Journal of Environmental Protection
Vol.3 No.11(2012), Article ID:25099,10 pages DOI:10.4236/jep.2012.311176
Bioassessment of the Rio Grande Upstream and Downstream of Los Alamos National Laboratory, New Mexico, USA
![]()
1Environmental Stewardship Group, Los Alamos National Laboratory, Los Alamos, USA; 2Jacobi and Associates, Santa Fe, USA.
Email: fresquezp@lanl.gov
Received September 12th, 2012; revised October 11th, 2012; accepted November 8th, 2012
Keywords: Benthic Macroinvertebrates; Water Quality, Radionuclides; Polychlorinated Biphenyls; Mercury; Rio Grande; Monitoring
ABSTRACT
Benthic macroinvertebrates (aquatic insects) were collected from the Rio Grande upstream and downstream of Los Alamos Canyon (LAC), a major drainage that crosses Los Alamos National Laboratory (LANL) lands in northern New Mexico, USA. LAC contains legacy waste, including radionuclides and polychlorinated biphenyls, and occasionally discharges storm water and snowmelt flows to the Rio Grande. The Rio Grande is the major waterway that flows southward across the state. In 2009, rock baskets were placed in waters 61- to 76-cm-deep within each reach (five per reach), and, after approximately 6 weeks of colonization, the rock baskets were retrieved. All samples were sorted completely and organisms were identified to the lowest possible taxonomic level. Both reaches in 2009 were dominated by the collector filtering net-spinning caddisfly, Hydropsyche occidentalis. In 2011, benthic macroinvertebrates were collected using D kick nets from shallow riffle locations (15- to 31-cm depth) from each reach (six per reach). These samples were collected after post- (Las Conchas) fire flooding events moved sediment and ash through the two study areas—the downstream reach, however, was affected by higher flows and greater number of flooding events than those affecting the upstream reach. Each kick net sample consisted of ten 1-m (kick) samples. The 10 subsamples were composited and organisms were picked from randomly selected cells in a sorting pan until 500 organisms had been identified to the lowest possible taxonomic level. Both reaches in 2011 were dominated by the collector-gathering mayfly, Baetis tricaudatus. A bioassessment of the downstream reach compared with the upstream (reference) reach was conducted by scoring 10 metrics related to the structure and function of the benthic macroinvertebrate community. While 2009 ranked at the highest level (nonimpaired), 2011 ranked a level lower (slightly impaired). The slightly lower bioassessment score of the downstream reach in 2011 may be a result of flooding impacts following the Las Conchas fire rather than of LANL operations. Overall, based on the similarity of benthic macroinvertebrate metrics between reaches and the composition of benthic macroinvertebrates favoring pollution intolerant taxa, LANL influences, if any, via the LAC system to the Rio Grande are not significantly impacting water quality of the Rio Grande.
1. Introduction
The use of benthic macroinvertebrates (aquatic insects) to evaluate the presence and extent of environmental pollution in aquatic environments is well established [1-3]. Because of their intimate contact with the substrate, relative immobile state, feeding habits, and extended residence time in aquatic systems, benthic macroinvertebrates may provide an indication of the quality of the water [4,5]. In general, large numbers of individuals and small numbers of species may occur with an increase in pollution or disturbance; unpolluted/undisturbed waters exhibit the converse [6,7]. Additionally, the types of benthic macroinvertebrates may be influenced by pollution conditions; the numbers of species in the orders most often associated with clean waters—Ephemeroptera (mayflies), Plecoptera (stoneflies), and Trichoptera (caddisflies)—generally decrease with an increase in metal [8,9] and organic [1] pollution, whereas the populations of Tubificidae (worms), Chironomidae (midges), and Gastropoda (snails) generally increase [10,11].
Los Alamos National Laboratory (LANL), a multidisciplinary research institution established in 1943, is situated on a large mesa with many deep, mostly ephemeral, west-to-east-oriented drainage canyons (Figure 1). Dur-
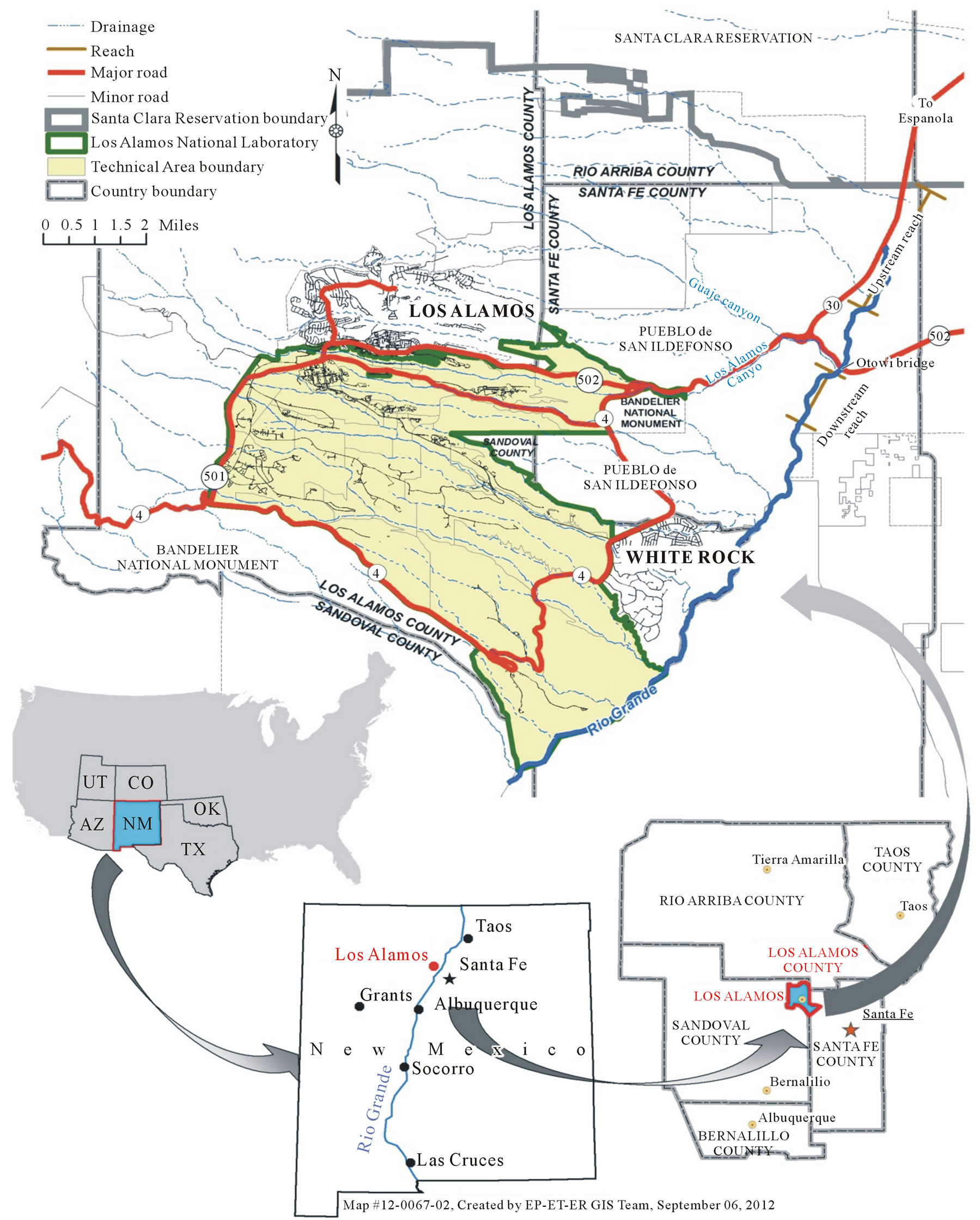
Figure 1. Location of upstream and downstream sample locations in relation to Los Alamos Canyon and Los Alamos National Laboratory.
ing the early years of LANL operations (mostly nuclear weapons work), some of these canyon drainage systems received various amounts of radioactive and chemical waste effluents [12,13]. Of the major drainages that cross LANL lands, the Los Alamos Canyon (LAC) drainage system has been identified as containing the highest amounts of radionuclides and polychlorinated biphenyls (PCBs) [14-16] and also has the highest potential of transporting these chemicals to the Rio Grande, which is 8 km away [17]. As a result of this and initiated by the prospect of increased storm water flows caused by a large wildfire west of LANL in 2000 (Cerro Grande Fire), a gabion rock-filled, low-head weir in LAC was constructed in 2001 to reduce the transport of sediments past the northeastern boundary of LANL [18].
The Rio Grande is the main drainage in New Mexico and traverses approximately 1207 river kilometers from its headwater in the San Juan Mountains in southwestern Colorado, southward through the entire State of New Mexico, to El Paso, Texas [19]. PCBs and mercury are the two dominant pollutants in fish inhabiting the river [20,21]. Average PCB concentrations are 24,575 pg/g wet and 17,813 pg/g wet in fish (fillets) collected directly upstream and downstream of LAC, respectively, and mercury concentrations in fish from both reaches regularly approach the US Environmental Protection Agency (EPA) screening level of 0.30 mg/kg [22]. Most radionuclides, with the exception of strontium-90, in fish (muscle plus bone) collected downstream of LAC were either not detected or similar to upstream fish [20,22].
Benthic macroinvertebrates are collected using a variety of methods—nets [2], dredges [23], and artificial substrates [24,25] are the most widely used—and the method of choice is largely dependent on resources, the size and type of the lotic system, subject benthic macroinvertebrates, and the goals of the study [26-28]. Few studies have been conducted on benthic macroinvertebrates inhabiting the mainstream Rio Grande in northern New Mexico [29,30], and basically no published studies exist that directly employ the use of benthic macroinvertebrates within the Rio Grande to evaluate defined or potential contamination from upstream point sources. The objective of this study was to determine, using two sampling methodologies (rock baskets and kick nets), if there was any difference in the structure and function of the benthic macroinvertebrate community upstream and downstream of the confluence of LAC with the Rio Grande.
2. Methods and Materials
In late July of 2009, 5 rock baskets were placed in the Rio Grande along the length of each of 2 reaches relative to LAC (and LANL) (10 total baskets) (Figure 1). The reference reach, along a 5-km upstream stretch, was located about 3.2 km north of the LAC confluence, and the downstream reach, along a 1.5-km stretch, was located directly south of the LAC confluence. The rock baskets, which were constructed of PVC-coated galvanized wire mesh, 17 by 28 cm in size, and containing 45 5- to 8-cm-diameter rocks, were attached to t-posts located in approximately 61- to 76-cm-deep water. They were set on top of a 2.5-cm-thick flat rock in a vertical position on the bottom of the river. After a colonization period of approximately 6 weeks (late July to early September), the baskets were collected; each rock basket was approached from the downstream side, enclosed with a large net (0.50-mm mesh opening), and carefully lifted out of the water. At the water’s edge, each rock, basket, and net was gently scrubbed and rinsed clean of benthic macroinvertebrates inside a 19-L plastic bucket. The water plus organisms in the bucket were then poured onto a Standard No. 35 (0.50-mm size) sieve. All material (debris and organisms) remaining on the sieve were placed into a 500-mL polyethylene bottle and preserved with 70% ethanol. After 24 hours, the old ethanol was replaced with a fresh mix.
In mid October of 2011, benthic macroinvertebrates were collected using a 0.23- by 0.46-m Turtox® bottom kick net (0.50-mm mesh) from six upstream locations and from six downstream locations of LAC on the Rio Grande; they were generally collected in the same locations as the rock basket samplers. Samples were collected after flooding event(s) occurred within both reaches of the Rio Grande; the upstream reach was influenced mostly by Santa Clara Canyon storm waters, a tributary to the Rio Grande approximately 12 km upstream of LAC, and the downstream reach was influenced by both the Santa Clara Canyon and the LAC drainage systems. Flooding within the Rio Grande was a result of the Las Conchas fire, a large wildfire that burned nearly 65,000 hectares of mixed conifer timber just west and upgradient of LANL in a south-to-north direction from June 26 to August 3, 2011. There were approximately 4 storm water events of varying degrees affecting the upstream reach and 12 (8 directly from the LAC drainage) affecting the downstream reach; the most significant of these occurred on August 21, 2011, that affected both reaches and on September 4, 2011, that affected only the downstream reach [31].
At each sample location, 10 (kick net) subsamples were collected in a downstream direction along a 10 m long transect at the 15- to 30-cm depth. Each subsample was collected by holding the net approximately 1 m downstream and then waddling/shuffling towards the net, which lofted benthic macroinvertebrates into the net. The total sample area was approximately 5 m2 (0.46 m × 10 m = 4.6 m2). Each composite sample (net) was immersed and inverted in a 19-L plastic bucket filled one-half full of water and gently rinsed clean of benthic macroinvertebrates and accompanying debris. The contents were then processed and preserved the same as described for the rock baskets.
Organisms from the rock basket and kick nets were sorted from the debris and were identified to the lowest practical taxonomic level. Because of the larger sampling area used for the kick nets, the samples were counted until 500 organisms were identified. This was accomplished by pouring each sample into a 40- × 30- × 6.4-cm white gridded 3 × 3 sorting pan (9 cells) and spreading the contents evenly throughout the pan. Cells were randomly selected, and all the organisms were removed (separated from the debris) from the selected cells until approximately 500 organisms had been counted and identified. The number of cells sorted was recorded and used to calculate the total number of organisms in the sample. The contents of the pan were further examined for any other uncommon or infrequently collected organisms. These were then added to the organism count for that sample (this count occurrence was not included for the multiplier but was added to the total after the multiplier had been used).
Based on these data, a bioassessment of the downstream reach compared with the upstream (reference) reach was conducted by comparing 10 biological attributes (metrics) related to the structure and function of the benthic macroinvertebrate community. The 10 metrics included abundance (population size); taxa richness (number of taxa); Ephemeroptera, Plecoptera, and Trichoptera (EPT) richness (number of EPT taxa); EPT/EPT + Chironomidae ratio (a high ratio indicates a good biotic condition); community loss (the lower the value the more similarity exists between the two locations); percent dominant taxa; taxa diversity (Shannon-Weaver); Hilsenhoff Biotic Index (HBI) (0 - 3.5 = excellent, 3.5 - 4.5 = very good, 4.5 - 5.5 = good,… 8.5 - 10 = very poor water quality); scrapers/scrapers + filtering collector ratio (indicates the food base present; a low ratio indicates the abundance of filamentous algae); and shredders/total functional feeder ratio (gives an indication of the coarse particulate organic material present; a low ratio indicates a poor riparian environment). The relationship (percent similarity) of these metrics as they relate to the upstream reference site was used to calculate an overall biological condition score [32], which is compared with the following four designations: nonimpaired (>83% similar community attributes), slightly impaired (54% - 79%), moderately impaired (21% - 49%), and severely impaired (<19%).
3. Results and Discussion
A wide range of benthic macroinvertebrate taxa (49 total) representing many orders and families were identified from rock baskets in 2009 from the two reaches studied (Table 1). Both reaches contained a similar number of taxa (39 each), but the numbers of organisms were significantly higher (Wilcoxon Rank Sum test at the 0.05 probability level) in the downstream reach as compared with the upstream reach. The numbers of EPT taxa from both reaches were well represented and the net-spinning caddisfly, Hydropsyche occidentalis (57% upstream and 67% downstream), was the most abundant organism collected. Hydropshyche was followed by other EPT insects in the following order: Heptagenia sp. (mayfly), Baetis tricaudatus (mayfly), Brachycentrus occidentalis (caddisfly), and Cheumatopsyche sp. (caddisfly).
Most metrics calculated from the rock basket data in 2009 were similar between reaches; these included taxa richness (already mentioned), EPT richness, diversity, HBI, and various community and functional ratios (Table 2). The number of EPT taxa and the HBI, in particular, which represent pollution sensitive organisms and the degree of organic pollution [1], respectively, indicate collectively that the water quality of both reaches was generally good. The two metrics from the downstream reach that was most dissimilar to the reference reach (upstream) included abundance (211% of the upstream reach) and the scrapers/scrapers + filtering collector’s ratio (31% of the upstream reach). Indeed, the populations of the filtering collectors, like Hydropsyche occidentalis, were over two times higher in the downstream reach than the upstream reach and may indicate that the downstream reach may be influenced by a slightly higher nutrient enrichment source than the upstream reach which favors the growth of filamentous algae, the food base for filtering collectors [33]. Overall, however, based on the bioassessment score from the rock baskets in 2009, the downstream reach was approximately 85% similar to the reference reach, which resulted in the highest rating of nonimpaired.
There were no significant differences (p > 0.05) in the total numbers of organisms between the two locations using kick nets in 2011 (Table 1). In contrast, there was a significant difference (p < 0.05) between the numbers of taxa in each sample at the two locations; a total of 36 taxa were collected upstream versus 23 taxa at the downstream location. Fourteen taxa collected upstream were not present downstream while only one taxon was collected downstream but not collected upstream. Most of these taxa were infrequently occurring organisms, less than 12 per sample except for more numerous flatworms at the upstream location. Both locations were dominated by the mayfly, Baetis tricaudatus (78% upstream and 82% downstream), followed by Culoptila sp. (caddisfly) and Heptagenia sp. (mayfly) in significantly lower amounts. Because of this dominance by one organism, both diversity indices were low, less than 1.6 (Table 2). The bioassessment summary showed that the downstream location was rated as slightly impaired compared with the upstream location. It contained 72% of the attributes of the upstream location.
The slightly lower metric designations within the downstream reach compared with the upstream reach in 2011 may be a result of the greater flow/number of flooding events at the downstream reach rather than of LANL influences. Both reaches, for example, were dominated by EPT taxa and the degree of organic pollu-
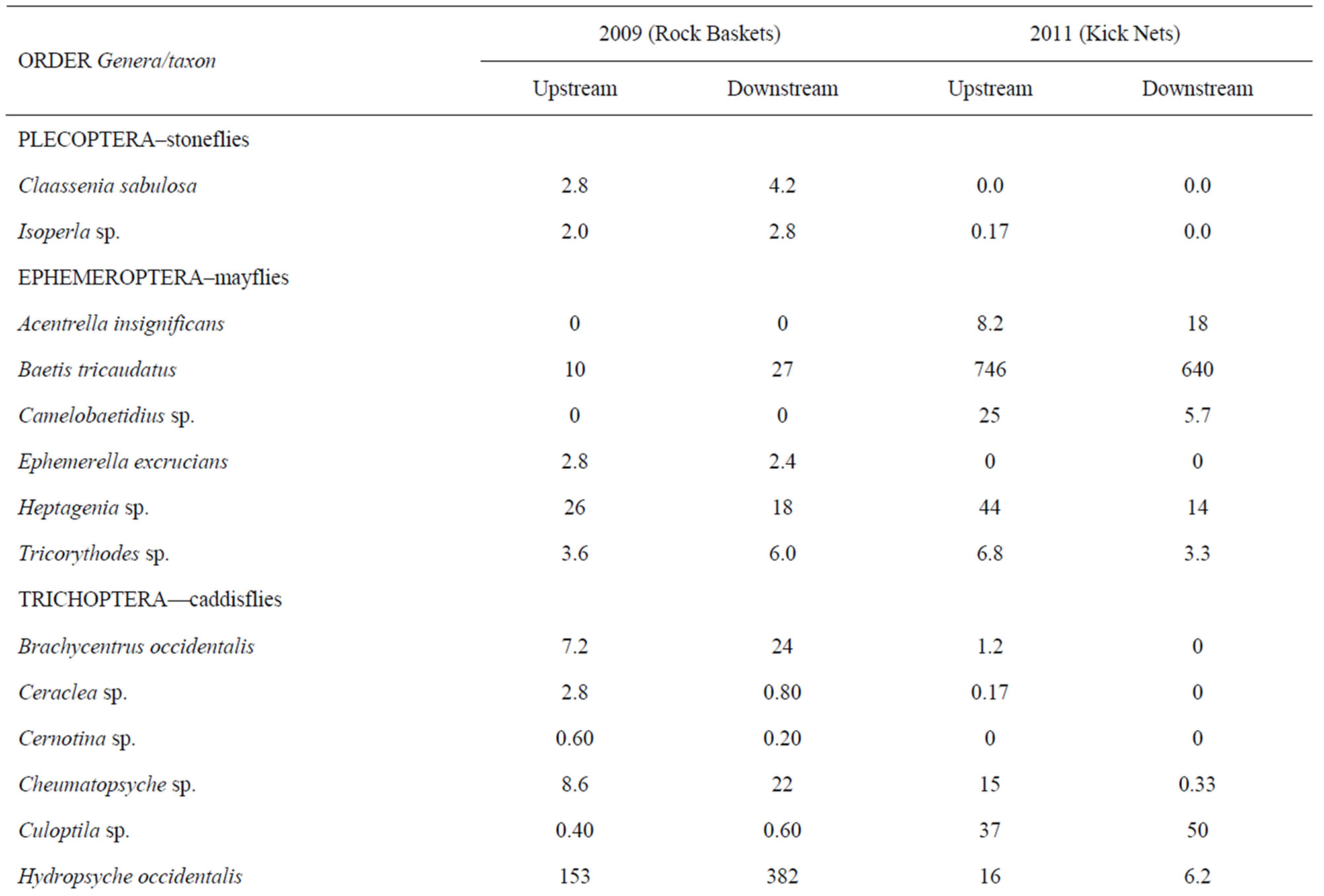
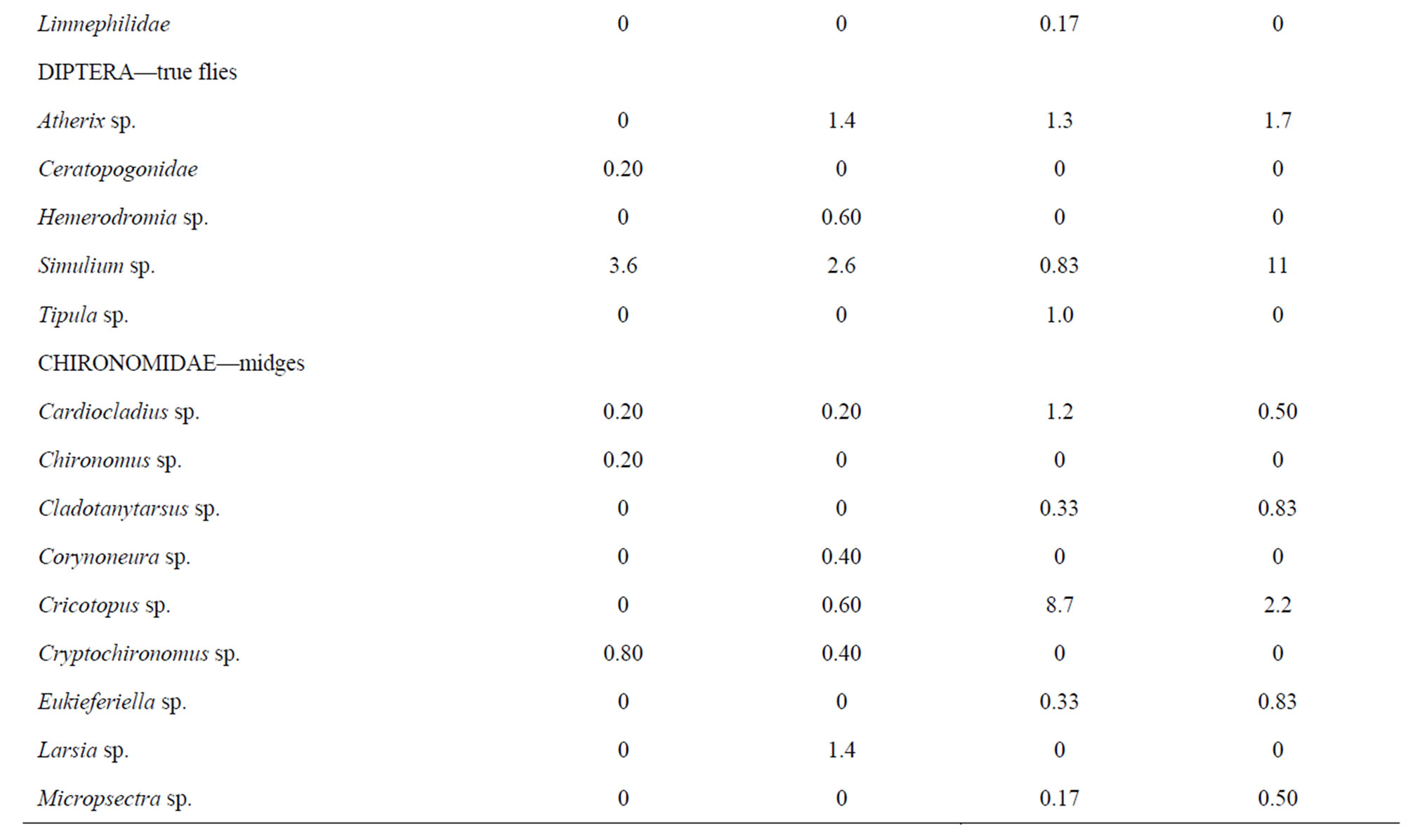

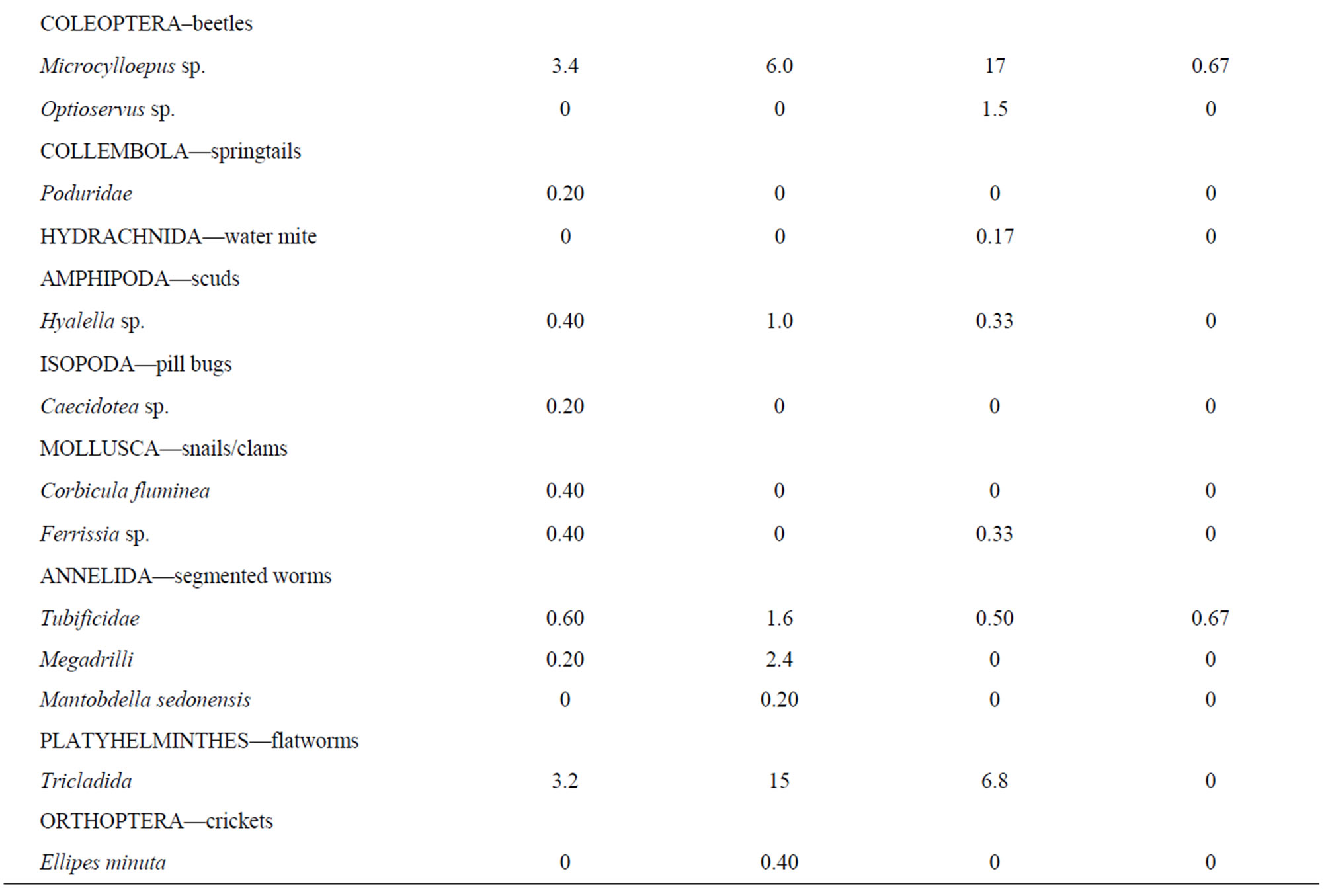
Table 1. Average benthic macroinvertebrate populations collected from the Rio Grande in 2009 using rock baskets and in 2011 using kick nets upstream and downstream of LANL.
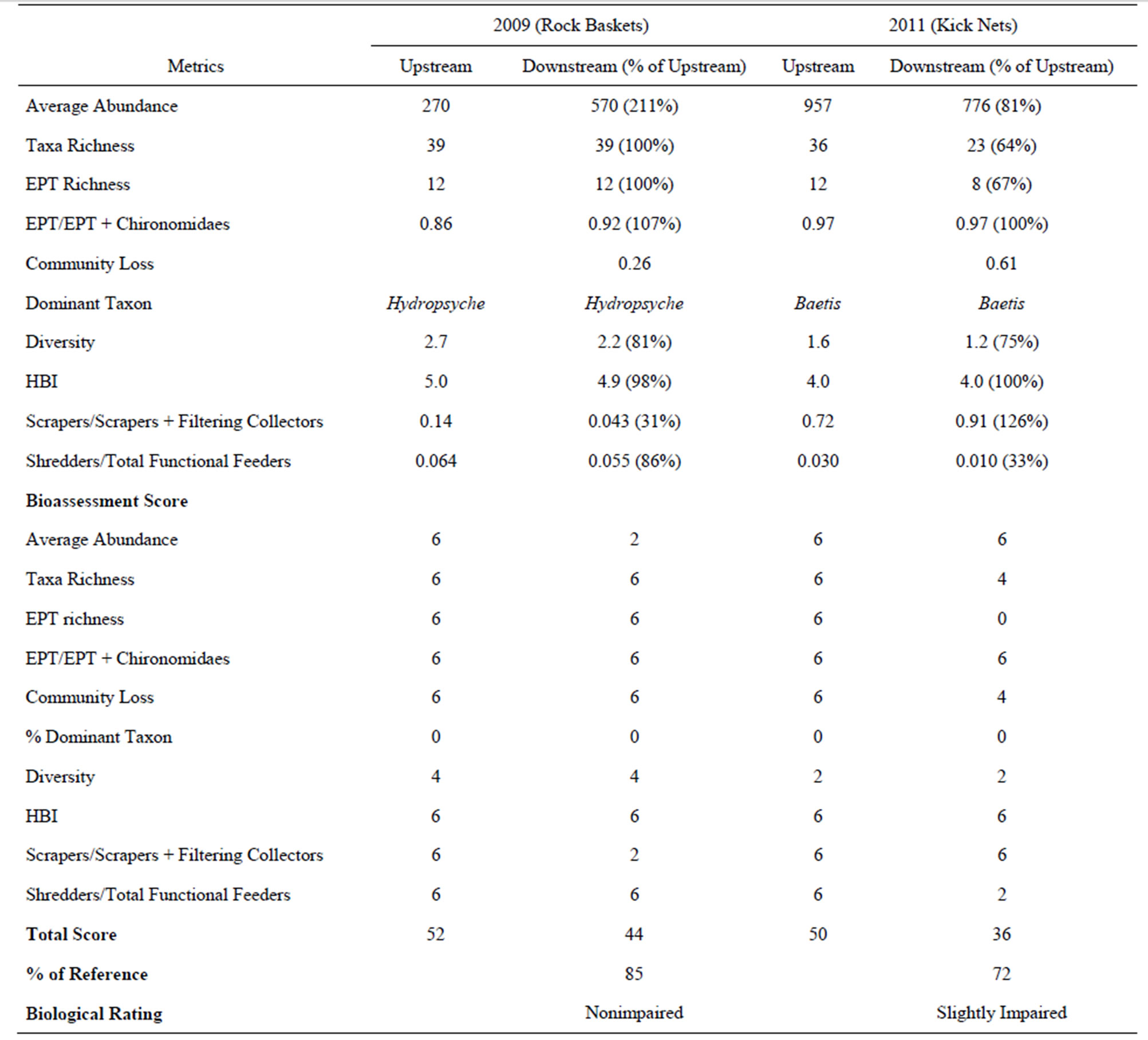
Table 2. Metrics and bioassessment score based on benthic macroinvertebrates collected from the Rio Grande in 2009 using rock baskets and in 2011 using kick nets upstream and downstream of LANL.
tion as measured by the HBI was indicative of very good water quality with possible slight organic pollution [1]. On the other hand, many studies have shown that flooding, particularly as a result of fire, which increases the amount of sediment and ash, significantly decreases the number and types of aquatic organisms [34-38]. The average suspended sediment concentration in waters of the Rio Grande within the upstream and downstream reaches from the flooding event of August 21, 2011, for example, was 28,000 (n = 3, a result of Santa Clara storm water measured upstream of LAC at the Otowi Bridge) and 35,230 mg/L (n = 4, measured approximately 3 river miles downstream from LAC), respectively [31]; the higher suspended sediment concentration in the downstream reach was from the added sediment in storm water from the LAC drainage (mostly from the Guaje Canyon tributary), which was 2 to 24 times higher than normal. Guaje Canyon is located north of LANL in an east-towest direction and empties into LAC approximately 1 mile west of the Rio Grande (Figure 1). Both the Santa Clara and Guaje Canyon drainages are largely unrelated to lab property. There were approximately 12 storm water events of varying degrees downstream from LAC (8 directly from the LAC drainage) to the Rio Grande prior to the benthic macroinvertebrate sampling as compared to about 4 from the upstream reach; the most significant of these occurred on August 21, 2011, that affected both reaches and on September 4, 2011, that affected only the downstream reach [31].
The numbers and types of organisms collected in 2009 using rock basket samplers were generally different from the benthic macroinvertebrate assessment conducted in 2011 using kick nets. This was expected, owing to the different collection methods employed between the years plus the significant flooding event(s) caused by the Las Conchas fire that occurred less than 2 months before the collection of benthic macroinvertebrates in 2011. In particular, taxa richness and diversity were higher (from both reaches) in 2009 than in 2011 and the ratio of scrapers/scrapers + filtering collectors was higher in 2011 than in 2009 (this means that filtering collectors like Hydropsyche were high in abundance in 2009 and low in abundance in 2011). The densities of generalist feeders (collector gatherers) like Baetis, on the other hand, was higher in 2011 than in 2009. Baetis are the first to colonize after fire induced flooding events [37].
Both Baetis and Hydropsyche are collectors of fine particulate organic matter [39]. Whereas Baetis is a collector-gatherer, scurrying over rock surfaces in search of fine particles (either debris or algae), Hydropsyche is a highly specialized collector-filterer using a fixed net to capture food. During sediment/ash flow, Hydropsyche, which was dominant in 2009, would probably be more impacted than Baetis, which was dominant in 2011, as its net would clog with debris; hence it couldn't function (survive) and thus be most vulnerable to this catastrophic event. Baetis, on the other hand, is an opportunist and may seek refuge from the sediment/ash flow by moving into interstices to avoid the inundation of the scouring and clogging sediment and ash.
4. Conclusion
A wide range of benthic macroinvertebrates, including pollution intolerant taxa, were collected in the Rio Grande upstream and downstream of LAC in 2009 using rock baskets and 2011 using kick nets. Most of the 10 metrics in 2009 were similar between reaches resulting in a bioassessment score of nonimpaired; the 2011 bioassessment ranked a level lower (slightly impaired). The slightly lower bioassessment score of the downstream reach in 2011 may be a result of the more severe flooding impacts than the upstream reach following the Las Conchas fire rather than of LANL influences because pollution intolerant taxa were still the dominant organisms (and related metrics). Overall, LANL influences, if any, via the LAC system to the Rio Grande are not significantly impacting water quality of the Rio Grande.
5. Acknowledgements
We thank Louie Naranjo and Rhonda Robinson of LANL and Raymond Martinez of the Pueblo of San Ildefonso for help in this study. Gerald Jacobi identified the benthic macroinvertebrates with assistance on the Chironomidae from Daniel McGuire (McGuire Consulting).
REFERENCES
- W. L. Hilsenhoff, “An Improved Biotic Index of Organic Stream Pollution,” Great Lakes Entomology, Vol. 20, 1987, pp. 31-39.
- M. T. Barbour, J. Gerritsen, B. D. Snyder and J. B. Striblingm, “Rapid Bioassessment Protocols for Use in Streams and Wadeable Rivers: Periphyton, Benthic Macroinvertebrates, and Fish,” 2nd Edition, US Environmental Protection Agency, Office of Water, Washington DC, 1999.
- L. S. Fore, J. R. Karr and R. W. Wisseman, “Assessing Invertebrate Responses to Human Activities: Evaluating Alternative Approaches,” Journal of the North American Benthological Society, Vol. 15, No. 2, 1996, pp. 212-231. doi:10.2307/1467949
- Environmental Protection Agency, “Biological Criteria: National Program Guidance for Surface Waters,” US Environmental Protection Agency, Report EPA-440/5-90- 004, 1990.
- Environmental Protection Agency, “Lake and Reservoir Bioassessment and Biocriteria,” US Environmental Protection Agency Technical Guidance Document, Office of Wetlands, Oceans, and Watershed, EAP 841-B-98-007, 1998.
- J. L. Wilhm, “Comparison of Some Diversity Indices Applied to Populations of Benthic Macroinvertebrates in a Stream Receiving Organic Wastes,” Journal of Water Pollution Control Federation, Vol. 39, No. 1, 1967, pp. 1673-1683.
- J. L. Wilhm and T. C. Dorris, “Biological Parameters for Water Quality Criteria,” BioScience, Vol. 18, No. 6, 1968, pp. 477-481. doi:10.2307/1294272
- R. W. Winner, B. W. Boesel and M. P. Farrell, “Insect Community Structure as an Index of Heavy-Metal Pollution in Lotic Ecosystems,” Canadian Journal of Aquatic Science, Vol. 37, No. 4, 1980, pp. 647-655. doi:10.1139/f80-081
- J. R. Deacon, N. E. Spahr, S. V. Mize and R. W. Boulger, “Using Water, Bryophytes and Macroinvertebrates to Assess Trace Element Concentrations in the Upper Colorado River Basin,” Hydrobiologia, Vol. 455, No. 1-3, 2001, pp. 29-39. doi:10.1023/A:1011931216906
- P. R. Cosser, “Macroinvertebrate Community Structure and Chemistry of an Organically Polluted Creek in South-East Queensland,” Australian Journal Marine and Freshwater Research, Vol. 39, No. 5, 1988, pp. 671-683. doi:10.1071/MF9880671
- J. G. Rae, “Chironomid Midges as Indicators of Organic Pollution in the Scioto River Basin, Ohio,” Ohio Journal Science, Vol. 89, No. 1, 1989, pp. 5-9.
- W. D. Purtymun, “Storm Runoff and Transport of Radionuclides in DP Canyon, Los Alamos County, New Mexico,” Los Alamos Scientific Laboratory Report LA-5744, 1974.
- T. E. Hakonson, G. C. White, E. S. Gladney and M. Dreicer, “The Distribution of Mercury, Cesium-137, and Plutonium in an Intermittent Stream at Los Alamos,” Journal of Environmental Quality, Vol. 9, No. 2, 1980, pp. 289-292. doi:10.2134/jeq1980.00472425000900020026x
- B. M. Gallaher and D. E. Efurd. “Plutonium and Uranium from Los Alamos National Laboratory in Sediments of the Northern Rio Grande Valley,” Los Alamos National Laboratory Report LA-13974, 2002.
- S. L. Reneau and R. J. Koch, “Watershed Monitoring,” In: Environmental Surveillance at Los Alamos during 2007, Los Alamos National Laboratory report LA-14369-ENV, 2008, pp. 201-248.
- P. R. Fresquez, C. Hathcock and D. Keller, “Foodstuffs and Biota Monitoring,” In: Environmental Surveillance at Los Alamos during 2007, Los Alamos National Laboratory Report LA-14369-ENV, 2008, pp. 267-290.
- W. V. Abeele, M. L. Wheeler and B. W. Burton, “Geohydrology of Bandelier Tuff,” Los Alamos Scientific Laboratory Report, LA-8962-MS, 1981.
- P. R. Fresquez, “The Characterization of Biotic and Abiotic Media Upgradient and Downgradient of the Los Alamos Canyon Weir: Revision 1,” Los Alamos National Laboratory Report LA-14308, 2006.
- S. R. Ellis, G. W. Levings, L. F. Carter, S. F. Richey and M. J. Radell, “Rio Grande Valley, Colorado, New Mexico, and Texas,” Water Resource Bulletin, American Water Resources Association, Vol. 29, No. 4, 1993, pp. 617-646. doi:10.1111/j.1752-1688.1993.tb03230.x
- P. R. Fresquez, D. H. Kraig, M. A. Mullen and L. Naranjo Jr., “Radionuclides and Trace Elements in Fish Collected Upstream and Downstream of Los Alamos National Laboratory and the Doses to Humans from the Consumption of Muscle and Bone,” Journal of Environmental Science and Health, Vol. B34, No. 5, 1999, pp. 885-899.
- G. J. Gonzales and P. R. Fresquez, “Polychlorinated Biphenyls (PCBs) in Catfish and Carp Collected from the Rio Grande Upstream and Downstream of Los Alamos National Laboratory: Revision 1,” Los Alamos National Laboratory Report, LA-14362, 2008.
- P. R. Fresquez, C. Hathcock, and D. Keller, “Foodstuffs and Biota Monitoring,” In: Environmental Surveillance at Los Alamos during 2008, Los Alamos National Laboratory Report LA-14407-ENV, 2009, pp. 267-296.
- J. B. Anderson and W. T. Mason Jr., “A Comparison of Benthic Macroinvertebrates Collected by Dredge and Basket Sampler,” Journal of Water Pollution Control Federation, Vol. 40, No. 1, 1968, pp. 252-259.
- J. Cairns Jr., “Artificial Substrates,” Ann Arbor Science Publications, Ann Arbor, 1982.
- G. Z. Jacobi, “A Quantitative Artificial Substrate Sampler for Benthic Macroinvertebrates,” Transactions of the American Fisheries Society, Vol. 100, No. 1, 1971, pp. 136-138. doi:10.1577/1548-8659(1971)100<136:AQASSF>2.0.CO;2
- J. R. Karr and D. R. Dudley, “Ecological Perspectives on Water Quality Goals,” Environmental Management, Vol. 5, No. 1, 1981, pp. 55-68. doi:10.1007/BF01866609
- D. J. Klemm, P. A. Lewis, F. Fulk and J. M. Lazorchak, “Macroinvertebrate Field and Laboratory Methods for Evaluating the Biological Integrity of Surface Waters,” EPA/600/4-90-030, US Environmental Protection Agency, Office of Water, Washington DC, 1990.
- R. J. Casey and S. A. Kendall, “Comparisons among Colonization of Artificial Substratum Types and Natural Substratum by Benthic Macroinvertebrates,” Hydrobiologia, Vol. 341, No. 1, 1996, pp. 57-64. doi:10.1007/BF00012303
- New Mexico Environment Department, “Water Quality Survey Summary for the Upper Rio Grande Watershed, Part I, 2000,” New Mexico Environment Department Report NMED/SWQB, Santa Fe, New Mexico, 2004.
- New Mexico Environmental Department, “Water Quality Survey Summary for the Upper Rio Grande Watershed, Part II, 2001,” New Mexico Environment Department report NMED/SWQB, Santa Fe, New Mexico, 2004.
- New Mexico Environment Department, Correspondence from S. Yanicak, New Mexico Environment Department, to A. Duran, US Department of Energy, Entitled, “Submittal 2011 and 2012 post Las Conchas Fire Sampling Results for Stormwater, Farm Soils, Post-Flood Muck and Precipitation,” New Mexico Environment Department, 2012.
- G. Z. Jacobi, L. R. Smolka and M. D. Jacobi, “Use of Biological Assessment Criteria in the Evaluation of a High Mountain Stream, the Rio Hondo, New Mexico, USA,” Verhandlung Internationale Vereinigung Limnologie, Vol. 26, 1998, pp. 1227-1234.
- D. L. Klemm, P. A. Lewis, F. Funk and J. M. Lazorchak, “Macroinvertebrate Field and Laboratory Methods for Evaluating the Biological Integrity of Surface Waters,” Environmental Monitoring and Support Laboratory, EPA- 600-4-90-030, US Environmental Protection Agency, Cincinnati, 1990.
- M. D. Molles, “Recovery of a Stream Invertebrate Community from a Flash Flood in Tesuque Creek, New Mexico,” Southwestern Naturalist, Vol. 30, No. 2, 1985, pp. 279-287. doi:10.2307/3670741
- J. N. Rinne, “Short-Term Effects of Wildfire on Fishes and Aquatic Macroinvertebrates in the Southwest United States,” North American Journal of Fisheries Management, Vol. 16, No. 3, 1996, pp. 653-658. doi:10.1577/1548-8675(1996)016<0653:MBSTEO>2.3.CO;2
- G. W. Minshall, C. T. Robinson and D. E. Lawrence, “Postfire Responses of Lotic Ecosystems in Yellowstone National Park, USA,” Canadian Journal of Fisheries and Aquatic Sciences, Vol. 54, 1997, pp. 2509-2525. doi:10.1139/cjfas-54-11-2509
- N. K. M. Vieira, W. H. Clements, L. S. Guevara and B. F. Jacobs, “Resistance and Resilience of Stream Insect Communities to Repeated Hydrologic Disturbances after a Wildfire,” Freshwater Biology, Vol. 49, No. 10, 2004, pp. 1243-1259. doi:10.1111/j.1365-2427.2004.01261.x
- N. K. M. Vieira, T. R. Barnes, and K. A. Mitchell. “Effects of Wildfire and Postfire Floods on Stonefly Detritivores of the Pajarito Plateau, New Mexico,” Western North American Naturalist, Vol. 71, No. 2, 2011, pp. 257-270. doi:10.3398/064.071.0213
- R. W. Merritt and K. W. Cummins, “An Introduction to the Aquatic Insects of North America,” Kendall/Hunt Publishing Company, Dubuque, 1978.

