Agricultural Sciences
Vol. 4 No. 12 (2013) , Article ID: 41168 , 9 pages DOI:10.4236/as.2013.412099
Proximate composition and seed lipid components of “kabuli”-type chickpea (Cicer arietinum L.) from Argentina
![]()
1Laboratorio Calidad Nutricional de Granos, Estación Experimental Agropecuaria (EEA), Instituto Nacional de Tecnología Agropecuaria (INTA) Manfredi, Córdoba, Argentina; *Corresponding Author: mjmartinez@manfredi.inta.gov.ar
2Facultad de Ciencias Agropecuarias (F.C.A.), Universidad Nacional de Córdoba (U.N.C.), Córdoba, Argentina
3Centro de Excelencia en Productos y Procesos (CEPROCOR-MINCyT), Córdoba, Argentina
Copyright © 2013 Carla G. Marioli Nobile et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 1 October 2013; revised 8 November 2013; accepted 27 November 2013
Keywords: Chickpea; Pulse Crop; Fatty Acids; Tocopherols; Mineral Elements
ABSTRACT
Chickpea is an important pulse crop with a wide range of potential nutritional benefits because of its chemical composition. The purpose of the current work was to provide the chemical composition of “kabuli”-type chickpea (Cicer arietinum L.) developed in Argentina for nutritional purpose. Protein, oil and ash contents, fatty acid, tocopherol and mineral element compositions were studied. Among the studied genotypes, protein content ranged from 18.46 to 24.46 g/100g, oil content ranged from 5.68 to 9.01 g/100g and ash from 3.55 to 4.46 g/100g. Linoleic, oleic and palmitic acids were the most abundant fatty acids. The average oleic-to-linoleic ratio was 0.62 and average iodine value was 117.82. Tocopherols, well-established natural antioxidants, were found in chickpea seeds in relatively similar amounts across all genotypes. Mineral element analysis showed that chickpea was rich in macronutrients such as K, P, Mg and Ca. The nutritional composition of chickpea genotypes developed and grown in Argentina provides useful information for breeding programs, food marketing and consumers and establishes chickpea as component of a balanced human diet.
1. INTRODUCTION
Chickpea (Cicer arietinum L.), originally domesticated in Middle Eastern, African and Asian countries, is the third largest pulse crop in the world [1]. As a source of vegetable protein, carbohydrates, dietary fiber, vitamins and minerals, the demand for chickpea has increased over the last few years due to its notable nutritional value [2]. Additional health benefits include low allergenic properties and high in vitro protein digestibility [3-9].
The oil fraction in chickpea is the highest among dry pulses and represents the 3% to 10% of total dry seed weight [10-12]. Chickpea oil is mainly composed of unsaturated fatty acids [13]. Omega-6 linoleic fatty acid is the major fatty acid present in chickpea oil (46% - 62% of total acids) followed by omega-9 oleic acid [12]. Omega-6 fatty acid is one of the essential unsaturated fatty acids for human metabolism that must be incorporated through diet [14]. Omega-9 oleic fatty acid is desired in grains since it confers low oxidation properties during storage [15]. Incorporating chickpeas into a healthy diet helps to increase the polyunsaturated fatty acids (PUFA) intake, as well as the polyunsaturated to saturated fatty acid ratio. Both parameters are associated with reduced total serum cholesterol levels [16].
Chickpea oil is rich in tocopherols and it contains the highest amount of alpha tocopherol among pulses (up to 13.7 mg/100g) [12,17]. The major tocopherol in chickpea is gamma tocopherol, a natural seed antioxidant [17].
Mineral macronutrients, such as potassium (K), calcium (Ca), phosphorous (P) and magnesium (Mg), and micronutrients, such as ferrum (Fe), zinc (Zn), copper (Cu), manganese (Mn), are required in the human diet. A single 100 g serving of cooked chickpeas can provide 24%, 43% and 39% of the recommended dietary allowance (RDA) for the macronutrient P and for the micronutrients Mn and Cu, respectively [12]. Therefore, chickpea has become an important source of vitamins and minerals to the cereal-based daily diet of millions of people in under-developed countries [2].
In recent years, the number of chickpea hectares (ha) grown in Argentina has rapidly increased from roughly 5000 ha in 2008 to 42,000 ha in 2010 (+840%) [18]. Similarly, total chickpea production in Argentina increased from 8700 tn in 2008 to 78,000 tn in 2010 (+896%) [18]. In 2011, Argentina exported chickpea to 44 countries raising its exports by 42% since 2007. This expansion has fuelled the need to develop new genotypes that better meet the needs of the local and global market supply for human consumption.
An Argentinean chickpea breeding program was started in 1972 at the Facultad de Ciencias Agropecuarias, Universidad Nacional de Córdoba (Córdoba, Argentina). In 1990, the National Institute of Agricultural Technology (INTA) (Agricultural Experimental Station in Cerrillos, Argentina) joined to expand the program. The local chickpea landrace, Sauco, is believed to have been introduced from Spain [19] and the Argentinean commercial chickpea genotypes are Chañaritos S-156 and Norteño [19]. Currently, chickpea breeding programs are aimed at obtaining higher yields and better crop adaptability [20,21]. The chemical composition of chickpea genotypes provides useful information for breeding programs and scientists intended to work in this area. The objective of the current study was to determine the chemical and nutriational composition of the 14 chickpea genotypes from Argentina developed for human nutrition.
2. MATERIALS AND METHODS
2.1. Chickpea Samples
Seeds of 14 “kabuli”-type chickpea (Cicer arietinum L.) genotypes were provided by the chickpea breeding program at the National University of Córdoba (UNC) Argentina and the National Institute of Agricultural Technology (INTA) of Salta, Argentina. The studied material was G112, G58, G101, G47, P44, P39, P41, P98, P102, P56, L9, L2, Chañaritos S-156 and Norteño, being G = genotype; P = population; L = Line respectively. The agronomic features are listed in Table 1. Experimental field trial was laid out as a randomized complete-block design, with three replications in Chalacea (30˚45'52.24"S, 63˚40'19.86"O), Province of Córdoba, Argentina, during the winter season of 2010. Germplasm registration information was generated by the breeding program. Crops were grown under rain fed conditions and following cultural practices recommended by the chickpea breeding program. These practices include disease, insect, and weed control (with specific products for chickpea crop) to prevent any biotic factor that could alter the chemical quality of the kernel. Plant density for the trials was 25 pl/m2 at 52 cm row spacing. Seed was harvested and stored at room temperature until analysis.
2.2. Proximate Composition
Samples, fifty g each, were finely ground (200 μm sieve) and stored in a fridge at −20˚C until analyze.
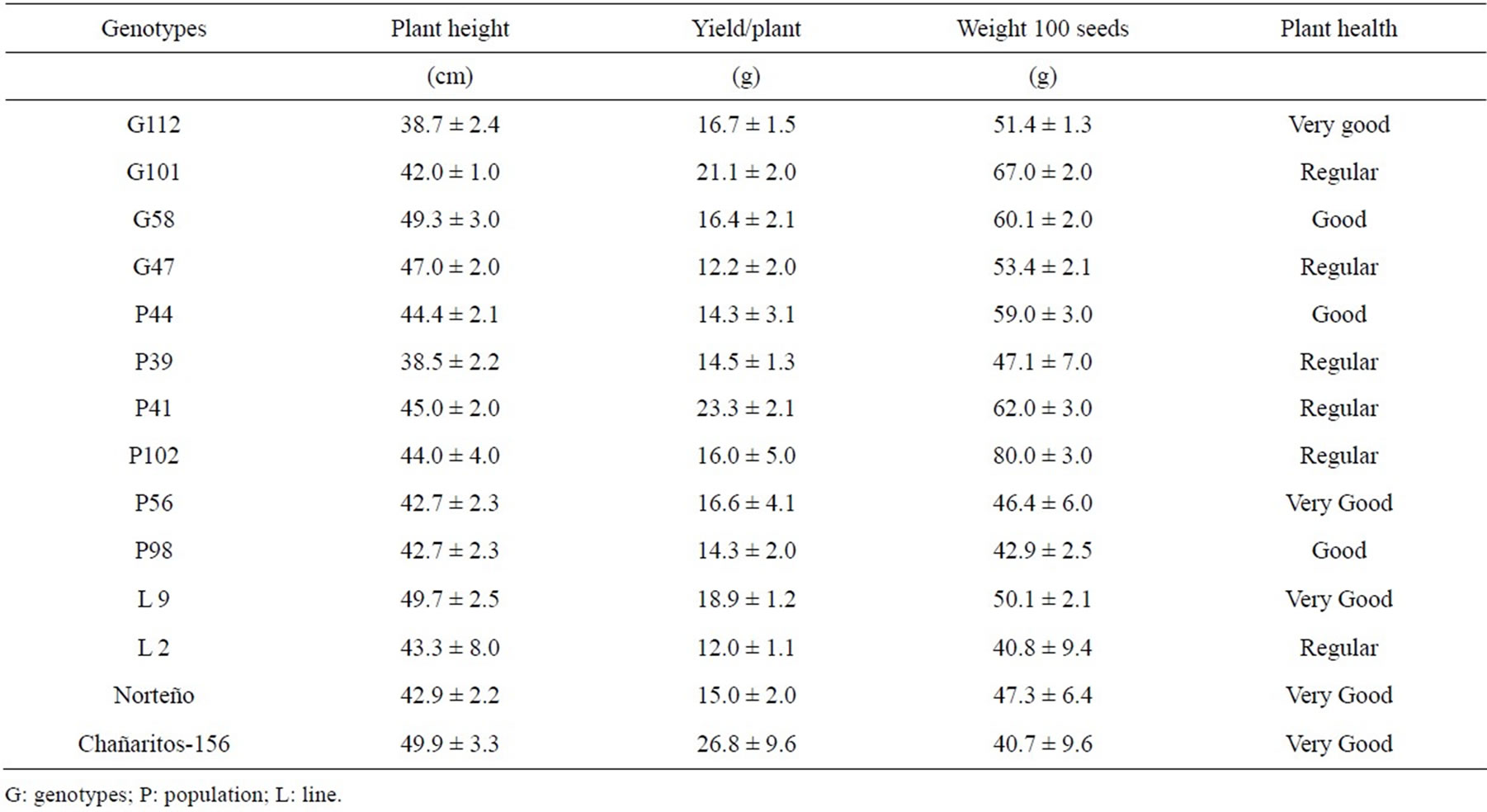
Table 1. Agronomic features of 14 “kabuli”-type chickpea genotypes (Cicer Arietinum L.) genotypes from Argentina.
Moisture (method Ab 2 - 49), protein N × 6.25 (method Ab 4 - 91), fat (method Am 2 - 93) and ash (method Bc 5 - 49) were determined following the procedures detailed by AOCS [22]. Total nitrogen was measured using the Kjeldahl method and protein content was calculated as N × 6.25. A digestion unit TecatorTM Auto 1001 3844/Rev 1 (Foss Tecator; Höganäs, Sweden), scrubber unit TecatorTM 1001 4329/Rev 1 (Foss Tecator; Höganäs, Sweden), and distillation unit K-350 (Büchi; Switzerland) were used. The fat fraction was extracted from finely ground chickpeas samples (10 g) with n-hexane in a Soxhlet apparatus for 12 h. The recovered oil was filtered to remove any possible meal contamination, before its quantification, and then, saved for fatty acid and tocopherol analysis. Ash content was determined after incineration of the sample in a muffle furnace at 550˚C for 6 h. Moisture content was measured based on weight loss after oven-drying for 2 h. Results were expressed as a percentage of total dry matter (g/100g). Total carbohydrates were calculated by difference following the equation:
% Carbohydrates = % Protein + % Oil + % Ashes (1)
2.3. Fatty Acids Composition
Methyl esters of fatty acids were prepared from the extracted oil following the specifications of the official method ISO 5509.2000 [23]. Fatty acid composition was then quantified by capillary gas chromatography, method Ce 1e-91 [22]. A gas chromatographer (Hewlett-Packard 6890, Wilmington, DE, USA) was used, equipped with a flame ionization detector and HP-INNOWAX capillary column (Crosslinked Polyethylene Glycol). Nitrogen was used as the carrier gas. The flow rate was 1.5 mL/min (12 min), and then, it was kept at 3.8 mL/min to the end of the run program. The temperature of inlet and detector was 260˚C. Oven temperature started at 200˚C and ramped at 2.5˚C/min to a final temperature of 230˚C. Results were recorded in a ChemStation Data System. Standard fatty acid mixture (FAME Mix Rapeseed, AOCS) was purchased from Sigma-Aldrich (St. Louis, MO) and used as a calibration standard for peak identification and quantification. Fatty acids were expressed as g/100g of total fatty acids. The oleic-to-linolenic acid ratio (O/L) and iodine values were calculated from fatty acid results, providing a general indication of oil quality. Iodine values were calculated using the following formula: IV = (% oleic × 0.8601) + (% linoleic × 1.7321) + (% eicosenoic × 0.7854) [24].
2.4. Tocopherol Composition
Tocopherol determination was performed according to AOCS recommended practice Ce 8 - 89 [22]. The oil was diluted in a proper volume of n-hexane and analyzed by High Performance Liquid Chromatography (HPLC) Agilent Technology 1100 Serie (Wilmington, DE), equipped with a diode array detector (DAD). UV absorbance was measured at 292 nm. A Zorbax RX-Sil column was used and maintained at 25.5˚C during analysis. The separation of the tocopherols was performed in isocratic mode with n-hexane:isopropanol (99.5:0.5, v/v) as mobile phase. The injection volume was 20 μL and flow rate was 1 mL/min. Calibration curves were obtained using commercial alpha tocopherol (AT) and delta tocopherol (DT) standards purchased from Sigma-Aldrich (St. Louis, MO, USA). Response factors of beta tocopherol (BT) and gamma tocopherol (GT) were calculated from AT and DT, respectively, and corrected for their molar extinction coefficient and molecular mass. Total tocopherol (TT) was calculated by adding up the content of individual isomers. Tocopherol results were expressed as g/100g oil.
2.5. Mineral Composition
Mineral elements were analyzed as described by Inga et al. [25]. Approximately 2 g of chickpea seeds were finely ground in a porcelain mortar (to avoid metal contamination) and placed in a digestion crucible in a muffle furnace at 550˚C. After incineration, the resulted ash was diluted with 50% HNO3 and heated to 80˚C to complete the digestion. After digestion, 1mL of 50% HNO3 was added to the crucible to remove the ash from the walls. The sample solution was transferred to a 50 mL plastic tube and diluted with deionized Millipore water to a known volume (50 mL). Mineral elements were analyzed from the solution by inductively coupled plasma-mass spectrometry (ICP-MS) with argon ionization. Mg, P, K, Ca, Fe, Mn, Cu and Zn were determined with a H2/He collision cell to control isobaric interferences. All chemicals used were of analytical grade or higher purity. Mineral measurements were validated using NIST standard reference material N˚ SRM 2387 PEANUT BUTTER [26]. Results were expressed as mg/100g seed dry weight.
2.6. Statistical Analysis
All analyses of each chickpea genotype were done in triplicate and means and standard deviations were calculated. An analysis of variance was performed to identify differences among genotypes. Means were separated using a Fisher LSD test with a statistical significance of 5% [27]. Statistic analyses were performed with InfoStat [28].
3. RESULTS AND DISCUSSION
Protein, oil, ash and carbohydrate content of the different chickpea genotypes included in this study are shown in Table 2. Significant differences were observed among the genotypes. As shown in Table 2, carbohydrates represent the main fraction of chickpea seed com-
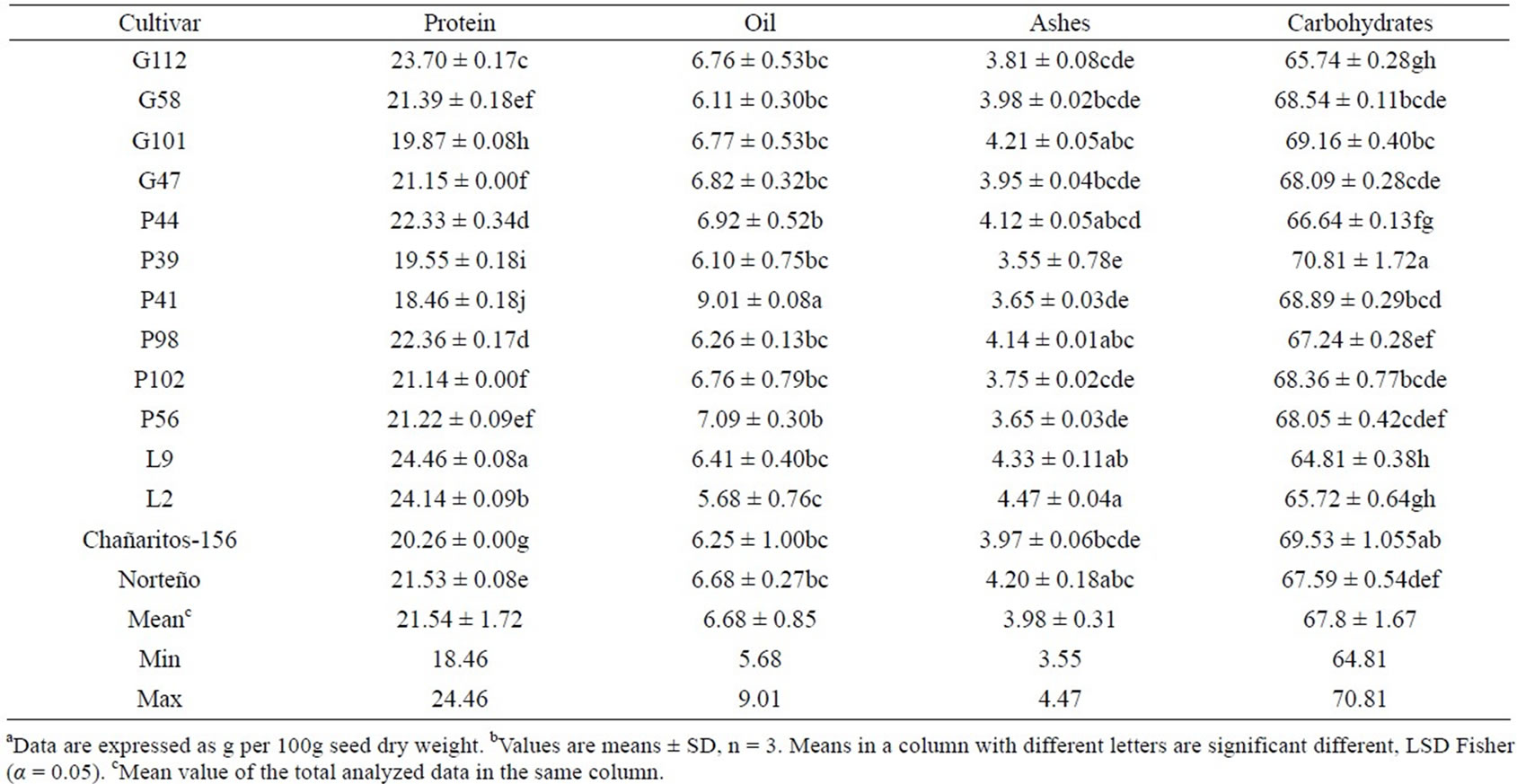
Table 2. Proximate composition of 14 “kabuli” type ckickpea (Cicer Arietinum sp) genotypes from Argentina (g/100 g)ab.
position (64.81 - 70.81 g/100g). In the present study, the mean total carbohydrate value of 14 Argentinean chickpea genotypes was 67.8% ± 1.67%, slightly higher than 66% total carbohydrates obtained by Shad et al. [21]. Legumes are rich in complex carbohydrates and oligosaccharides, important components to human diet for keeping a healthy intestine flora [9,12]. P39 showed the highest carbohydrate percentage.
Protein mean value was 21.54 g/100g. Genotypes L9 and L2 exhibited the highest protein content (mean = 24.46 g/100g and 24.13 g/100g, respectively). ChañaritosS156 and Norteño, the commercial Argentinean cultivars analyzed in this study, had lower values of protein (20.26 g/100g and 21.53 g/100g, respectively) than L9 and L2 genotypes. Similarly, El-Adawy [29] reported differences among genotypes on protein content (23.64 g/100g for Egyptian genotypes and 18.5 g/100g for Brazilian genotypes).
Oil content mean value of the evaluated genotypes was 6.68 ± 0.85 g/100g (Table 2). Similar results were reported by De Almeida Costa et al. [11] for Brazilian genotypes, Alajaji and El-Adawy [30] for local Egyptian chickpea seeds, and Boschin and Arnoldi [17] for commercially available seed in the Italian market. The P41 genotype exhibited a significantly higher oil content (mean = 9.01 g/100g) than the other genotypes.
The ash content ranged from 3.55 g/100g to 4.47g/ 100g in the 14 Argentinean chickpea genotypes, showing significant differences between them. L2 contained the highest ash content (mean = 4.47 g/100g) and P39 had the lowest ash content (mean = 3.55 g/100g). Shad et al. [9] also reported significant differences in ash content for Pakistani “desi”-type chickpea genotypes.
A summary of fatty acid composition is presented in Table 3. Significant variability in fatty acid composition was observed among the 14 Argentinean chickpea genotypes. Linoleic (18:2 omega-6) and linolenic (18:3 omega-3) are essential fatty acids that cannot be synthesized by the human body and must be supplied through diet [14]. Linoleic (18:2 omega-6) was the major unsaturated fatty acids in all chickpea genotypes (Table 3). G101 showed a significantly higher amount of linoleic acid (mean = 58.73 g/100g) than the other genotypes, while P39 contained the highest linolenic acid (mean = 3.06 g/100g). Finally, L9 had the highest oleic acid (18:1 omega-9) content (mean = 43.01 g/100g). Results from these analyses indicate that the Argentinean chickpeas are rich in omega-3 and -6 essential fatty acids, as well as omega-9.
Iodine value (IV) and the oleic-to-linoleic ratio (O/L) are both indicators of oil stability and shelf life in oil from legume seeds such as peanut oil [31]. The iodine value of Argentinean chickpea genotypes ranged from 110.43 p 121.02, which is higher than values reported for peanut oil that ranged from 99.2 - 110.4 [32]. Similar results to the current study were obtained by Zia-Ul-Haq et al. [33] and Shad et al. [9]. Genotype L9 showed the greatest oleic-to-linoleic ratio (1.02 ± 0.01) hence, it may have better oil stability and shelf life.
Gül et al. [13] showed that variability of chickpea fatty acid composition could be attributed to genotypic variation and climate conditions. Furthermore, planting date can also affect unsaturated fatty acid content [18].
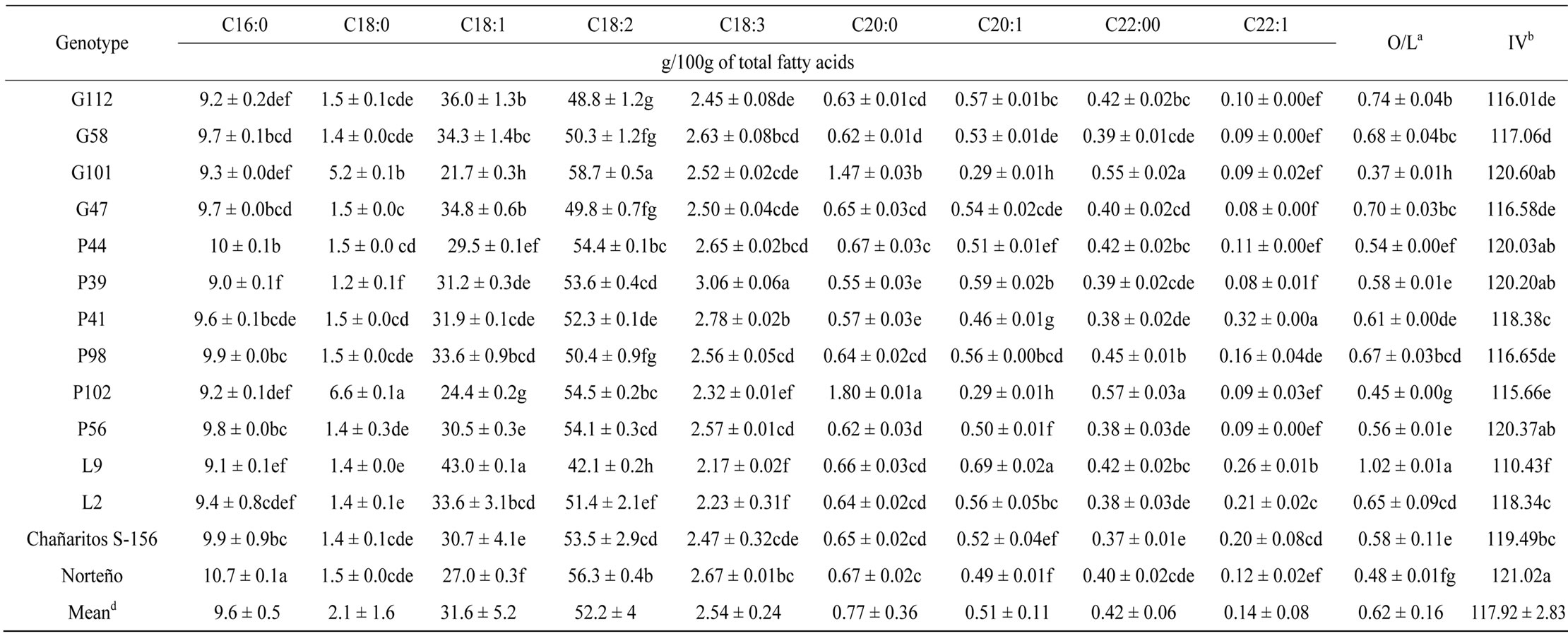
Table 3. Fatty acid composition, oleic-to-linoleic (O/L) ratio and Iodine Value (IV) of 14 “kabuli” type chickpea(Cicer arietinum L.) genotypes from Argentina.c
aO/L: Oleic-to-linoleic ratio. bIV: Iodine Value. cValues are means ± SD, n = 3. Means in a column with different letters are significant different, LSD Fisher (α = 0.05). dMean value of the total analyzed data in the same column.
Additional studies are needed to characterize fatty acid genotypic variation in Argentinean chickpeas among different environments.
Tocopherols are bioactive compounds known for their antioxidant activity [34]. Zia-Ul-Haq et al. [33] have found four different forms of tocopherols (alpha, beta, gamma and delta) in chickpea oil, with relatively constant values among genotypes. In our study (Table 4) there were not significant differences among genotypes for alpha, beta, gamma, delta and total tocopherols and, it was consistent with the previous study [33]. Similar to other legumes, gamma tocopherol was the most abundant tocopherol isomer in Argentinean chickpeas [13,17].
Mineral elements need to be incorporated through diet [35]. Mg, P, K and Ca were the main mineral elements in chickpeas (Table 5), consistent with information from the USDA database [36] and results previously reported by Petterson et al. [37] for Australian chickpea seeds. In our study, Mg and Ca mean content were 156.91 mg/ 100g and 160.58 mg/100g, respectively, which were similar to values observed for Canadian “kabuli”-type chickpea seeds [38]. Mn, Fe, Cu, and Zn were also analyzed (Table 5). Mn content was higher in G58 and G47 than in most of the other genotypes evaluated. In agreement with previous studies (e.g. [38]), no significant differences were found for Fe content and Cu content was relatively constant across the genotypes as well. Conversely, significant variability was observed in Zn values among genotypes.
On the one hand, L2 showed consistently higher values for all mineral elements (Table 5) analyzed and also had the highest ash content (Table 2) compared to the rest of the genotypes. On the other hand, P39 exhibited a consistently lower mineral content (Table 5) and had the lowest ash content (Table 2) among all genotypes. Bueckert et al. [38] concluded in their study that combined genotype and environmental effects determined the differences on chickpea seed mineral content. According to them, further studies would be needed to determine that the observed differences are consistent through environments.
4. CONCLUSION
To our knowledge, this is the first publication of the chemical composition of Argentinean chickpeas. The chemical composition of the 14 chickpea genotypes developed in Argentina suggests that Argentinean chickpeas are an important vegetable source of carbohydrates, proteins and mineral nutrients and, their oily fraction is rich in unsaturated essential fatty acids and vitamin E. Most notably, genotypes L9 and L2 had the highest protein content, and genotype P41 contained the highest oil content among genotypes tested meanwhile P39 had the highest amount of carbohydrates. Genotype G101 had the highest linoleic acid content, while Genotype P39 contained the highest amount of linolenic acid, but the lowest mineral nutrient content in general. The greatest oleic-to-linoleic ratio was found in genotype L9, suggesting better oil stability and shelf life. Tocopherols, well-established natural antioxidants, are found in chickpea seeds in relatively similar amounts across all genotypes. Mg, P, K, Ca, Zn and Mn are the main macro and micronutrient essential minerals found in Argentinean chickpea seeds. The values found for Mn and Fe in chickpeas were higher than the typical values for these elements found in peanuts. This study provides useful information for breeding programs to further optimize the nutritional value of chickpea as they are intended for human consumption.
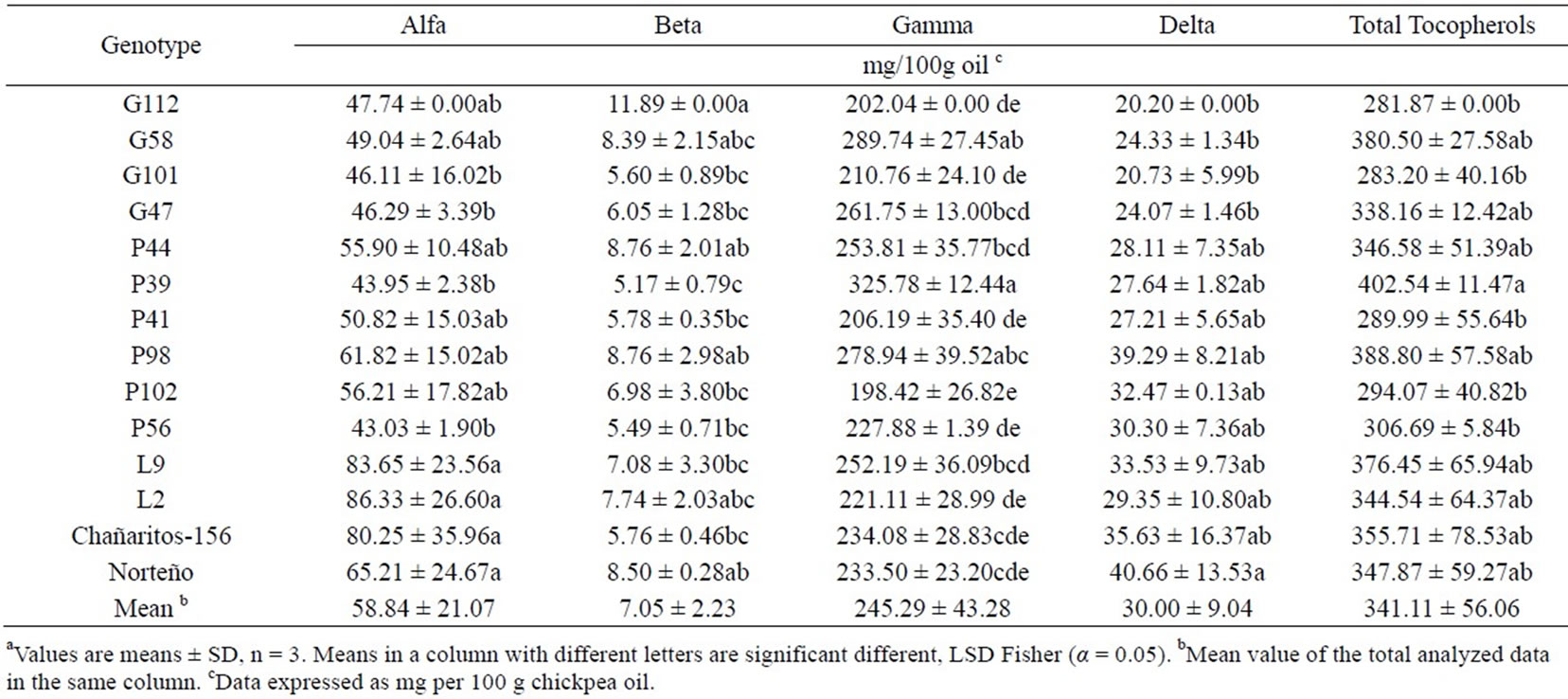
Table 4. Tocopherol composition of 14 “kabuli” type chickpea (Cicer arietinum L.)genotypes from Argentina.a
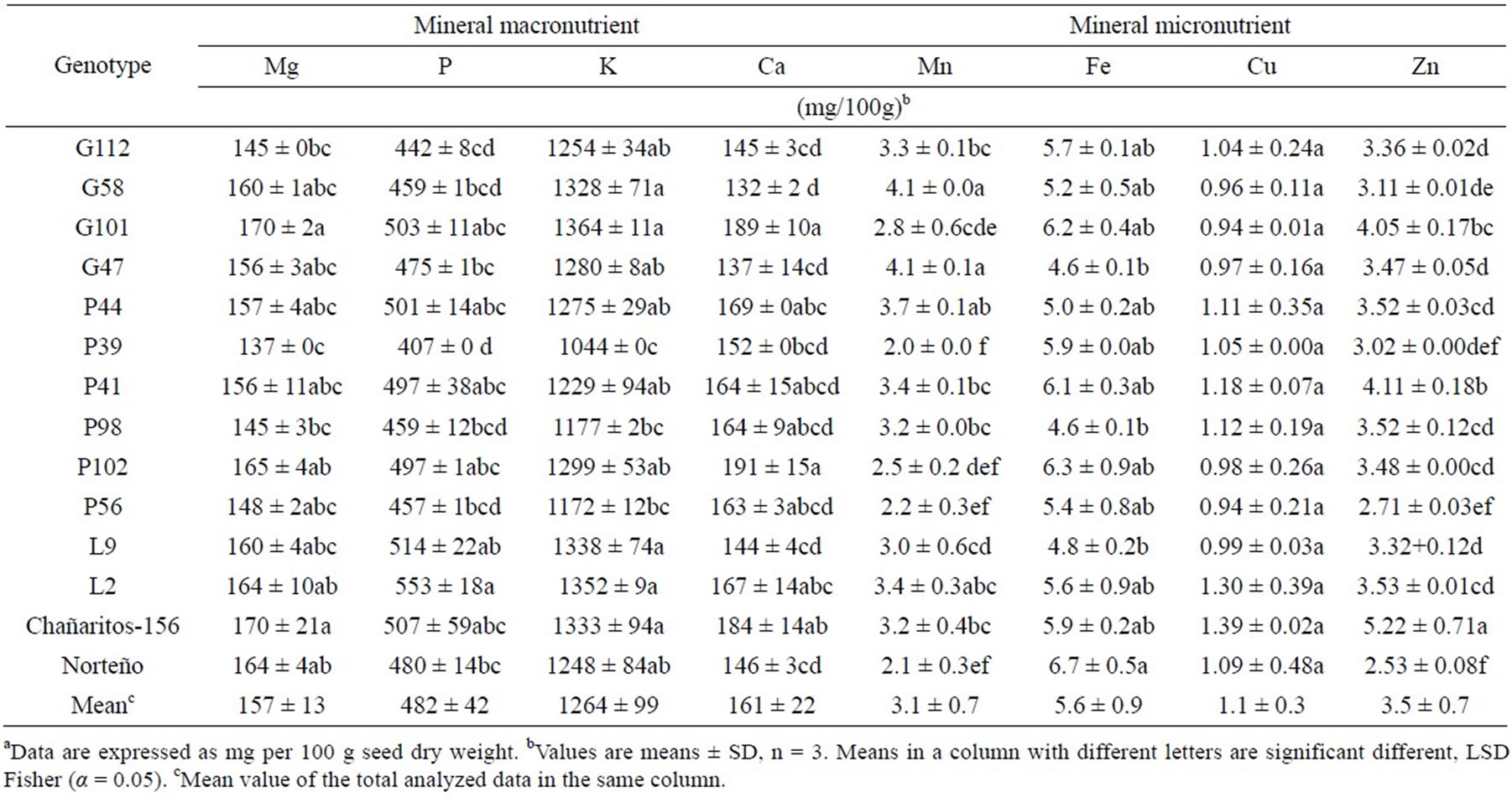
Table 5. Mineral composition of 14 chickpea (Cicerarietinum L.) genotypes from Argentina.a
5. ACKNOWLEDGEMENTS
This work was financially supported by INTA (Instituto Nacional de Tecnología Agropecuaria), Facultad Ciencias Agropecuarias Universidad Nacional de Córdoba, Argentina and CONICET (Consejo Nacional de Investigaciones Cientificas y Tecnicas). We also wish to thank to Agustin Martellotto for his help in the manuscript edition.
REFERENCES
- Food and Agriculture Organization of the United NationsStatistics Division (2011) http://faostat.fao.org/
- Jukanti, A.K., Gaur, P.M., Gowda, L.L.C. and Chibbar, R.N. (2012) Nutritional quality and health benefits of chickpea (Cicer arietinum L.): A review. British Journal of Nutrition, 108, S11-S26. http://dx.doi.org/10.1017/S0007114512000797
- Ulloa, J.A., Valencia, M.E. and García, Z.H. (1988) Protein concentrate from chickpea: Nutritive value of a protein concentrate form chickpea (Cicer arietinum L.) obtained by ultra filtration and its potential use in an infant formula. Journal of Food Science 53, 1396-1398. http://dx.doi.org/10.1111/j.1365-2621.1988.tb09285.x
- Morrow, B. (1991) The rebirth of legumes: Legume production, consumption and export are increasing as more people become aware of legumes nutritional benefits. Food Technology, 9, 715-723.
- Cordle, C.T. (1994) Control of food allergies using protein hydrolysates. Journal of Food Technology, 48, 72-76.
- P. Clemente, A. Vioque, J. Sanchez-Vioque, R. Predroche, J. Bautista, J. and Millan, F. (1999) Protein quality of chickpea (Cicer arietinum L.) protein hydrolysates. Food Chemistry, 67, 269-274. http://dx.doi.org/10.1016/S0308-8146(99)00130-2
- Tharanathan, R.N. and Mahademavamma, S. (2003) Grain legumes—A boon to human nutrition. Trends Food & Science Technology, 14, 507-518. http://dx.doi.org/10.1016/j.tifs.2003.07.002
- Amjad, I., Iqtidar, A.K., Nadia, A. and Khan, M.S. (2006) Nutritional quality of important food legumes. Food Chemistry, 97, 331-335. http://dx.doi.org/10.1016/j.foodchem.2005.05.011
- Shad, M.A., Pervez, H., Zafar, Z.I., Zia-Ul-Haq, M. and Nawaz, H. (2009) Evaluation of biochemical composition and physicoquemical parameters of oil from seeds of desi chickpea varieties cultivated in arid zone of Pakistan. Pakistan Journal of Botany, 41, 655-662.
- http://www.pakbs.org/pjbot/tcontents/tcontent41(2)11-20.html
- Williams, P.C. and Singh, U. (1987) Nutritional quality and the evaluation of quality in breeding programs. In: Saxena, M.C. and Singh, K.B., Eds., The Chickpea, CAB International, Wallingford, 329-356.
- De Almeida Costa, G.E., Da Silva Queiroz-Monici, K., Pissini Machado Reis, S. and Costa De Oliveira, A. (2006) Chemical composition, dietary fibre and resistant starch contents of raw and cooked pea, common bean, chickpea and lentil legumes. Food Chemistry, 94, 327-330. http://dx.doi.org/10.1016/j.foodchem.2004.11.020
- Wood, J.A. and Grusak, M.A. (2007) Nutritional value of chickpea. In: Yadav, S.S., Redden, B., Chen, W. and Sharma, B., Eds., Chickpea Breeding and Management, CAB International, Wallingford, 101-142. http://dx.doi.org/10.1079/9781845932138.005
- Gül, M.K., Egesel, C.M. and Turham, H. (2008) The effects of planting time on fatty acids and tocopherols in chickpea. European Food Research and Technology, 226, 517-522. http://dx.doi.org/10.1007/s00217-007-0564-5
- Simopoulos, A. (1999) Essential fatty acids in health and chronic disease. American Journal of Clinical Nutrition, 70, 560s-569s.
- Turkulov, J., Dirnic, E., Karlovic, D., Percic, I. and Zubic, Z. (1996) Quality of edible nonrefined sunflower oil with different fatty acid composition. Proceedings of the 14th International Sunflower Conference, Beijing/Shenyang, 12-20 June 2006.
- Pittaway, J.K., Robertson, I.K. and Ball, M.J. (2008) Chickpeas may influence fatty acid and fiber intake in an ad libitum diet, leading to small improvements in serum lipid profile and glycemic control. Journal of the American Dietetic Association, 108, 1009-1013. http://dx.doi.org/10.1007/s00217-007-0564-5
- Boschin, G. and Arnoldi, A. (2011) Legumes are valuable sources of tocopherols. Food Chemistry, 127, 1199-1203. http://dx.doi.org/10.1016/j.foodchem.2011.01.124
- Bolsa de Cereales (2011) Argentinean Chickpea Production 2010/2011. http://www.bccba.com.ar/bcc/images/semillas/Garbanzo%202010_2011.pdf
- Biderbost, E. and Carreras, J. (2005) Registration of Cha- ñaritos S-156. Chickpea. Crops Science, 45, 1653. http://dx.doi.org/10.2135/cropsci2003.033
- Carreras, J., Allende, M., García, S., Panadero, C., Millán, T. and Gil, J. (2006) Selections of chickpea (Cicer arietinum L.) genotypes for direct threshing. Proceedings of the 29th Argentine Congress of Horticulture, Catamarca, 88.
- Carreras, J., Allende, M., Avalos, S., Ateca, N., Mazzuderi, V., García, S. and Rubbio, J. (2010) The importance of chickpea breeding programs for the local economies of semiarid Chaco of Argentina. Proceedings of the 1st Plant Breeding Congress of Peru, Lima, 106-108.
- AOCS (1998) Official methods and recommended practices of the American Oil Chemists’ Society. American Oil Chemists’ Society, Champaign.
- ISO 5509 (2000) Animal and vegetable fats and oils: Preparation of methyl esters of fatty acids. http://www.iso.org/iso/home/store/catalogue_ics/catalogue_detail_ics.htm?csnumber=11560
- Hashim, I.B., Koehler, P.E., Eitenmiller, R.R. and Kvien, C. (1993) Fatty acid composition and tocopherol content of drought stressed Florunner peanuts. Peanut Science, 20, 21-24. http://dx.doi.org/10.3146/i0095-3679-20-1-6
- Inga, C.M., Poliotti, M.V., Spahn, J.G., Badini, R.G., Martinez, M.J., Silva, M.P. and Aguilar, R.C. (2010) Non essential element maximum levels in runner and high oleic raw peanuts of Córdoba-Argentina by ICPMS. Proceedings of the 11th Rio Symposium on Atomic Spectrometry, Mar del Plata, 24-29 October 2010.
- Poliotti, M.V., Inga, C.M., Spahn, J.G., Badini, R.G., Martinez, M.J., Aguilar, R.C. and Silva, M.P. (2010) Nutritional and mineral content in high oleic raw runner peanuts of Cordoba, Argentina, by atomic spectroscopy techniques. Proceedings of the 11th International Rio Symposium on Atomic Spectrometry, Mar del Plata, 24-29 October 2010.
- Montgomery, D.C. (1991) Experiments with a single factor: The analysis of variance. In: Design and Analysis of Experiments, 3rd Edition, Willey, New York, 78.
- Di Rienzo J.A., Casanoves, F., Balzarini, M.G., Gonzalez, L., Tablada, M. and Robledo, C.W. (2011) InfoStat. Infostat group from the National University of Córdoba, Argentina. http://www.infostat.com.ar
- El-Adaway, T. (2002) Nutritional composition and nutriational factors of chickpeas (Cicer arietinum L.) undergoing different cooking methods and germination. Plant Foods for Human Nutrition, 57, 83-97. http://dx.doi.org/10.1023/A:1013189620528
- Alajaji, S.A. and El-Adawy, T.A. (2006) Nutritional composition of chickpea (Cicer arietinum L.) as affected by microwave cooking and other traditional cooking methods. Journal of Food Composition and Analysis, 19, 806- 812. http://dx.doi.org/10.1016/j.jfca.2006.04.007
- Ahmed, E.H. and Young, C.T. (1982) Composition, nutrition and flavor of peanut. In: Pattee, H.E. and Young, C.T., Eds., Peanut Science and Technology, American Peanut Research and Education Society, Inc., Yoakum, 655-687.
- Grosso, N.R., Nepote, V. and Guzmán, C.A. (2000) Chemical composition of some wild peanut species (Arachis L.) seeds. Journal of Agricultural and Food Chemistry, 48, 806-809. http://dx.doi.org/10.1021/jf9901744
- Zia-Ul-Haq, M., Ahmad, S., Ahmad, M., Iqbal, S. and Khawar, K.M. (2009) Effects of cultivar and row spacing on tocopherol and sterol composition of chickpea (Cicer arietinum L.) seed oil. Journal of Agricultural Science (Tarim Bilimleri Dergisi), 15, 25-30.
- Bramley, P.M., Elmadfa, I., Kafatos, A., Kelly, F.J., Manios, Y. and Roxborough, H.E., et al. (2000) Vitamin E (review). Journal of the Science of. Food and Agriculture, 80, 913-938. http://dx.doi.org/10.1002/(SICI)1097-0010(20000515)80:7<913::AID-JSFA600>3.0.CO;2-3
- Graham, R.D., Welch, R.M., Saunders, D.A., Ortiz-Monasterio, I., Bouis, H.E., Bonierbale, M., Haan, S. De, Burgos, G., Thiele, G., Liria, R., Meisner, C.A., Beebe, S.E., Potts, M.J., Kadian, M., Hobbs, P.R., Gupts, R.K. and Twomlow, S. (2007) Nutritious subsistence food systems. In: Advances in Agronomy, Elsevier Inc., 92. http://www.askforce.org/web/Biofortification/Graham-Subsistence-Food-Systems-2008.pdf
- United States Department of Agriculture-Agricultural Research Service (2012) Nutrient database for standard reference. http://ndb.nal.usda.gov/ndb/foods/show/4671?fg=Legumes+and+Legume+Products&man=&lfacet=&format=&count=&max=25&offset=50&sort=&qlookup=
- Petterson, D.S., Sipsas, S. and Mackintosh, J.B. (1997) The chemical composition and nutritive value of Australian pulses. 2nd Edition, Grains Research and Development Corporation, Kingston.
- Bueckert, R.A., Thavarajah, D., Thavarajah, P. and Pritchard, J. (2011) Phytic acid and mineral micronutrients in field-grown chickpea (Cicer arietinum L.) cultivars from western Canada. European Food Research and Technology, 233, 203-212. http://dx.doi.org/10.1007/s00217-011-1495-8

