Applied Mathematics
Vol.07 No.10(2016), Article ID:67882,24 pages
10.4236/am.2016.710105
The Bistability Theorem in a Model of Metastatic Cancer
Jens Christian Larsen
Vanløse Alle 50 2 mf tv, 2720 Vanløse, Copenhagen, Denmark

Copyright © 2016 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 2 May 2016; accepted 27 June 2016; published 30 June 2016
ABSTRACT
The main theorem of the present paper is the bistability theorem for a four dimensional cancer model, in the variables 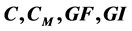 representing primary cancer C, metastatic cancer
representing primary cancer C, metastatic cancer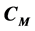 , growth factor GF and growth inhibitor GI, respectively. It says that for some values of the para- meters this system is bistable, in the sense that there are exactly two positive singular points of this vector field. And one is stable and the other unstable. We also find an expression for
, growth factor GF and growth inhibitor GI, respectively. It says that for some values of the para- meters this system is bistable, in the sense that there are exactly two positive singular points of this vector field. And one is stable and the other unstable. We also find an expression for 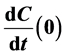 for the discrete model T of the introduction, with variables
for the discrete model T of the introduction, with variables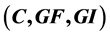 , where C is cancer,
, where C is cancer, 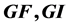 are growth factors and growth inhibitors respectively. We find an affine vector field Y whose time one map is T2 and then compute
are growth factors and growth inhibitors respectively. We find an affine vector field Y whose time one map is T2 and then compute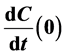 , where
, where 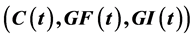 is an integral curve of Y through
is an integral curve of Y through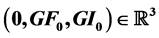 . We also find a formula for the first escape time for the vector field associated to T, see section four.
. We also find a formula for the first escape time for the vector field associated to T, see section four.
Keywords:
Bistability, Cancer, Mass Action Kinetic System, Discrete Dynamical System

1. Introduction
1.1. Summary of the Paper
We continue the study of the cancer model from Larsen (2016) [1] . The model is
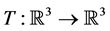
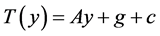
where
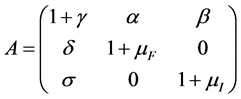
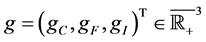 are birth rates and T denotes transpose. Here
are birth rates and T denotes transpose. Here 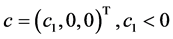 is chemotherapy
is chemotherapy
and 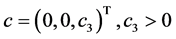 is immune therapy. The parameters
is immune therapy. The parameters ,
, 




when 
The eigenvalues are
In section two we prove the Bistability Theorem for a mass action kinetic system of metastatic cancer 







For 








The phase space of our model T is






1.2. The Litterature
uPAR (urokinase plasminogen activator receptor) is a cell surface protein, that is associated with invasion and metastasis of cancer cells. In Liu et al. (2014) [2] a cytoplasmic protein Sprouty1 (SPRY1) an inhibitor of the (Ras-mitogen activated protein kinase) MAPK pathway is shown to interact with uPAR and cause it to be degraded. Overexpression of SPRY1 in HCT116 or A549 xenograft in athymic nude mice, led to great suppression of tumor growth. SPRY1 is an inhibitor of the MAPK pathway. Several cancer cells have a low basal expression of SPRY1, e.g. breast, prostate and liver cancer. SPRY1 promotes the lysosomal mediated degradation of uPAR. SPRY1 overexpression results in a decreased expression of uPAR protein. This paper suggests that SPRY1 regulates cell adhesion through an uPAR dependant mechanism. SPRY1 inhibits proliferation via two distinct pathways: 1) SPRY1 is an intrinsic inhibitor of the Raf/MEK/ERK pathway; 2) SPRY1 promotes degradation of uPAR, which leads to inhibition of FAK and ERK activation.
According to Luo and Fu (2014), [3] EGFR (endoplasmic growth factor receptor) tyrosine kinase inhibitors (TKIs) are very efficient against tumors with EGFR activating mutations in the EGFR intracytoplasmic tyrosin kinase domain and cell apoptosis was the result. However some patients developed resistance and this reference aimed to elucidate molecular events involved in the resistance to EGFR-TKIs. The first EGFR-TKI s to be approved by the FDA (Food and Drug Administration, USA) for treatment of NSCLC (non small cell lung cancer) were gefitinib and erlotinib. The mode of action is known. These drugs bind to the ATP binding site of EGFR preventing autophosphorylation and then blocking downstream signalling cascades of pathways RAS/ RAF/MEK/ERK and PI3K/AKT with the results, proliferation inhibition, cell cycle progression delay and cell apoptosis.
There are several important monographs relevant to the present paper, see Adam & Bellomo (1997), [4] , Geha & Notarangelo (2012), [5] , Murphy (2012), [6] , Marks (2009), [7] , Molina (2011), [8] .
2. A mass Action Kinetic Model of Metastatic Cancer
The main result of this section is Theorem 1 below that proves the bistability of the mass action kinetic system (1) to (8). Consider then the mass action kinetic system from Larsen (2016), [9] , in the species 








The complexes are 


all 
But first we state a theorem, we shall next prove. A positive (nonnegative) singular point
of f is a singular point of f, such that 

Theorem 1 Assume 


where 





is stable when
Consider a singular point 
Setting the last coordinate of f equal to zero gives
when 

and

When 
and from (10) we get
This means that B simplifies to
Let 
Also
The characteristic polynomial of 
Finally
In Larsen (2016) [9] , we found two cubic polynomials 
whenever 
Lemma 1 Assume 
where
Proof. The coefficient to 




Everything cancels out and leaves a zero. The coefficient to 
Square 

Everything cancels out except
The coefficient to 
Multiply
Everything cancels out except
Finally the constant term is
The lemma follows.
Theorem 2 Assume 


where
Proof. We have
where
and
due to symmetry of 


in P and
in

are singular points of f and that

are not singular points of f. Here
and
Also


We have

and logically equivalent

where 
So
and from this the formula follows. And (16) is a similar computation.
We shall insert (15), (16) in the first coordinate of f, multiplied with
Now abbreviate 
Multiply with 
But this amounts to
and this vanishes due to the formula for roots of quadratic polynomials. That the second coordinate vanishes is logically equivalent. So (11) are singular points of f.
We shall now argue, that
is not a singular point of f. To this end define
Insert the formulas (15), (16) for 

Multiply with 


But (17) is zero by the above and (18) is nonzero. So 

In the remainder of the proof of Theorem 1, we assume, that
We shall now verify that 
But we have
Simply insert (15) and (16) in the numerator
Now we use that
so
is equivalent to
The right hand side here is negative and the left hand side is positive. Thus 

We shall now show that 


But this amounts to
which is equivalent to
and this again is equivalent to
and from this it follows that 
And a formula for
Define
so that
Now introduce these two formulas in the formulas for
Notice that 

is negative for small 






This equation holds for small


where 
And from this it follows that 








Consider the mass action kinetic system in the species 







The complexes are 


see Horn and Jackson (1972), [10] . Notice that (24), (25) are the Brusselator, which is known to have oscillating solutions for some values of the parameters, see Sarmah et al. (2015), [11] . Subtracting 






3. Eigenvalues with Negative Real Part
In this section 
Figure 1. The oscillating mass action kinetic system. I have plotted P versus C.
on the hyperplane 




and compute, when
If 

By Larsen (2016), [1] ,
Then
Define the vector field


where 


where 
If
then
Assume that 
But this means that
because we have

i.e. 
So assuming
We want to have
and
such that
Here 

Then
Thus
Now
Define
Let 
where 



Now suppose 

Then
when 


Then
Define the vector field



and the time one map is
and we want this to be
Then define the vector field
This vector field has time one map
Then arguing as before
and
We can now find
Next consider the chemo therapy model
and initially, that 
We want this vector field to have time one map
Then we find
Now compute arguing as above
Finally we can find
and this becomes
Now consider the chemo therapy model, when 

The second coordinate here should be equal to
while the third coordinate should be equal to
in order that the time one map of 

and this is simplified to
Remark 1 When 


by the above you can find an affine vector field whose time one map is




4. Escaping Phase Space
In this section 





and 
U as in section 3. This vector field, B, has time one map T, see Larsen (2016), [1] , or argue as in Section 3.
The purpose of this section is to prove, that there exists a first escape time

When 
or there exists a smallest 
Proposition 3 Suppose 



Proof. We have the following formula for the flow of B
Here
and
Define
Since 

It follows that we have the following formula
Since 
Remark 2 By the proof we have
implies 


When 
These formulas are explained in the proof of Proposition 4.
Let 
D as in section 3. B has time one map T, see Larsen (2016), [1] , or argue as in section three.
Proposition 4 Suppose 



If 
for all
(ii) If 

If 
for all
Proof. First of all the flow of F is
We have the following formula
where 
From this formula, (ii) follows.
Remark 3 In case (i) of the proposition, if 
implies
In case (ii) of the proposition, if 
implies
We shall now derive a formula for the first escape time 


and
where
i.e.
Compute
where
If 



Then we have the following formulas


Assume that 

for 

we claim that there are atmost finitely many such solutions and hence that there exists a smallest 
Assume for contradiction, that there are infinitely many solutions to
By (31) there are exactly 
Since there are infinitely many solutions to 
in 
By the mean value theorem, there exists 






If 
Since 



so
By 





let 

By 

Suppose 






If 





We shall now find the first escape time when 
and
where
i.e.
Assume in the notation of Proposition 4, that 
If 

and
There are atmost two solutions to 









5. Summary and Discussion
In this paper we proved that the model of primary and metastatic cancer in Section 2 is bistable, in the sense, that there are exactly two positive singular points. One of them is unstable, and when one of the rate constants is small the other is stable. Then we found formulas for the rate of change of cancer growth for the model T of the introduction, when for 





It would be interesting to figure out what happens if the polynomials 
About the References
How do cancer cells coordinate glycolysis and biosynthesis. They do that with the aid of an enzyme called Phosphoglycerate Mutase 1. In the reference [12] , the authors suggest a dynamical system for their findings in a figure at the end of the paper. In the reference [13] , A. K. Laird showed that solid tumors do not grow exponentially, but rather like a Gompertz function. The publications of the author are concerned with semi Riemannian dynamical systems, e.g. Lorentzian Geodesic Flows, see [14] and electrical network theory of countable graphs, see [15] , [16] .
Cite this paper
Jens Christian Larsen, (2016) The Bistability Theorem in a Model of Metastatic Cancer. Applied Mathematics,07,1183-1206. doi: 10.4236/am.2016.710105
References
- 1. Larsen, J.C. (2016) Models of Cancer Growth. Journal of Applied Mathematics and Computing.
- 2. Liu, X., Lan, Y., Zhang, D., Wang, K. and Hua, Z.-C. (2014) SPRY1 Promotes the Degradation of uPAR and Inhibits uPAR-Mediated Cell Adhesion and Proliferation. American Journal of Cancer Research, 4, 683-697.
- 3. Luo, M. and Fu, L.-W. (2014) Redundant Kinase Activation and Resistance of EGFR-Tyrosine Kinase Inhibitors. American Journal of Cancer Research, 4, 608-628.
- 4. John, A. and Bellomo, N. (1997) A Survey of Models for Tumor-Induced Immune System Dynamics. Birkhauser, Boston.
- 5. Geha, R. and Notarangelo, L. (2012) Case Studies in Immunology. Garland Science, Hamden.
- 6. Murphy, K. (2012) Immunobiology. 8th Edition, Garland Science, Hamden.
- 7. Marks, F., Klingmüller, U. and Müller-Decker, K. (2009) Cellular Signal Processing. Garland Science, Hamden.
- 8. Molina-Paris, C. and Lythe, G. (2011) Mathematical Models and Immune Cell Biology. Springer Verlag, New York.
http://dx.doi.org/10.1007/978-1-4419-7725-0 - 9. Larsen, J.C. The Bistability Theorem in a Cancer Model.
- 10. Horn, F. and Jackson, R. (1972) General Mass Action Kinetics. Archive for Rational Mechanics and Analysis, 47, 81- 116.
http://dx.doi.org/10.1007/BF00251225 - 11. Sarmah, H.K., Das, M.C. and Baishya, T.K. (2015) HOPF Bifurcation in a Chemical Model. International Journal for Innovative Research in Science and Technology, 1, 23-33.
- 12. Hitosugi, T., et al. (2012) Phosphoglycerate Mutase 1 Coordinates Glycolysis and Biosynthesis to Promote Tumor Growth. Cancer Cell, 22, 585-600.
- 13. Laird, A.K. (1964) Dynamics of Tumor Growth. British Journal of Cancer, 18, 490-502.
http://dx.doi.org/10.1038/bjc.1964.55 - 14. Larsen, J.C. (1996) Lorentzian Geodesic Flows. Journal of Differential Geometry, 43, 119-170.
- 15. Larsen, J.C. (1991) Dynamical Systems and Semi Riemannian Geometry. Ph.D. Thesis, the Technical University of Denmark, Lyngby.
- 16. Larsen, J.C. (1997) Electrical Network Theory of Countable Graphs. IEEE Transactions, Curcuits and Systems: Fundamental Theory and Applications, 44, 1045-1055.
























































































































































































































