Health
Vol.6 No.8(2014), Article ID:44320,11 pages DOI:10.4236/health.2014.68100
Immunoglobulin: A Natural Way to Suppress Helicobacter pylori in Humans
Adham M. Abdou1,2*, Manal M. E. Ahmed2, Yusuke Yamashita3, Mujo Kim3
1Department of Food Control, Benha University, Moshtohor, Egypt
2Department of Research & Development, Pharma Foods International Co., Ltd., Middle East Office, Cairo, Egypt
3Department of Research & Development, Pharma Foods International Co., Ltd., Kyoto, Japan
Email: *dradham@yahoo.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 22 January 2014; revised 26 February 2014; accepted 7 March 2014
ABSTRACT
The use of immunoglobulin is successfully applied in different areas of research, diagnostics, medical application and biotechnology. Egg yolk immunoglobulin (IgY) can successfully compete with immunoglobulin (IgG) produced in the blood of mammals. Recently, successful progresses have been achieved in Japan through industrialization of IgY technology. Using IgY has been shown to provide a safer, more efficient and less expensive method for managing disease-causing pathogens. Helicobacter pylori (H. pylori), a spiral Gram-negative microaerophilic pathogen, it infects over 50% of the population worldwide, and is recognized as the etiologic agent of gastritis, peptic ulcer, and has been linked to the development of gastric adenocarcinoma and mucosa associated lymphoid tissue lymphoma. It is found that urease is the most abundant protein of H. pylori. Urease is recognized as an essential factor in the organism colonization of the gastric mucosa. The eradication of H. pylori by administration of oral antimicrobials is not always successful and may be associated with adverse effects. Therefore, several treatment regimens have emerged to cure H. pylori infection. Accordingly, a novel approach in prevention and reduction of H. pylori infection has been reported based on production of urease-specific immunoglobulin that can suppress the bacterial colonization through urease-binding by anti-H. pylori urease IgY (IgY-urease). The use of IgY against a pathogenic factor of H. pylori will be a prudent way to suppress the infection.
Keywords:Immunoglobulin; Egg Yolk Immunoglobulin (IgY); Helicobacter pylori; Urease; Food Ingredient

1. Introduction
Helicobacter pylori (H. pylori) is a Gram-negative, microaerophilic bacterium found in the stomach. It was identified in 1982 by Australian scientists Barry Marshall and Robin Warren, who found that it was present in patients with chronic gastritis and gastric ulcers, conditions that were not previously believed to have a microbial cause. It is also linked to the development of duodenal ulcers and stomach cancer. However, over 80 percent of individuals infected with the bacterium are asymptomatic and it has been postulated that it may play an important role in the natural stomach ecology [1] .
Up to 85% of people infected with H. pylori never experience symptoms or complications [2] . Acute infection may appear as an acute gastritis with abdominal pain (stomach ache) or nausea [3] . Where this develops into chronic gastritis, the symptoms, if present, are often those of non-ulcer dyspepsia: stomach pains, nausea, bloating, belching, and sometimes vomiting or black stool [4] .
Individuals infected with H. pylori have a 10% to 20% lifetime risk of developing peptic ulcers and a 1% to 2% risk of acquiring stomach cancer [5] . Inflammation of the pyloric antrum is more likely to lead to duodenal ulcers, while inflammation of the corpus (body of the stomach) is more likely to lead to gastric ulcers and gastric carcinoma [6] .
Many questions, however, still remain concerning the optimal diagnostic and therapeutic regimens with which to approach the organism. The eradication of H. pylori by administration of oral antimicrobials, especially proton pump inhibitor based triple therapy, has been the mainstay of therapy in the past two decades. Rising antibiotic resistance increases the need to search for new therapeutic strategies; this might include prevention in form of vaccination [7] . Several non-antibiotic approaches, including probiotics, phages and phytomedicines, had been shown to be effective in treating or preventing some infectious diseases [8] . Egg yolk was an inexpensive antibody source, and the therapeutic usefulness of egg yolk immunoglobulin (IgY) in oral passive immunization had been investigated recently [9] . Immunodominant H. pylori proteins with reactivity to H. pylori specific IgY were identified in 2003 [10] . In 2004, it has been reported the successful production of anti-H. pylori urease-specific IgY [11] . Oral administration of urease-specific IgY inhibited H. pylori disease activity in H. pylori-infected gerbils, and prevented its colonization in those not yet infected [12] . Seventeen asymptomatic volunteers diagnosed as H. pylori-positive by the C13-urea breath test were orally administered urease-specific IgY for 4 weeks and then the breath test values were significantly decreased [13] . In a 53-year-old female administered urease-specific IgY, when combined with antacids, ameliorated gastric inflammation [14] . This article will focus on introducing the novel approaches on developing an effective, safe, and natural IgY against the H. pylori that may enable the future eradication of the organism as a significant human pathogen.
2. Epidemiology
Numerous studies have tried to assess the incidence and prevalence of H. pylori infection, its mode of transmission, and any risk factors contributing to development of the infection. The annual incidence reported in 3 adult studies in developed countries was between 0.3% and 0.5% per year [15] -[17] . Prevalence estimates vary greatly, depending on the location of the study group and the characteristics of the population studied. In general, prevalence increases with age [18] and correlates positively with a low socioeconomic status during childhood [19] . Worldwide, but especially in developed nations, infection with H. pylori is declining [20] . The acquisition of H. pylori occurs during childhood, most often by a fecal-oral or oral-oral route.
Some studies have also indicated a role for a gastro-oral route of transmission [21] . The role played by other factors, including ABO blood type, alcohol and tobacco use, dietary and nutritional influences, and genetic predisposition to infection, has also been studied, but results have been inconsistent [22] . Interestingly, a recent study of 655 subjects from a teaching hospital in Rome found an overall prevalence of infection of 40%, with a higher prevalence among nurses and auxiliary employees than among physicians [23] .
3. Pathogenicity and Virulence Factors
The earliest descriptions of the organism classified it as predominately extracellular, Gram-negative, flagellated, and motile (Figure 1). With the advancement of biochemical techniques, new information about the pathogenicity and virulence factors of H. pylori has emerged, indicating that infection by H. pylori requires a complex interaction of both bacterial and host factors.

Figure 1. Helicobacter pylori.
Investigators have identified several bacterial proteins necessary for colonization of the gastric mucosa by H. pylori, including proteins active in the transport of the organism to the surface of the mucosa (e.g., flagellin, which is encoded on genes flaA and flaB) [24] .
Once in the presence of the gastric mucosa, bacteria induce a transient hypochlorhydria by an unknown mechanism. The urease enzyme produced by the bacteria alters the microenvironment of the organism to facilitate colonization [25] . Adherence then occurs via interaction between cell-surface glycolipids and adhesins specific to H. pylori [26] .
There also appears to be a role played by proteins called cecropins, which are produced by H. pylori and inhibit the growth of competing organisms [27] , as well as by a P-type adenosine triphosphatase, which helps prevention excessive alkalinization of the microenvironment by urease [28] . Once attached to gastric mucosa, H. pylori causes tissue injury by a complex cascade of events that depends on both the organism and the host. Like all Gram negative bacteria, H. pylori has in its cell wall lipopolysaccharide, which acts to disrupt mucosal integrity [29] . Furthermore, the organism releases several pathogenic proteins that induce cell injury. For example, the CagA protein, produced by cytotoxic-associated gene A (CagA), is a highly immunogenic protein that may be associated with more severe clinical syndromes, such as duodenal ulcer and gastric adenocarcinoma (although this question is far from settled) [30] [31] . There is increasing evidence that CagA positivity is associated with an increased risk for distal, but not proximal, gastric adenocarcinoma [32] . In addition, protein products of the vacuolating cytotoxin A gene (VacA) and the A gene induced by contact with epithelium (IceA) are known to be associated with mucosal injury [33] [34] .
Once colonization of the gastric mucosa has taken place, the immunogenic properties of H. pylori induce an inflammatory reaction with neutrophilic gastritis that ultimately results in the clinical manifestations of the infection (Figure 2). This process is mediated by host factors, including interleukins 1, 2, 6, 8, and 12; interferon gamma; tumor necrosis factor-a; T and B lymphocytes; and phagocytic cells. These factors mediate injury through release of reactive oxygen species and inflammatory cytokines [35] . Also, H. pylori appears to increase the rate of mucosal programmed cell death (also known as apoptosis) [36] .
4. General Treatment Principles
Determining the optimum treatment of H. pylori infection is difficult, because the organism lives in an environment not easily accessible to many medications and because emerging bacterial resistance presents an added challenge. Moreover, many of the recommended regimens are difficult for patients to take, leading to problems with compliance; specifically, having to take a large number of pills at least twice daily and coping with unpleasant adverse effects do little to encourage patient cooperation. Despite these obstacles, current regimens can obtain cure rates in excess of 85% in most patient populations [37] .
Antibiotic based therapy, especially proton pump inhibitor based triple therapy, has been the mainstay of therapy in the past two decades [2] . Antibiotic resistance is a well-known issue and metronidazole resistance as high as 60% - 70% was reported in some areas [38] [39] . Resistance towards clarithromycin, which initially was very effective, was also an increasing problem when its use became more popular in past decade. Even with the latest levofloxacin-based regimens, the mean eradication rate is only 80% for the resistant cases [7] . Trying to prevent the emergence of antibiotic resistance, a paradigm shift in the treatment of infectious disease had been advocated. Several non-antibiotic approaches, including probiotics, phages and phytomedicines, had been shown to be effective in treating or preventing some infectious diseases [8] . Alternative approaches in managing H. pylori infection are also necessary.
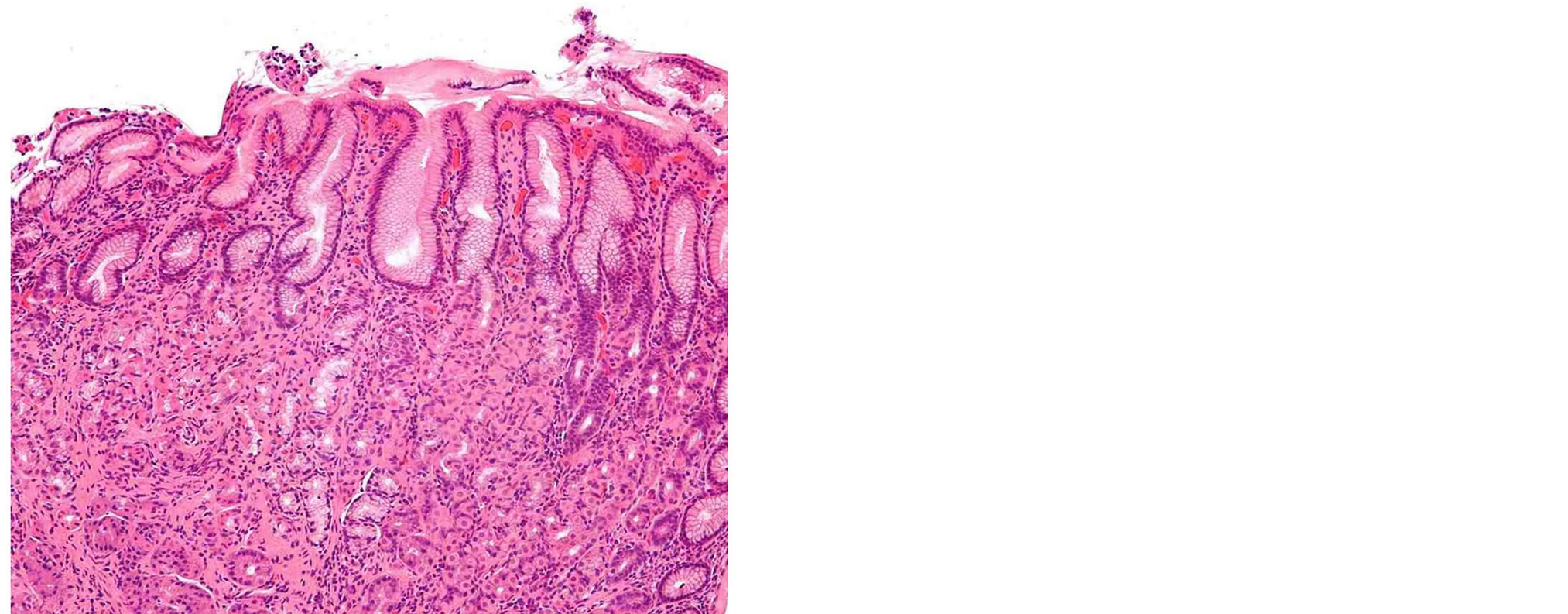
Figure 2. H & E stained section of an inflamed (infected with H. pylori) gastric antral biopsy.
5. Novel Trend in Eradication of H. pylori Using Immunoglobulin
5.1. Egg Yolk Immunoglobulin
Immunoglobulin from yolk (IgY) is the major antibody found in hen eggs. In 1893, Klemperer first described the acquisition of passive immunity in birds, by demonstrating the transfer of immunity against tetanus toxin from the hen to the chick [40] . Three immunoglobulin classes analogues to the mammalian immunoglobulin classes; IgA, IgM, and IgG, have been shown to exist in chicken. In the egg, IgA and IgM are present in the egg white, while IgG is present in the egg yolk [41] . Immunoglobulin G (IgG) in egg yolk has been referred to as IgY to distinguish it from its mammalian counterpart [42] .
The concentration of IgY in the yolk is essentially constant (10 - 20 mg/mL) through the oocyte maturation. Approximately 100 - 400 mg IgY is packed in an egg. The concentration of IgY in the yolk is 1.23 times to the serum concentration [43] . A delay of 3 to 4 days is observed for the appearance of specific IgY in yolk after first appearance of specific IgG in the serum of a hen.
5.2. Structure and Characteristics of Avian IgY versus Mammalian IgG
General structure of IgY molecule is the same as mammalian IgG with 2 heavy (Hv) chains with a molecular mass of 67 - 70 kDa each and two light (L) chains with the molecular mass of 25 kDa each (Figure 3). The major difference is the number of constant regions (C) in H chains: IgG has 3 C regions (Cy1-Cy3), while IgY has 4 C regions (Cv1-Cv4). Due to occurrence of one additional C region with two corresponding carbohydrate chains, molecular mass of IgY (180 kDa) is larger than mammalian IgG (150 kDa). IgY is less flexible than mammalian IgG due to the absence of the hinge between Cy1 and Cy2. There are some regions in IgY (near the boundaries of Cv1-Cv2 and Cv2-Cv3) containing proline and glycine residues enabling only limited flexibility. IgY has isoelectric point 5.7 - 7.6 and is more hydrophobic than IgG [42] [44] .
Most biological effectors’ functions of immunoglobulin are activated by the Fc region, where the major structural difference between IgG and IgY is located. Therefore, Fc-dependent functions of IgY are essentially different from those of mammalian IgG. First, IgY does not activate the mammalian complement system [45] , second, IgY does not bind to protein-A and G [46] , third, IgY is not recognized by mammalian antibodies [47] i.e. rheumatoid factors (RF, an autoantibody reacting with the Fc portion of IgG) or HAMA (human anti-murine antibodies), and fourth, it does not bind to cell surface Fc receptor [48] . These differences in molecular interactions bring great advantages to the application of IgY antibodies. Then that were IgY has been successfully applied into a variety of methods in different areas of research, diagnostics, and medical areas. For these applications, IgY can successfully compete with antibodies (IgG) isolated from the blood of mammals [49] .
The advantage of usage of the immunized hen is that it produces a large number of eggs. Approximately 40 g of IgY could be collected from egg of one hen each year, compared with about 1.3 g from blood of one rabbit [50] . An industrial scale production of IgY is possible because of the availability of large number of chicken farms and automation of egg breaking and processing.
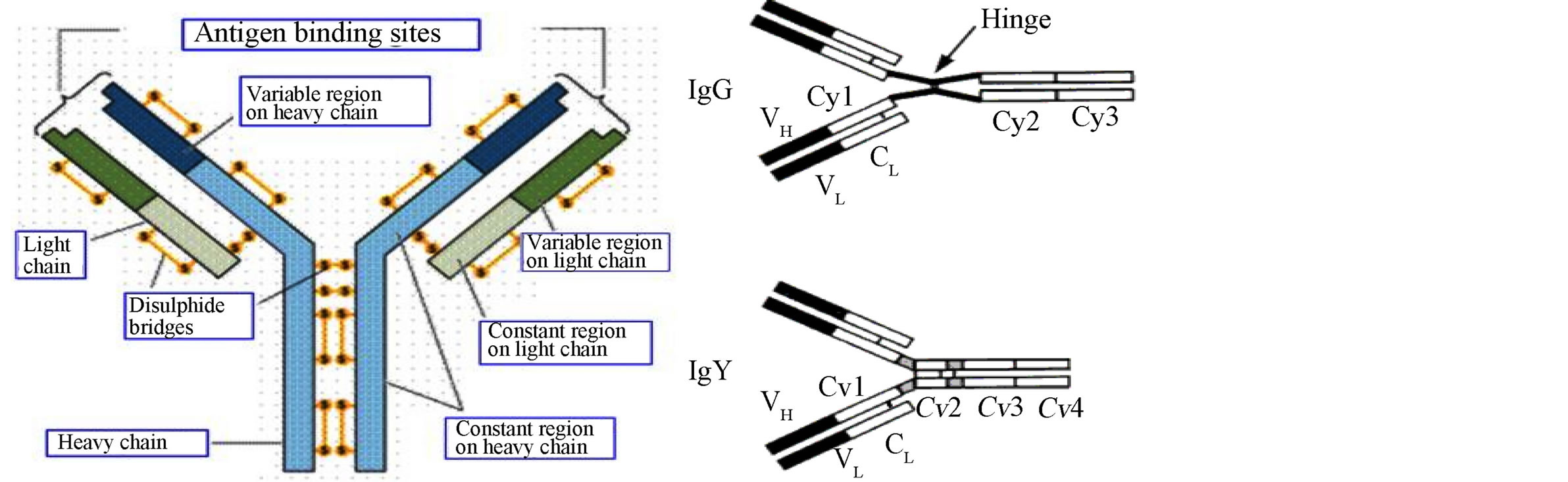
Figure 3. Structure of IgY and IgG.
5.3. Applications of IgY
Oral administration of antibodies specific to host pathogens is an attractive approach to establish protective immunity, especially against gastrointestinal pathogens both in human and animals. Eggs are normal dietary components and there is practically no risk of toxic side effects of IgY given orally. As mentioned above, IgY does not activate mammalian complement system nor interact with mammalian Fc-receptors that could mediate inflammatory response in the gastrointestinal tract.
On the basis of these facts, IgY has been used for suppression of growth and multiplication of food-borne pathogens [40] . It was prepared from the egg of the hens that had been immunized previously with a specific antigen. These IgY treatments have been shown to provide a safer, more efficient and less expensive method than those using conventional mammalian antibodies for managing disease-causing pathogens.
Recently, successful progresses in industrialization of IgY has been achieved in Japan, where IgY as a bioactive ingredient in food, nutraceuticals, cosmetics and other sectors is applied.
5.4. Anti-H. pylori IgY
In Japan, researchers developed two IgY, an anti-urease IgY and an anti-VacA IgY with the hope of controlling H. pylori problems.
5.5. Anti-H. pylori Urease IgY
For colonization of H. pylori in the gastric mucosa, it abundantly produces urease enzyme, which degrades urea into ammonia. The organism uses the produced ammonia to neutralize microenvironment in the gastric mucosa [28] .
Accordingly, anti-H. pylori urease IgY has been developed as a novel approach in treatment of H. pylori infection. It has been reported that oral administration of anti-H. pylori urease IgY could suppress the bacterial colonization [13] [51] .
Today, consumers prefer foods that promote good health and could reduce risk of diseases. Dairy products are excellent media to generate an array of products that fit into the current consumer demand for functional foods [52] .
Scientific and clinical evidence is mounting to corroborate the consumer perception of health from yogurt. Designing a yogurt fortified with anti-H. pylori urease IgY could supply passive immunization with a natural and specific attempt to decrease the H. pylori infection. Three clinical studies were done to examine the efficacy of a specially designed functional yogurt containing anti-H. pylori urease IgY on the suppression of H. pylori in humans.
Anti-H. pylori urease IgY containing yogurt (plain and drinking) have been prepared and in markets in Japan, Korea, and Taiwan.
A clinical study was conducted to determine the effect of anti-H. pylori urease IgY yogurt to decrease H. pylori in humans [53] . To assess presence of H. pylori in stomach, UBT method has been extensively used. This method based on the invasive detection of exhaled C13-labeled carbon dioxide resulting from H. pylori urease activity [54] . One hundred seventy-four volunteers were screened using a C13-urea breath test (UBT). Heavily infected volunteers (with UBT values over 30 per mill) were selected (16 subjects) and recruited. Each volunteer consumed 1 cups of yogurt twice daily (4 g/d egg yolk containing 40 mg anti-H. pylori urease IgY) for 12 weeks. Volunteers were tested after 4, 8 and 12 weeks. The UBT values obtained at week 8 and 12 were significantly different from those obtained at week 0 (P < 0.001), showing a 55.1% and 57.2% reduction in UBT values after 8 and 12 weeks, respectively (Figure 4).
Other clinical studies, using anti-H. pylori urease containing drinking yogurt, were carried out in Taiwan and Korea showed nearly similar results [13] [51] .
The three different studies demonstrated that administration of a specially designed yogurt with highly specific antibodies from egg yolk had a significant effectiveness against H. pylori because of its ability to inhibit H. pylori from adhering to the gastric mucosa in humans. During the study period of the three clinical studies, the ingestion regimen was well-tolerated and no adverse effects or any complications were observed.
Furthermore, because of anti-H. pylori urease IgY binds urease only, the functional efficacy observed was presumably via capture of bacterium-associated urease within the gastric mucus layer, which resulting in bacterial aggregation and clearance via the constant washing action of the gut. By such a mechanism, consumption of anti-H. pylori urease IgY yogurt may play a dual role in suppression and prophylaxis against H. pylori in humans (Figure 5).

Figure 4. UBT value change of volunteers-clinical study in Japan.
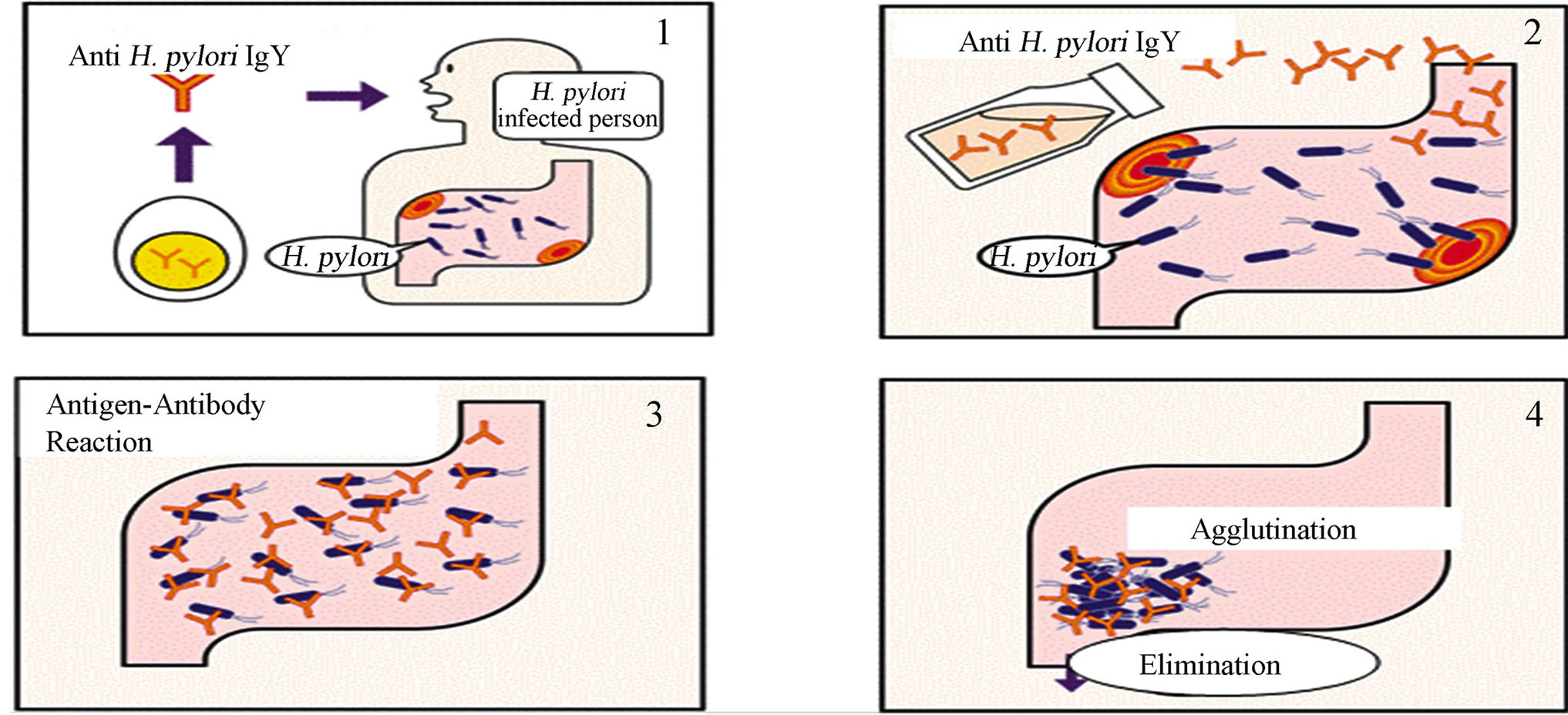
Figure 5. Suppressive mechanism of anti-H. pylori urease IgY.
These findings opened new gate of applications of anti-H. pylori urease IgY in the food industry to prevent H. pylori. Recently, a specially designed egg containing anti-H. pylori urease IgY was produced in Japan. This egg has been on the Japanese market under a trade name of “stomach friendly egg”. Moreover, anti-H. pylori urease IgY was incorporated in nutraceutical formulations that launched recently in the Japanese, Middle Eastern and EU market aiming to prevent and reduce H. pylori infection.
On the other hand, Siriya et al. [55] spot more light on the economic point of view. They applied a study aimed to establish an efficient procedure to produce anti-H. pylori urease IgY from the eggs of the laying hens. Among three extraction methods of dextran sulphate, isopropanol and water-soluble fraction (WSF), WSF method was the best method to recover and purify the total proteins. Further, high purity of the IgY could be obtained by ammonia sulfate precipitation. They could reach to a simple and inexpensive method to obtain a yield of 90% purity of the anti-H. pylori IgY in combinations of the WSF method, ammonia sulfate precipitation, and affinity chromatography. The highly purified IgY produced by this way is available for not only the nutraceutical but also the diagnosis use.
5.6. Anti-Helicobacter pylori VacA IgY
Helicobacter pylori secretes many proteinaceous factors that are important for initial colonization and subsequent persistence in the host stomach. One of the major protein toxins secreted by H. pylori is the VacA. It is one of the most important virulence factors produced by H. pylori even though neither its role nor its action mechanisms are completely understood. First considered as a toxin inducing only cell vacuolation, VacA causes apoptosis of gastric epithelial cells by targeting mitochondria. A hotly debated question about VacA action is its relationship with ammonia, which is produced in vivo by H. pylori urease [56] .
After VacA secretion from the bacteria via a type V autotransport secretion system, the 88 kDa VacA toxin binds to host cells and is internalized, causing severe “vacuolation” characterized by the accumulation of large vesicles that possess hallmarks of both late endosomes and early lysosomes. The development of “vacuoles” has been attributed to the formation of VacA anion-selective channels [57] .
It has been reported that mice deficient in receptor-like protein tyrosine phosphatases (RPTP), one of the receptors of VacA, are resistant to gastric ulcer induced by VacA [58] . This indicates that VacA has an essential role in gastric diseases by H. pylori.
In a Japanese study, researchers developed IgY against VacA with the hope of controlling H. pylori. The research team reported that the anti-VacA IgY decreased VacA activities, vacuolation (Figure 6) and apoptosis (cell death) of cells thereby significantly raised their viability. In addition, inflammatory cytokines from cells incubated with VacA were suppressed by anti-VacA IgY. As a result of the administration of anti-VacA IgY to H. pylori infected gerbils, the inflammation of stomach was improved [59] . Recently, in Korea, a commercial Yogurt containing the anti-VacA IgY is released for the purpose of suppressing inflammation derived from H. pylori in the stomach.
Anti-H. pylori recombinant VacA specific IgY has been prepared and added the appropriate proportions of sucralfate into the specific IgY in order to increase its tolerance to the acid and pepsin. Moreover, the protective effects of the specific IgY on H. pylori infection was fully played by utilizing the adhesiveness of sucralfate with
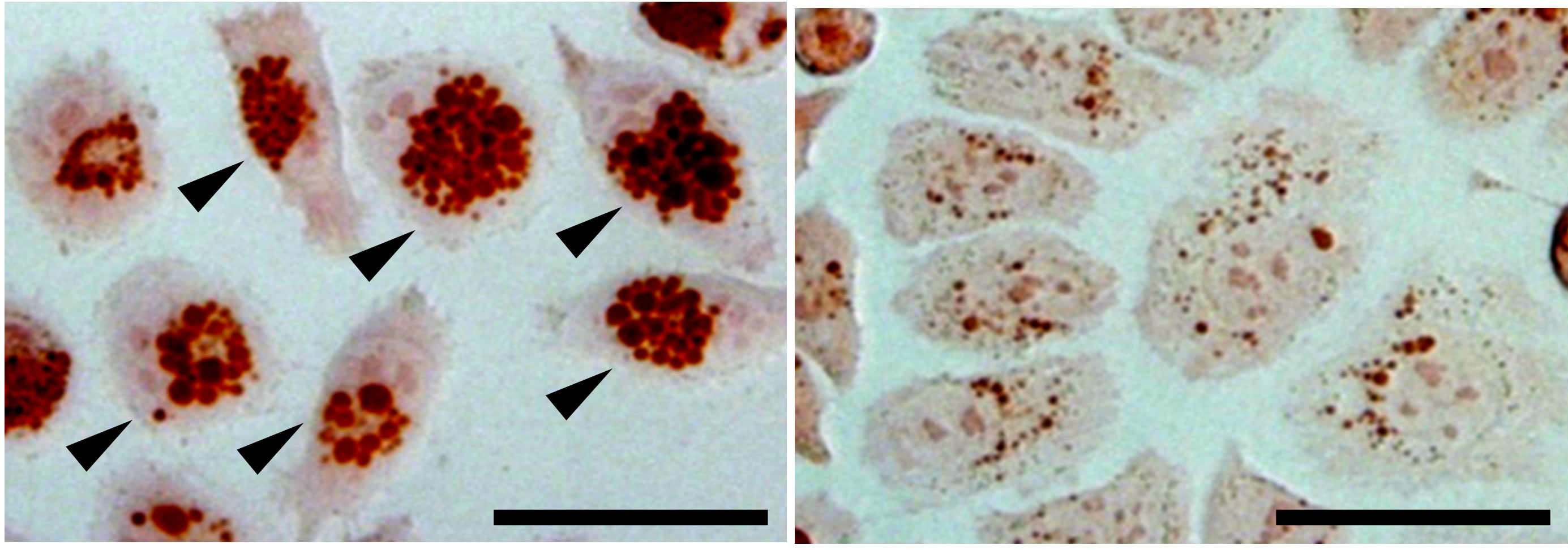
Figure 6. Inhibition by anti-VacA IgY of VacA induced cell vacuolation of AZ-521 cells (human gastric cell line). AZ-521 cells were treated with 50 μg/mL of VacA (left panel) or VacA with 1000 μg/mL of anti-VacA IgY (right panel). Arrow head indicates vacuolated cells. Bar, 50 μm.
mucus on the surface of gastric mucosa. The protective effect of sucralfate on the specific IgY was evaluated in vitro and intragastrically through comparison of stabilization between sucralfate and carbohydrate. Results showed that both sucralfate and many carbohydrates could increase the IgY stability in acidic environments and the protective effect of sucralfate on IgY was significantly superior to carbohydrates and also superior to liposomes-packaged IgY and chitosan-alginate microcapsules-packaged IgY. 30% - 50% sucralfate could effectively protect IgY activity and increase the anti-freeze thawing ability in simulated gastric fluid environment containing 0.02 mg/ml pepsin at pH 2. The in vivo experiment showed that 0.5 mg IgY plus 30% sucralfate/day could effectively prevent gastric mucosal damage caused by H. pylori infection and the effect increased eight folds as compared with the group without sucralfate [60] .
6. Future Research Directions
This review demonstrated that the IgY could effectively suppress H. pylori infection and inflammation. Therefore, IgY would be an important natural food ingredient to alternate or help in suppression and/or prophylaxis against H. pylori.
For all of the above, IgY could be a good candidate overcoming tolerance of antibiotics for the treatment of H. pylori-mediated gastric ulcers and other pathogens.
Also, further studies are needed to reach to more simple and less expensive methods of preparation of IgY and to obtain more purified ingredient.
Author Contributions
Abdou AM and Ahmed MME wrote the paper, Yamashita Y and Kim M revised the paper, Abdou AM drafted and submitted the paper
References
- Blaser, M.J. (2006) Who Are We? Indigenous Microbes and the Ecology of Human Diseases. EMBO Reports, 7, 956-960. http://dx.doi.org/10.1038/sj.embor.7400812
- Bytzer, P., Dahlerup, J.F., Eriksen, J.R., Jarbøl, D.E., Rosenstock, S. and Wildt, S. (2011) Diagnosis and Treatment of Helicobacter pylori Infection. Danish Medical Bulletin, 58, C4271.
- Butcher, G.P. (2003) Gastroenterology: An Illustrated Colour Text 2003. Elsevier Health Sciences.
- Ryan, Kenneth. (2010) Sherris Medical Microbiology 2010. McGraw-Hill, Boston, 573, 576.
- Kusters, J.G., van Vliet, A.H. and Kuipers, E.J. (2006) Pathogenesis of Helicobacter pylori Infection. Clinical Microbiology Reviews, 19, 449-490. http://dx.doi.org/10.1128/CMR.00054-05
- Suerbaum, S. and Michetti, P. (2002) Helicobacter pylori Infection. The New England Journal of Medicine, 347, 1175-1186. http://dx.doi.org/10.1056/NEJMra020542
- Selgrad, M. and Malfertheiner, P. (2008) New Strategies for Helicobacter pylori Eradication. Current Opinion in Pharmacology, 8, 593-597. http://dx.doi.org/10.1016/j.coph.2008.04.010
- Carson, C.F. and Riley, T.V. (2003) Non-Antibiotic Therapies for Infectious Diseases. Communicable Diseases Intelligence, 27, S143-S146.
- Suzuki, H., Nomura, S., Masaoka, T., Goshima, H., Kamata, N., Kodama, Y., Ishii, H., Kitajima, M., Nomoto, K. and Hibi, T. (2004) Effect of Dietary Anti-Helicobacter pylori-Urease Immunoglobulin Y on Helicobacter pylori Infection. Alimentary Pharmacology & Therapeutics, 20, 185-192. http://dx.doi.org/10.1111/j.1365-2036.2004.02027.x
- Shin, J.H., Nam, S.W., Kim, J.T., Yoon, J.B., Bang, W.G. and Roe, I.H. (2003) Identification of Immunodominant Helicobacter pylori Proteins with Reactivity to H. pylori-Specific Egg-Yolk Immunoglobulin. Journal of Medical Microbiology, 52, 217-222. http://dx.doi.org/10.1099/jmm.0.04978-0
- Shin, J.H., Roe, I.H. and Kim, H.G. (2004) Production of Anti-Helicobacter pylori Urease-Specific Immunoglobulin in Egg Yolk Using an Antigenic Epitope of H. pylori Urease. J Journal of Medical Microbiology, 53, 31-34. http://dx.doi.org/10.1099/jmm.0.05327-0
- Nomura, S., Suzuki, H., Masaoka, T., Kurabayashi, K., Ishii, H., Kitajima, M., Nomoto, K. and Hibi, T. (2005) Effect of Dietary Anti-Urease Immunoglobulin Y on Helicobacter pylori Infection in Mongoliangerbils. Helicobacter, 10, 43-52. http://dx.doi.org/10.1111/j.1523-5378.2005.00290.x
- Horie, K., Horie, N., Abdou, A.M., Yang, J.O., Yun, S.S., Chun, H.N., Park, C.K., Kim, M. and Hatta, H. (2004) Egg Yolk Immunoglobulin (IgY) on Helicobacter pylori in Humans. Journal of Dairy Science, 87, 4073-4079. http://dx.doi.org/10.3168/jds.S0022-0302(04)73549-3
- Suzuki, M., Maruyama, K., Suzuki, H., Tanaki, S., Suzuki, K. and Ishii, H. (2004) 13C-Ethanol Breath Test Reveals Impaired Alcohol Metabolism in Patients with Helicobacter pylori Infection. Alimentary Pharmacology & Therapeutics, 20, 109-115. http://dx.doi.org/10.1111/j.1365-2036.2004.02020.x
- Parsonnet, J., Blaser, M.J., Perez-Perez, G.I., et al. (1992) Symptoms and Risk Factors of Helicobacter pylori Infection in a Cohort of Epidemiologists. Gastroenterology, 102, 41-46.
- Cullen, D.J., Collins, B.J., Christiansen, K.J., et al. (1993) When Is Helicobacter pylori Infection Acquired? Gut, 34, 1681-1682. http://dx.doi.org/10.1136/gut.34.12.1681
- Sipponen, P., Kosunen, T.U, Samloff, I.M., et al. (1996) Rate of Helicobacter pylori Acquisition among Finnish Adults: A Fifteen Year Follow-Up. Scandinavian Journal of Gastroenterology, 31, 229-232. http://dx.doi.org/10.3109/00365529609004871
- Malaty, H.M., Evans, D.G., Evans, Jr., D.J. and Graham, D.Y. (1992) Helicobacter pylori in Hispanics: Comparison with Blacks and Whites of Similar Age and Socioeconomic Class. Gastroenterology, 103, 813-816.
- Malaty, H.M. and Graham, D.Y. (1994) Importance of Childhood Socioeconomic Status on the Current Prevalence of Helicobacter pylori Infection. Gut, 35, 742-745. http://dx.doi.org/10.1136/gut.35.6.742
- Kosunen, T.U., Aromaa, A., Knekt, P., et al. (1997) Helicobacter Antibodies in 1973 and 1994 in the Adult Population of Vammala, Finland. Epidemiology & Infection, 119, 29-34. http://dx.doi.org/10.1017/S0950268897007565
- Tone, M.A. (1999) Transmission of Helicobacter pylori. Postgraduate Medical Journal, 75, 198-200.
- Everhart, J.E. (2000) Recent Developments in the Epidemiology of Helicobacter pylori. Gastroenterology Clinics of North America, 29, 559-578. http://dx.doi.org/10.1016/S0889-8553(05)70130-8
- Gasbarrini, A., Anti, M., Franceschi, F., Armuzzi, A., Cotichini, R., Ojetti, V., Candelli, M., Lippi, M.E., Paolucci, M., Cicconi, V., Cammarota, G., Danese, S., Silveri, N.G., Catananti, C., Pola, P., Stroffolini, T. and Gasbarrini, G. (2001) Prevalence of and Risk Factors for Helicobacter pylori Infection among Healthcare Workers at a Teaching Hospital in Rome: The Catholic University Epidemiological Study. European Journal of Gastroenterology & Hepatology, 13, 185- 189. http://dx.doi.org/10.1097/00042737-200102000-00015
- Eaton, K.A., Suerbaum, S., Josenhans, C. and Krakowka, S. (1996) Colonization of Gnotobiotic Piglets by Helicobacter pylori Deficient in Two Flagellin Genes. Infection and Immunity, 64, 2445-2448.
- Rektorschek, M., Weeks, D., Sachs, G. and Melchers, K. (1998) Influence of pH on Metabolism and Urease Activity of Helicobacter pylori. Gastroenterology, 115, 628-641. http://dx.doi.org/10.1016/S0016-5085(98)70142-8
- Segal, E.D., Falkow, S. and Tompkins, L.S. (1996) Helicobacter pylori Attachment to Gastric Cells Induces Cytoskeletal Rearrangements and Tyrosine Phosphorylation of Host Cell Proteins. Proceedings of the National Academy of Sciences of the United States of America, 93, 1259-1264. http://dx.doi.org/10.1073/pnas.93.3.1259
- Putsep, K., Branden, C.I., Boman, H.G. and Nomark, S. (1999) Antibacterial Peptide from H. pylori. Nature, 398, 671- 672. http://dx.doi.org/10.1038/19439
- Meichers, K., Weitznegger, T., Steinhilber, W., Sachs, G. and Schäfer, K.P. (1995) A Novel P Type ATPase Cloned from Helicobacter pylori. Gastroenterology, 108, A165. http://dx.doi.org/10.1016/0016-5085(95)23322-9
- Moran, A.P. (1996) The Role of Lipopolysaccharide in Helicobacter pylori Pathogenesis. Alimentary Pharmacology & Therapeutics, 10, 39-50.
- Censini, S., Lange, C., Xiang, Z.Y., Crabtree, J.E., Ghiara, P., Borodovsky, M., Rappuoli, R. and Covacci, A. (1996) Cag, a Pathogenicity Island of Helicobacter pylori, Encodes Type I-Specific and Disease-Associated Virulence Factors. Proceedings of the National Academy of Sciences of the United States of America, 93, 14648-14653. http://dx.doi.org/10.1073/pnas.93.25.14648
- Graham, D.Y. and Yamaoka, Y. (2000) Disease-Specific Helicobacter pylori Virulence Factors: The Unfulfilled Promise. Helicobacter, 5, S3-S9, Discussion S27-S31.
- Wu, A., Crabtree, J., Bernstein, L., Hawtin, P., Cokbum, M., Sosnowska, D. and Forman, D. (2001) Role of Helicobacter pylori Cag A+ Strains and Risk of Adenocarcinoma of the Stomach and Esophagus. Gastroenterology, 120, A14. http://dx.doi.org/10.1016/S0016-5085(08)80070-4
- Cover, T.L. (1996) The Vacuolating Cytotoxin of Helicobacter pylori. Molecular Microbiology, 20, 241-246. http://dx.doi.org/10.1111/j.1365-2958.1996.tb02612.x
- Peek Jr., R.M., Thompson, S.A., Donahue, J.P., Tham, K.T., Atherton, J.C., Blaser, M.J. and Miller, G.G. (1998) Adherence to Gastric Epithelial Cells Induces Expression of a Helicobacter pylori gene, IceA that Is Associated with Clinical Outcome. Proceedings of the Association of American Physicians, 110, 531-544.
- Go, M.F. and Crowe, S.E. (2000) Virulence and Pathogenicity of Helicobacter pylori. Gastroenterology Clinics of North America, 29, 649-670. http://dx.doi.org/10.1016/S0889-8553(05)70136-9
- Vorobjova, T., Maaroos, H.I., Sipponen, P., Villako, K. and Uibo, R. (2001) Apoptosis in Different Compartments of Antrum and Corpus Mucosa in Chronic Helicobacter pylori Gastritis. An 18-Year Followup Study. Scandinavian Journal of Gastroenterology, 36, 136-143. http://dx.doi.org/10.1080/003655201750065870
- Fennerty, M.B., Lieberman, D.A., Vakil, N., Magaret, N., Faigel, D.O. and Helfand, M. (1999) Effectiveness of Helicobacter pylori Therapies in a Clinical Practice Setting. JAMA Internal Medicine, 159, 1562-1566. http://dx.doi.org/10.1001/archinte.159.14.1562
- Iovene, M.R., Romano, M., Pilloni, A.P., Giordano, B., Montella, F., Caliendo, S. and Tufano, M.A. (1999) Prevalence of Antimicrobial Resistance in Eighty Clinical Isolates of Helicobacter pylori. Chemotherapy, 45, 8-14. http://dx.doi.org/10.1159/000007159
- Ling, T.K., Cheng, A.F., Sung, J.J., Yiu, P.Y. and Chung, S.S. (1996) An Increase in Helicobacter pylori Strains Resistant to Metronidazole: A Five Year Study. Helicobacter, 1, 57-61. http://dx.doi.org/10.1111/j.1523-5378.1996.tb00009.x
- Sunwoo, H.H. and Sim, J.S. (2003) IgY Technology for Human Health: Antimicrobial Activities of IgY Preparations against Foodborne Pathogens. The 225th ACS National Meeting, New Orleans, 23-27 March 2003. http://oasys2.confex.com/acs/225nm/techprogram/P581665.HTM
- Hatta, H., Tsuda, K., Akachi, S., Kim, M. and Yamamoto, T. (1993) Productivity and Some Properties of Egg Yolk Antibody (IgY) against Human Rotavirus Compared with Rabbit IgG. Bioscience, Biotechnology, and Biochemistry, 57, 450-454. http://dx.doi.org/10.1271/bbb.57.450
- Sun, S., Mo, W., Ji, Y. and Lius, S. (2001) Preparation and Mass Spectrometric Study of Egg Yolk Antibody (IgY) against Rabies Virus. Rapid Communications in Mass Spectrometry, 15, 708-712. http://dx.doi.org/10.1002/rcm.271
- Woolley, J.A. and Landon, J. (1995) Comparison of Antibody Production to Human Interlukin-6 (IL-6) by Sheep and Chickens. Journal of Immunological Methods, 178, 253-265. http://dx.doi.org/10.1016/0022-1759(94)00263-V
- Shimizu, M., Nagashima, H., Sano, K., Hashimoto, K., Ozeki, M. and Tsuda, K. (1992) Molecular Stability of Chicken and Rabbit Immunoglobulin G. Bioscience, Biotechnology, and Biochemistry, 56, 270-274. http://dx.doi.org/10.1271/bbb.56.270
- Campbell, R.D., Dodds, A.W. and Porter, R.R. (1980) The Binding of Human Complement Component C4 to AntibodyAntigen Aggregates. Biochemical Journal, 189, 67-80.
- Fischer, M. and Hlinak, A. (2000) The Lack of Binding Ability of Staphylococcal Protein A and Streptococcal Protein G to Egg Yolk Immunoglobulins of Different Fowl Species. Berliner und Münchener tierärztliche Wochenschrift, 113, 94-96.
- Larsson, A., Karlsson-Parra, A. and Sjoquist, J. (1991) Use of Chicken Antibodies in Enzyme Immunoassays to Avoid Interferences by Rheumatoid Factors. Clinical Chemistry, 37, 411-414.
- Rubinstein, E., Kouns, W.C., Jennings, L.K., Boucheix, C. and Carroll, R.C. (1991) Interaction of two GPIIb/IIa Monoclonal Antibodies with Platlet Fc Receptor (FcγRII). British Journal of Haematology, 78, 80-86. http://dx.doi.org/10.1111/j.1365-2141.1991.tb04386.x
- Narat, M. (2003) Production of Antibodies in Chickens. Food Technology and Biotechnology, 41, 259-267.
- Zeidler, G. (1998) Eggs Vital to Human and Animal Medicine. World’s Poultry, 14, 33-34.
- Chen, J.P. and Chang, M.C. (2003) Effect of Anti-Helicobacter pylori Urease Antibody (IgY) as a Food Ingredient on the Decrease of H. pylori in the Stomach of Humans Infected with H. pylori. Taiwanese Journal of Agricultural Chemistry and Food Science, 41, 408-414.
- Chandan, R.C. (1999) Enhancing Market Value of Milk by Adding Cultures. Journal of Dairy Science, 82, 2245-2256. http://dx.doi.org/10.3168/jds.S0022-0302(99)75472-X
- Yamane, T., Saito, Y., Takizawa, S., Goshima, H., Kodama, Y., Horie, N. and Kim, M. (2003) Development of AntiHelicobacter pylori Urease IgY and Its Application for Food Product. Food Processing and Ingredients, 38, 70. (in Japanese)
- Chang, Y., Min, S., Kim, K., Han, Y. and Lee, J. (2003) Delta (13)C-Urea Breath Test Value Is a Useful Indicator for Helicobacter pylori Eradication in Patients with Functional Dyspepsia. Journal of Gastroenterology and Hepatology, 6, 726-731. http://dx.doi.org/10.1046/j.1440-1746.2003.03049.x
- Siriya, P., Chu, C., Chen, M., Lo, C., Huang, S. and Lien, T. (2013) Extraction and Purification of Anti-Helicobacter pylori IgY. Journal of Agricultural Science, 5, 132-138.
- Chiozzi, V., Mazzini, G., Oldani, A., Sciullo, A., Ventura, U., Romano, M., Boquet, P. and Ricci, V. (2009) Relationship between VacA Toxin and Ammonia in Helicobacter pylori-Induced Apoptosis in Human Gastric Epithelial Cells. Journal of Physiology and Pharmacology, 60, 23-30.
- Palframan, S.L., Kwok, T. and Gabriel, K. (2012) Vacuolating Cytotoxin A (VacA), a Key Toxin for Helicobacter pylori Pathogenesis. Frontiers in Cellular and Infection Microbiology, 2, 92. http://dx.doi.org/10.3389/fcimb.2012.00092
- Fujikawa, A., Shirasaka, D., Yamamoto, S., Ota, H., Yahiro, K., Fukuda, M., Shintani, T., Wada, A., Aoyama, N., Hirayama, T., Fukamachi, H. and Noda, M. (2003) Mice Deficient in Protein Tyrosine Phosphatase Receptor Type Z Are Resistant to Gastric Ulcer Induced by VacA of Helicobacter pylori. Nature Genetics, 33, 375-381. http://dx.doi.org/10.1038/ng1112
- Yamashita, Y., et al. (2010) Egg Yolk Antibodies Suppress H. pylori Infection and Gastritis. European Helicobacter Study Group. XXIII International Workshop on Helicobacter and Related Bacteria in Chronic Digestive Inflammation and Gastric Cancer, Rotterdam, 16-18 September 2010, 339.
NOTES

*Corresponding author.

