raised the possibility that DDW might be one of the agents which can reduce the risk of cancer and acts as an effective modality in cancer therapy [10].
Prostate cancer (PC) is placed third in cancer mortality among men in Europe. The estimated mortality rate in 2011 is between 12.6 and 8.1/100,000 men [11]. In the years 2001-2005 the mean deaths of prostate cancer was 1265 in Hungary [12]. PC is the most frequently screened out malignant transformation; the incidence is 214 out of 1000 men. PC often lies behind the cases that are categorized as “died from other tumour” [13,14].
Decline in PC mortality is expected through Europe likely due to the attributable improvement in screening, diagnosis and predominantly treatment of the disease [11]. Treatment options for PC may have severe side effects, therefore an optimal choice attains a balance between deteriorations in quality of life and therapeutic benefit [15-17].
Hormonal therapy is a mainstay of treatment for patients with advanced PC [18]. The recently published clinical trials indicate a median survival time (MST) of 15 to 21 months in patients suffering from advanced hormone refractory PC [19-22].
In order to investigate whether DDW might exert an anticancer effect in humans and improve the results of conventional treatments, a four-month-long double-blind phase II placebo controlled clinical trial was conducted on PC patients. The primary outcome was the best response, and the agent’s safety was also assessed. In addition, the course of the disease was retrospectively evaluated in 91 DDW-treated patients.
2. Patients and Methods
DDW was produced from normal tap water using fractional distillation [23] as described earlier [24].
2.1. Prospective Study
The four-month-long phase II clinical trial was conducted (under the permission of the Hungarian Institute of Pharmacology: No. 5621/40/95) in four Hungarian trial sites in a double blind, randomized, placebo controlled setting according to GCP principles. The daily water intake of PC patients was replaced with DDW of 85 ppm D concentration (treated group), or remained normal with 150 ppm D (placebo group). The protocol contained no restrictive requirements with respect to the conventional treatments. The endpoints were the best overall response and the change in prostate size. Urination complaints, PSA value and safety were also evaluated. All patients were followed after closing their files for years, and the impact of DDW on survival was determined as well. Eligible patients had histologically confirmed primary or relapsed PC and their life expectancy was longer than six months. Forty-four patients were evaluated in the Intention-to-Treat analysis (ITT Population), 22 patients were involved in the treated and 22 patients in the placebo group. Thirty-three patients completed the trial in full accordance with the protocol requirements (PP Population), 17 of them (51.5%) were treated with the agent and 16 patients (48.5%) were taking the placebo. Here we present the evaluation of the ITT population alone, since the analysis of the ITT and PP population showed no substantive difference in the outcome. There was no statistical difference between the test groups in staging, histological diagnosis and the conventional treatments applied, so these factors were irrelevant in the statistical analysis.
The volume of the prostate gland was calculated with the formula
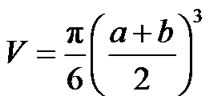
where a and b were the two diameters (cm) determined by transrectal ultrasonography. The prostate volume at the first visit (baseline) and the value calculated at the last (6th) visit were compared in both groups. Urination problems were also categorized. The PSA value at the last visit was compared to the baseline PSA level. During the extended follow-up, the mortality rates were compared in the test groups with Fisher’s Exact test.
Adverse events were to be also reported and characterized. At the time of involvement and the last visit hematologic values and serum chemical parameters were measured for screening liverand renal function according to GLP requirements.
2.2. Retrospective Study
Ninety-one PC patients were evaluated retrospectively. Eligible patient was who: 1) was histologically diagnosed with PC; 2) started consuming DDW at the time of the diagnosis or after that; 3) was taking DDW for at least 90 days in addition to the conventional forms of treatment. Patients were provided with all the available information regarding DDW-treatment. In this study a) D concentration of DDW was gradually decreased from 105 to 25 ppm; b) duration was longer than a year in most cases; c) the cure was interrupted and then continued several times. In order to gain comparable data a new rate, deuterium depletion unit (DdU), was introduced:
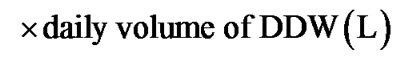
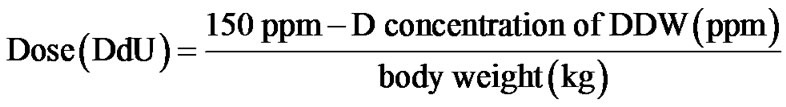
The time pattern of PSA changes and DDW dose was evaluated in detail in two patients.
The statistical analysis were performed using the SAS statistical package. The result was considered statistically significant at p < 0.05. The graphs were generated in R. Survival curves were created using the method of Kaplan and Meier.
Based on the presence or absence of distant metastasis, homogenous groups were made up. Forty-five patients had no metastasis. Among patients with metastasis (46 subjects) there were 32 patients who had exclusively bone metastasis and made up two homogenous subgroups; 20 of them developed bone metastasis within a year and 12 other patients later than one year after the diagnosis of PC. Fourteen patients were not involved in the evaluation due to the heterogeneity of metastases.
3. Results
3.1. Prospective Study
Seven patients in the treated group, and one patient in the placebo group achieved partial response (PR) (significant difference, p = 0.046). No change (NC) was verified in 11 patients in the treated, and in 13 patients in the placebo group (non-significant difference). Progression of disease (PD) was diagnosed in 4 cases in the treated group and in 8 patients of the control group. Because of the duration of the clinical trial (only 4 months), complete response (CR) was not verified in any of the patients during the study.
Prostate volume decreased in 18 out of 22 patients in the treated arm. No change was detected in one patient while the prostate volume increased in two subjects. There was no available data on the prostate size in one patient. In the placebo group, prostate size decreased in 11 patients, no change was recorded in 5 patients, and increased prostate size was found in another 5. One patient was excluded because the decrease in prostate size was not in connection with the improvement of the disease (the patient died after the 2nd visit). In the treated group, the cumulated decrease of prostate size was 171.6 cm3 and the increase was 11.3 cm3, while in the placebo group 108.1 cm3 decrease and 54.1 cm3 increase were found. The net decrease was 160.3 cm3 in the treated, and 54 cm3 in the control group (p = 0.0019). Furthermore, in those 7 patients who achieved PR, the prostate volume became smaller by 125.2 cm3 in total. One patient in the control group showing PR achieved 13.4 cm3 decrease in prostate volume.
Urination complaints ceased in 8 patients of the treated group, but in the placebo group none of the patients experienced changes in their complaints (p = 0.0041). Changes in the PSA values are summarized in Table 1.
It became evident that the PSA and prostate size were changing concomitantly in numerous cases. Cumulative PSA showed decrease in the test groups in the fourmonth-long study. The baseline value was 406.4 ng/mL and 521 ng/mL in the treated and placebo group, respectively. By the end of the trial the cumulative PSA value decreased to 80.3 ng/mL in the treated and 277.4 ng/mL in the placebo group.
Survival data were obtained during the three-year-long extended follow-up: in the first year 2 patients (9.1%) died in the treated group and 9 patients (40.9%) in the placebo group (p = 0.034, Fisher’s exact test). After twoyear follow-up, 7 patients died in the treated, and 12 in the placebo group, by the end of the third year the ratio was 8:12.
3.2. Retrospective Study
The cumulative time period that elapsed from the diagnosis of PC till the start of DDW treatment was 142.5 years (52,003 days) in the 91 DDW-treated PC patients. The cumulative duration of DDW treatment was 139.2 years (50,808 days), and the follow-up period from the initial diagnosis was 350.1 years (127,800 days).
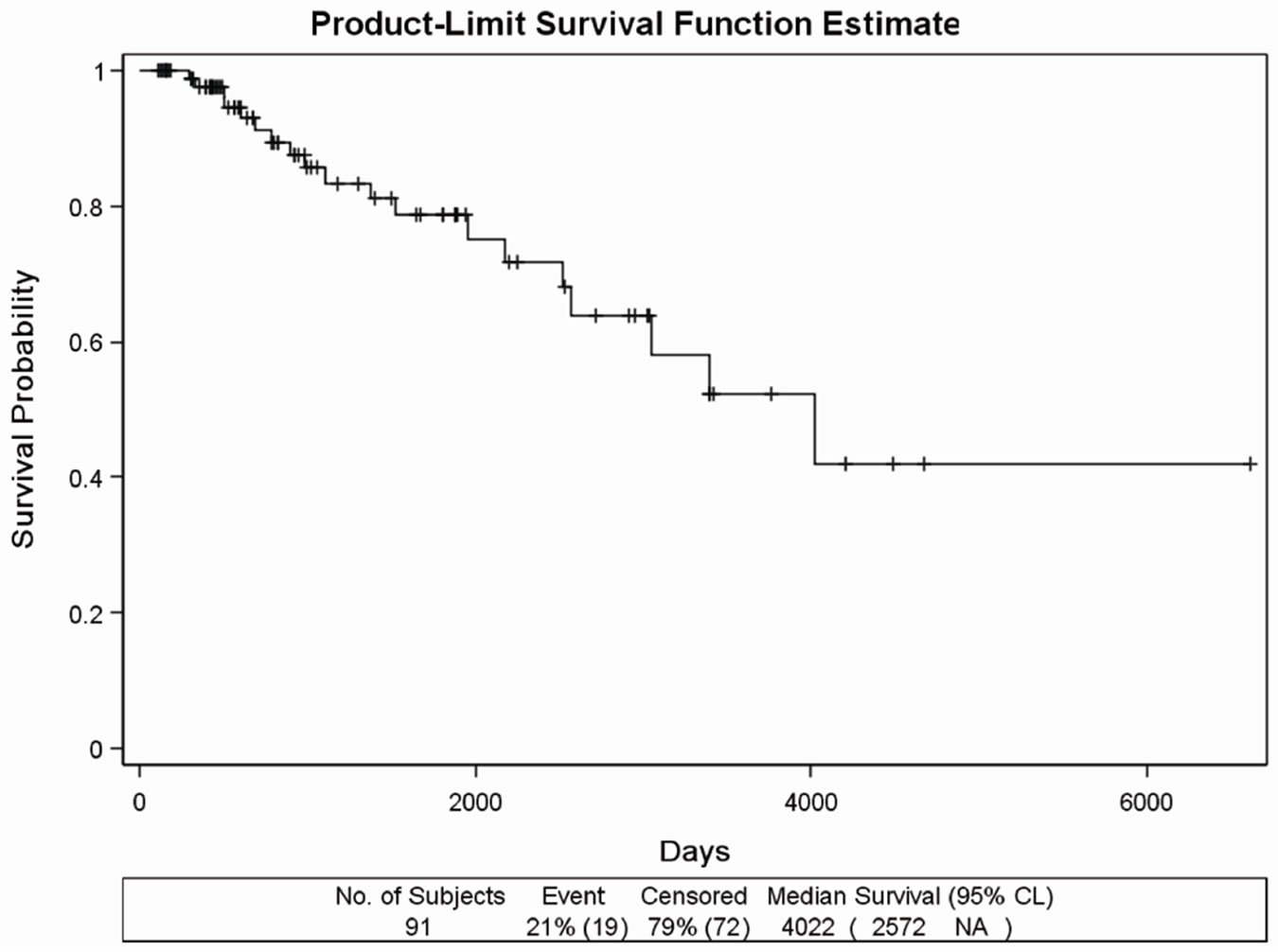
The MST was 11.02 years (4022 days), and 19 deaths (21 %) were detected (Figure 1(a)).
The Kaplan Meier curve of the 45 patients having no metastasis (Figure 1(b)) showed that only 4 patients (9%) died.
Due to the extremely long survival, the data were not suitable for calculating MST. Out of the 46 patients having distant metastasis, the subgroup of 20 patients whose bone metastasis was verified within one year from the diagnosis of PC was separated. On this subgroup, we addressed the question whether DDW extended MST; considering the poor prognosis at this stage. During the
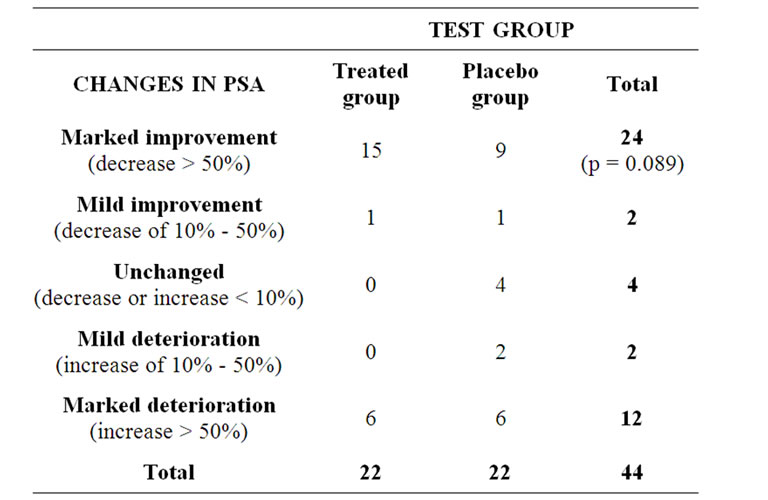
Table 1. Distribution of changes in PSA values during the four-month-long clinical trial in the DDW treated and placebo group.
 (a)
(a)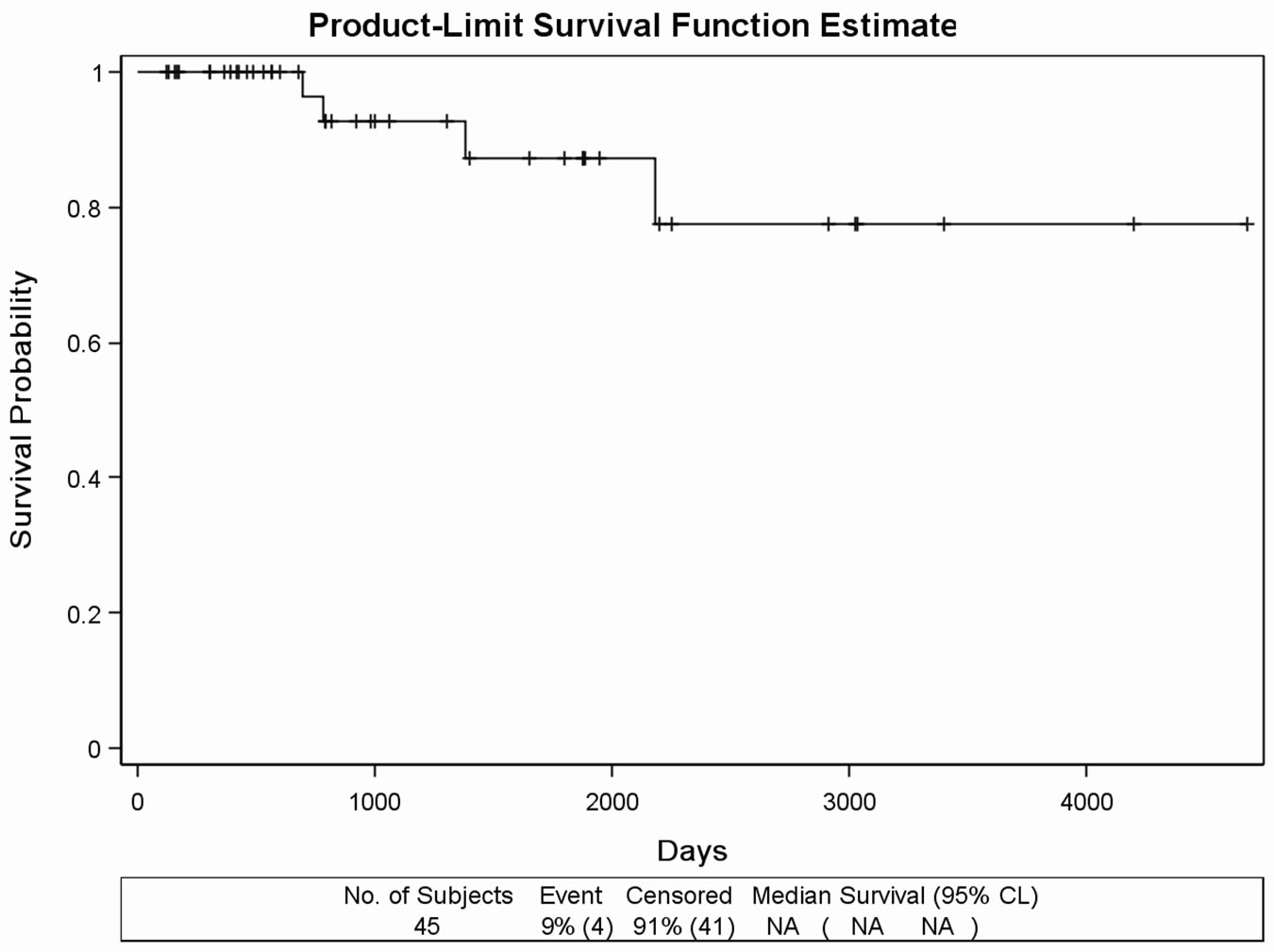 (b)
(b)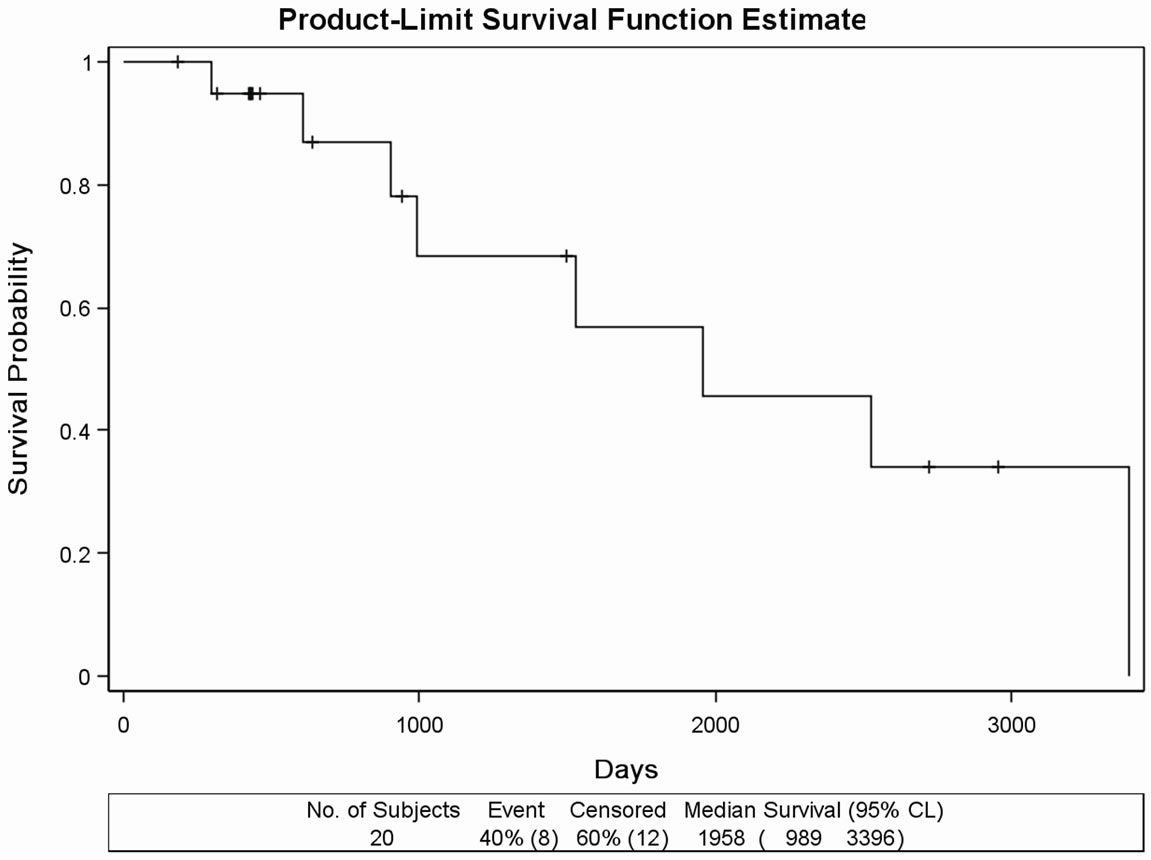 (c)
(c)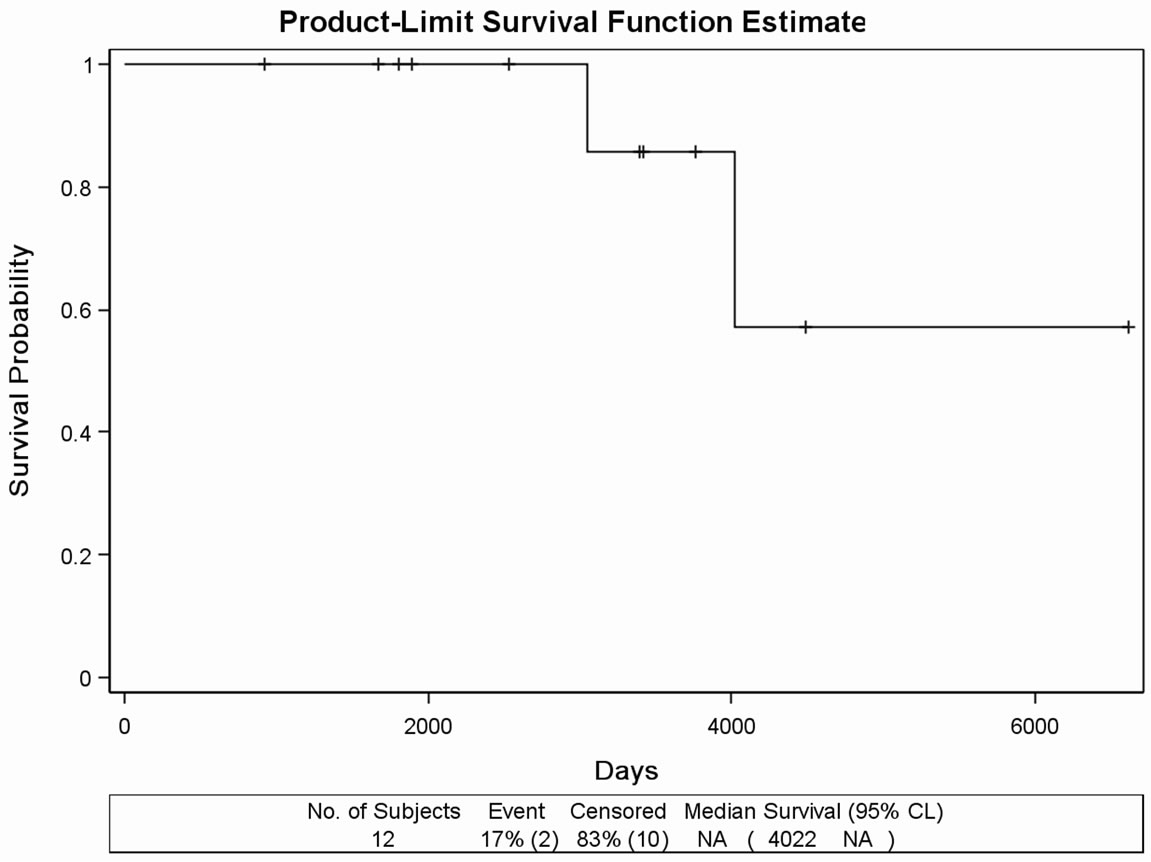 (d)
(d)
Figure 1. (a) Kaplan Meier survival curves in the retrospectively evaluated 91 DDW-treated PC patients. Days from the diagnosis of prostate cancer to the end of the follow-up. MST was 11.02 years (4022 days). (b) Survival curve of the 45 DDWtreated PC patients with no metastasis during the follow-up. Days from the diagnosis of prostate cancer to the end of the follow-up. MST cannot be calculated due to the long survival. (c) Survival curve of 20 DDW-treated PC patients, who developed bone metastasis within one year from the diagnosis of PC. Days from the diagnosis of prostate cancer to the end of the followup. MST was 65.27 months (1958 days). (d) Survival curve of 12 DDW-treated patients, who developed bone metastasis later than one year from the diagnosis. Days from the diagnosis of prostate cancer to the end of the follow-up. MST could not be calculated because of the low number of incidences (2 deaths).
follow-up, 8 patients (40%) died. MST from the diagnosis of PC was 1958 days (65.27 months), with individual values varying between 989 days (32.97 months) and 3396 days (113.2 months) (Figure 1(c)). In another subgroup, consisting of 12 patients who developed bone metastasis later than one year after the diagnosis of PC, MST could not be calculated because of the low incidence rate (2 deaths) and the small number of subjects (Figure 1(d)). The remaining 14 patients were not evaluated as a subgroup because of the heterogeneity, metastases in different organs or other type of cancer diagnosed beside PC.
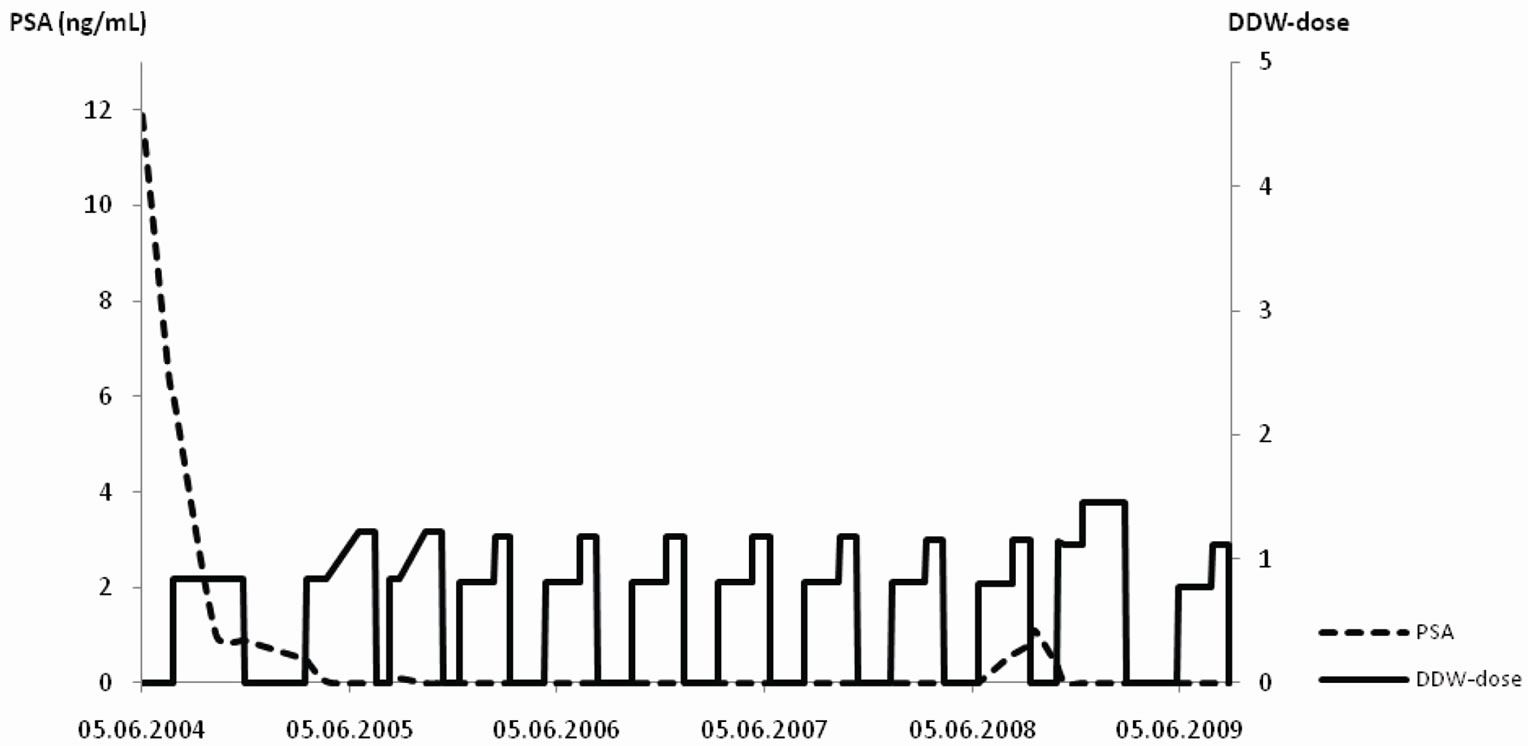
The time pattern of PSA changes in relation to DDW dose is exemplified on the data of two patients. Patient 1. started DDW at a relatively low PSA level following prostatectomy. Patient 2. started the cure with bone metastasis and highly elevated PSA level (over 1.000 ng/mL).
Patient 1. (53 years old; Figure 2(a)): Elevated PSA (11.9 ng/mL) and no bone metastasis were present at the time of diagnosis. The patient underwent prostatectomy followed by antiandrogen therapy, PSA still remained elevated (6 ng/mL). DDW treatment resulted in a decrease in PSA level. The patient repeated 12 - 16 weeks long DDW cures with interruptions of 8 weeks, whereby DDW dose was increased gradually during each cure but the DdU value was kept steady close to 1.0 which was sufficient to keep PSA close to 0.0 ng/mL. Four years later progression was detected therefore the DDW dose was increased up to 1.45 DdU that resulted in regression again. Five years after the initial diagnosis, PSA level is still steady below 1.0 ng/mL.
Patient 2. (61 years old; Figure 2(b)): Bone metastasis was confirmed at the diagnosis of PC; PSA level was over 1.000 ng/mL at that time. The conventional treatment plus DDW―the latter was initiated 21 months after the diagnosis―resulted in a striking decrease in PSA. The patient interrupted DDW consumption for six months that resulted in a slight increase of PSA. Then, the repeated cures with DDW resulted in a decrease of PSA again and the low level remained steady for years. Despite the potentially poor initial prognosis, the patient remained in regression for 7.5 years.
3.3. Safety of DDW Treatment
During the prospective trial, 16 adverse events were reported in 9 patients: three of them received the agent and six patients belonged to the placebo group. In a single case the detected symptoms (i.e. nausea, fatigue and weakness) were classified as treatment-related, in the other patients no causality was provable. In the treated group there was no significant change in the examined laboratory parameters.
During the 17-year-long history of the DDW treated PC patients, there were absolutely no adverse events related to DDW, apart from the concomitant phenomena of the healing process such as general weakness, despondency, drowsiness, flush, occasional high temperature, temporary increase of pain, alleviation and cessation of pain, and warming of the affected area. Those patients who started to use DDW in remission did not experience the above symptoms. There was no significant change in blood count even if the patient had taken high dose of DDW during the years.
4. Discussion
Application of DDW is a new opportunity in cancer therapy. Growing evidence suggests that D-depletion might play a role both in treatment and prevention of cancer. In vitro and in vivo experiments confirmed the inhibition of proliferation of cancer cells [25] and the possible cancer preventive effect [26]. Numerous other experiments, including the study had been conducted on mice xenotransplanted with PC-3 cells, proved the anticancer effect of D-depletion induced by the application of DDW [8]. Because of the novelty of this research area there are still a limited number of publications concerning D-depletion and first of all the human clinical application of DDW. In 2008 a case series of four lung cancer patients have been published [24]. The present study is the first evaluation of the effects of D-depletion in humans that was conducted on a substantially higher population of cancer patients. A human phase II clinical trial was launched to evaluate the possible anticancer effect of DDW in humans. Forty-four patients—a homogenous groups with respect to staging and the applied conventional forms of treatment—were evaluated in the treated and the placebo group. The only (non-significant) difference was that anaplastic adenocarcinoma was diagnosed in a single patient in the treated, and in four patients in the placebo group and therefore the final outcome of the trial could have been slightly altered. While the four patients in the control group, expectedly, did not show any improvement, the patient in the treated group with anaplastic adenocarcinoma achieved PR. These data suggest that D-depletion might be effective even in those cases where histological diagnosis predicts poor prognosis. The distribution of the best response (PR) to treatments differed significantly after four-month-long DDW or placebo treatment in the test groups (7 subjects vs. 1 subject).
The three times higher net decrease in prostate volume in the DDW treated group explains why the urination complaints ceased at a significantly higher rate (8 patients vs. 0 patients, p = 0.0041) in the treated group. Furthermore, this was in line with the fact that 15 out of
 (a)
(a)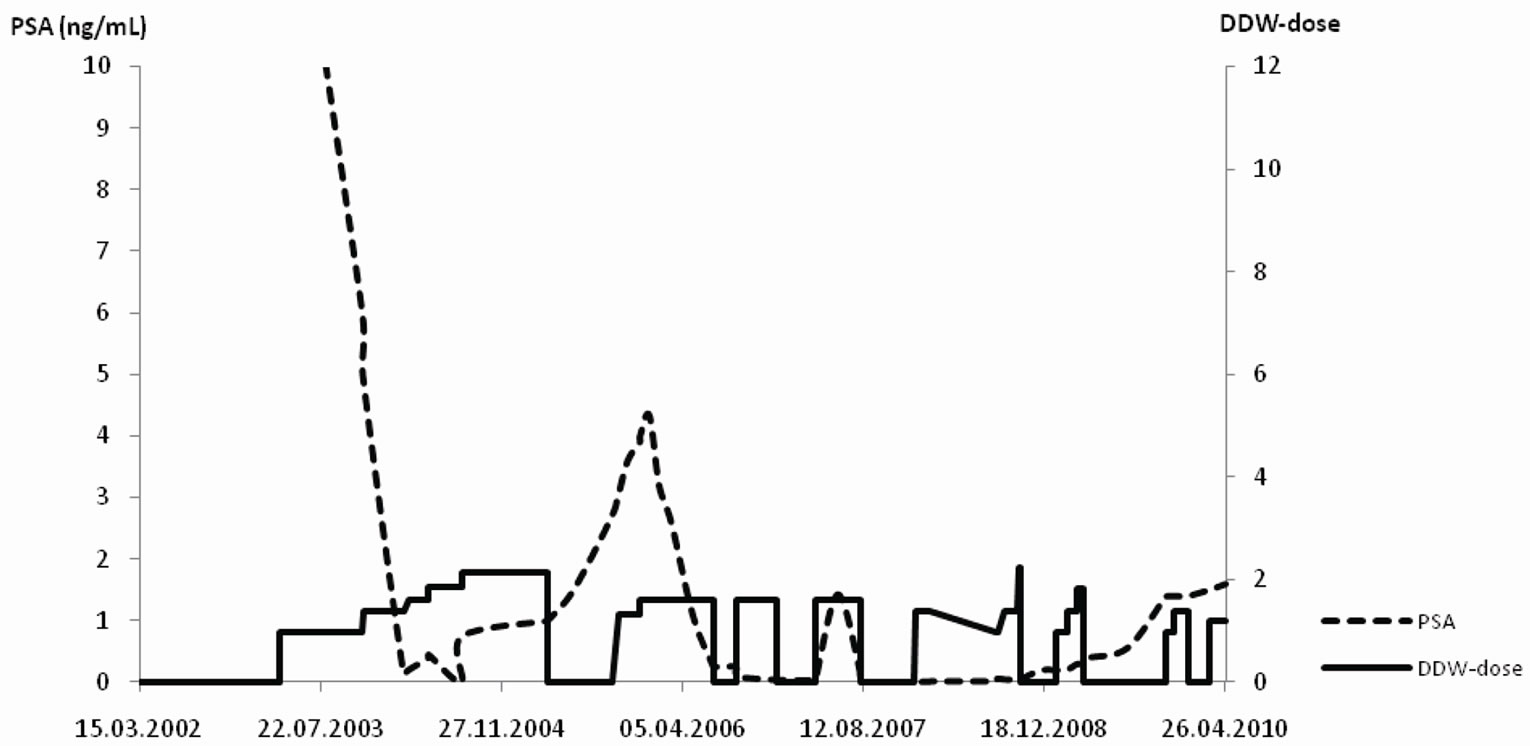 (b)
(b)
Figure 2. a-b The time course of DDW dose (DdU) and PSA levels (ng/mL) in two patients. The Y axis shows the DDW dose (right side) and the PSA value (left side). The pecked line depicts the timeline of PSA values, while the solid line represents the DDW dose. Notes: DdU = deuterium depletion unit, that was calculated considering the difference in D-concentration between plain tap water and the DDW used, the daily volume of the DDW, and body weight. (a) Patient 1: Five-years follow up of a 53 years old PC patient, who underwent prostatectomy followed by antiandrogen therapy. Further decrease in PSA was achieved, when DDW treatment was introduced. Repeated, 12 - 16 weeks long DDW cures kept PSA close to 0.0 ng/mL, and after the detection of an increase in PSA value the higher DDW dose ensured further reduction of PSA again. (b) Patient 2: Follow-up of a 61 years old PC patient with bone metastasis and highly elevated PSA (1000 ng/mL). DDW treatment was introduced in addition to the conventional forms of treatment. Despite the potentially poor prognosis the patient remained in regression for 7.5 years.
22 patients showed over 50% decrease in PSA level in the treated group while in the placebo group only 9 patients showed such result. All these results clarify the impressive difference in the death rate in the two groups (2:9, treated vs. placebo group) within the first year.
In addition to the prospective study, we had the possibility to follow 91 PC patients after the diagnosis for a cumulative time of 350 years. Patients without distant metastasis showed extremely low death rate during the cumulative follow-up period of 157 years (Figure 1(b)). In patients who suffered from early developing distant metastasis, D-depletion was able to extend MST to 64.8 months (Figure 1(c)), while other studies indicated a 15 - 20 month-long MST for progressive metastatic PC [22]. Considering also the MST in all prostate cancer patients that was published in the Hungarian National Cancer Registry [27] we suggest that D-depletion contributed to the long overall MST of 11 years calculated in the present study (Figure 1(a)).
Examining the PSA values in certain patients’ causality was observed between DDW cures and the time pattern of PSA-changes (Figures 2(a) and (b)). DDW-consumption could also prolong the progression-free interval in the early stages of PC.
Based upon the preclinical toxicological investigations [28], the prospective and retrospective clinical studies, the application of DDW at a concentration of 25 to 105 ppm D seems to be completely innocuous, and can act as a highly effective tool in cancer therapy which can be easily integrated in the treatment regimens. Consequently, the application of DDW in the most affected population might reduce the mortality of PC, since it is able to delay progression as well as to prolong MST in patients with histologically confirmed PC.
5. Acknowledgements
The authors wish to acknowledge the valuable contribution of the following clinicians: Gábor Árpási, Béla Böcskei, Gábor Csúsz, Tibor Kázmér, Jorgosz Szapanidisz (St. Johns Hospital, Budapest, Hungary); Dénes Répássy (St. István Hospital, Budapest, Hungary); Barnabás Ruszinkó, György Sára (Uzsoki Hospital, Budapest, Hungary).
REFERENCES
- J. J. Katz and H. L. Crespi, “Isotope Effects in Biological Systems,” In: C. J. Collins and N. S. Bowman, Eds., Isotope Effects in Chemical Reactions, Van Nostrand Reinhold, New York, 1971, pp. 286-363.
- P. W. Rundel, J. R. Ehleringer and K. A. Nagy, “Stable Isotopes in Ecological Research,” Springer, New York, 1988.
- G. Jancsó, “Isotope Effects,” In: A. Vértes, S. Nagy and Z. Klencsár, Eds., Handbook of Nuclear Chemistry, Kluwer Academic Publishers, Dordrecht, 2003, pp. 85-116.
- D. M. Czajka, A. J. Finkel, C. S. Fischer and J. J. Katz, “Physiological Effects of Deuterium on Dogs,” American Journal of Physiology, Vol. 201, 1961, pp. 357-362.
- J. J. Katz, H. L. Crespi, D. M. Czajka and A. J. Finkel, “Course of Deuteration and Some Physiological Effects of Deuterium in Mice,” American Journal of Physiology, Vol. 203, 1962, pp. 907-913.
- G. Somlyai, G. Jancsó, G. Jákli, K. Vass, B. Barna, V. Lakics and T. Gaál, “Naturally Occurring Deuterium Is Essential for the Normal Growth Rate of Cells,” FEBS Letters, Vol. 317, No. 1-2, 1993, pp. 1-4. doi:10.1016/0014-5793(93)81479-J
- G. Somlyai, G. Laskay, T. Berkényi, Z. Galbács, G. Galbács, S. A. Kiss, G. Jákli and G. Jancsó, “Naturally Occurring Deuterium May Have a Central Role in Cell Signalling,” In: J. R. Heys and D. G. Melillo, Eds., Synthesis and Applications of Isotopically Labelled Compounds, John Wiley and Sons Ltd, New York, 1998, pp.137-141.
- G. Somlyai, G. Laskay, T. Berkényi, Z. Galbács, G. Galbács, S. A. Kiss, G. Jákli and G. Jancsó, “The Biological Effects of Deuterium-Depleted Water, a Possible New Tool in Cancer Therapy,” Journal of Oncology, Vol. 30, 1998, pp. 91-94.
- Z. Gyöngyi and G. Somlyai, “Deuterium Depletion Can Decrease the Expression of C-Myc, Ha-Ras and p53 Gene in Carcinogen-Treated Mice,” In Vivo, Vol. 14, No. 3, 2000, pp. 437-439.
- G. Somlyai, M. Molnár, G. Laskay, M. Szabó, T. Berkényi, I. Guller and A. Kovács, “Biological Significance of Naturally Occurring Deuterium: The Antitumor Effect of Deuterium Depletion,” Orvosi Hetilap, Vol. 151, No. 36, 2010, pp. 1455-1460. doi:10.1556/OH.2010.28865
- M. Malvezzi, A. Arfé, P. Bertuccio, F. Levi, C. La Vecchia and E. Negri, “European Cancer Mortality Predictions for the Year 2011,” Annals of Oncology, Vol. 22, No. 4, 2011, pp. 947-956. doi:10.1093/annonc/mdq774
- F. Bray, J. Lortet-Tieulent, J. Ferlay, D. Forman and A. Auvinen, “Prostate Cancer Incidence and Mortality Trends in 37 European Countries: An Overview,” European Journal of Cancer, Vol. 46, No. 17, 2010, pp. 3040-3052. doi:10.1016/j.ejca.2010.09.013
- P. Boyle and J. Ferlay, “Cancer Incidence and Mortality in Europe 2004,” Annals of Oncology, Vol. 16, No. 3, 2005, pp. 481-488. doi:10.1093/annonc/mdi098
- A. Jemal, R. Siegel, E. Ward, Y. Hao, J. Xu, T. Murray and M. J. Thun, “Cancer Statistics, 2008,” CA: A Cancer Journal for Clinicians, Vol. 58, No. 2, 2008, pp. 71-96. doi:10.3322/CA.2007.0010
- A. B. Barqawi and E. D. Crawford, “The Current Use and Future Trends of Focal Surgical Therapy in the Management of Localized Prostate Cancer,” Cancer Journal, Vol. 13, No. 5, 2007, pp. 313-317. doi:10.1097/PPO.0b013e318156eb99
- D. T. Marshall, “Options and Recent Advances in Permanent Brachytherapy for Prostate Cancer,” Canadian Journal of Urology, Vol. 14, No. 14, Suppl. 1, 2007, pp. 28-31.
- T. J. Eichler, “Radiotherapy for Localized Prostate Cancer,” Canadian Journal of Urology, Vol. 14, Suppl. 1, 2007, pp. 19-23.
- C. Falandry, M. Bonnefoy, B. You and G. Freyer, “Metastatic Prostate Cancer Treatment in the Elderly,” Bull Cancer, Vol. 94, Suppl. 7, 2007, pp. F89-F96.
- W. H. Kruit, G. Stoter and J. G. Klijn, “Effect of Combination Therapy with Aminoglutethimide and Hydrocortisone on Prostate-Specific Antigen Response in Metastatic Prostate Cancer Refractory to Standard Endocrine Therapy,” Anticancer Drugs, Vol. 15, No. 9, 2004, pp. 843- 847. doi:10.1097/00001813-200410000-00004
- R. Nayvar, N. Sharma and N. P. Gupta, “Docetaxel-Based Chemotherapy with Zoledronic Acid and Prednisone in Hormone Refractory Prostate Cancer: Factors Predicting Response and Survival,” International Journal of Urology, Vol. 16, No. 9, 2009, pp. 726-731. doi:10.1111/j.1442-2042.2009.02351.x
- A. Fontana, L. Galli, A. Fioravanti, P. Orlandi, C. Galli, L. Landi, S. Bursi, G. Allegrini, E. Fontana, D. R. Marsico, A. Antonuzzo, M. D’Arcangelo, R. Danesi, M. Del Tacca, A. Falcone and G. Bocci, “Clinical and Pharmacodynamic Evaluation of Metronomic Cyclophosphamide, Celecoxib, and Dexamethasone in Advanced Hormonerefractory Prostate Cancer,” Clinical Cancer Research, Vol. 15, No. 15, 2009, pp. 4954-4962. doi:10.1158/1078-0432.CCR-08-3317
- O. Smaletz, H. I. Scher, E. J. Small, D. A. Verbel, A. McMillan, K. Regan, W. K. Kelly and M. W. Kattan, “Nomogram for Overall Survival of Patients with progressive Metastatic Prostate Cancer after Castration,” Journal of Clinical Oncology, Vol. 20, No. 19, 2002, pp. 3972-3982. doi:10.1200/JCO.2002.11.021
- H. London, “Separation of Isotopes,” George Newnes Limited, London, 1961.
- K. Krempels, I. Somlyai and G. Somlyai, “A Retrospective Evaluation of the Effects of Deuterium Depleted Water Consumption on 4 Patients with Brain Metastases from Lung Cancer,” Integrative Cancer Therapies, Vol. 7, No. 3, 2008, pp. 172-181. doi:10.1177/1534735408322851
- F. S. Cong, Y. R. Zhang, H. C. Sheng, Z. H. Ao, S. Y. Zhang and J. Y. Wang. “Deuterium-Depleted Water Inhibits Human Lung Carcinoma Cell Growth by Apoptosis,” Experimental and Therapeutic Medicine, Vol. 1, No. 2, 2010, pp. 277-283. doi:10.3892/etm_00000043
- I. E. Siniak, V. S. Turusov, A. I. Grigoriev, D. G. Zaridze, V. B. Gaidadymov, E. I. Gus’kova, E. E. Antoshina, T. G. Gor’kova and L. S. Trukhanova, “Consideration of the Deuterium-Free Water Supply to an Expedition to Mars,” Aerospace and Environmental Medicine, Vol. 37, 2003, pp. 60-63.
- G. Tusnády, I. Gaudi, L. Rejtő, M. Kásler and Z. Szentirmay, “Survival Chances of Hungarian Cancer Patients in the National Cancer Registry,” Magyar onkologia, Vol. 52, No. 4, 2008, pp. 339-349.
- G. Török, M. Csík, A. Pintér, et al., “Effects of Different Deuterium Concentrations of the Media on the Bacterial Growth and Mutagenesis,” Egészségtudomány/Health Science, Vol. 44, 2000, pp. 331-338.

