Food and Nutrition Sciences
Vol. 3 No. 2 (2012) , Article ID: 17530 , 5 pages DOI:10.4236/fns.2012.32037
Effects of Walnut on Lipid Profile as Well as the Expression of Sterol-Regulatory Element Binding Protein-1c(SREBP-1c) and Peroxisome Proliferator Activated Receptors α (PPARα) in Diabetic Rat
![]()
1Department of Laboratory Science, Islamic Azad University, Borūjerd, Iran; 2Afzalipour School of Medicine, Kerman University of Medical Sciences, Kerman, Iran.
Email: *7abbasi@gmail.com
Received November 2nd, 2011; revised December 21st, 2011; accepted December 30th, 2011
Keywords: Walnut; Cholesterol; SREBP-1c; PPARα
ABSTRACT
Diabetes Mellitus has appeared as a universal burden. Studies have reported that mortality from Coronary Heart Disease (CHD) in diabetic patients is 2 - 4 times higher than nondiabetics. In this respect, walnut is a treatment which has beneficial effects on CHD risk factors. PPARα and SREBP-1c play an important role in the regulation of lipid metabolism. This study was aimed to evaluate the effects of walnut on lipid profile as well as SREBP-1c and PPARα protein levels in rats. Animals were randomly divided into 3 groups (n = 6); Group 1: Received chow only (control), Group 2: Diabetic rats + chow, Group 3: Diabetic rats + chow supplemented with 4% of whole walnuts. After four weeks rats were sacrificed, blood was collected; lipid profiles as well as SREBP-1c and PPARα protein levels were determined. Compared with diabetic rats walnut significantly decreased serum cholesterol (P < 0.01), LDL-c (P < 0.01), triglyceride (P < 0.001) and VLDL-c (P < 0.001) and also increased HDL-c (P < 0.05) compared with diabetic. Moreover, SREBP-1c protein level significantly decreased (P < 0.05) and PPARα significantly increased in walnut group compared with diabetic group (P < 0.05). The findings showed that walnut administration in diet clinically decreases atherosclerosis risk factors. Lipid profile reduction might be due to the rise of PPARα and the reduction of SREBP-1c by this medical treatment in liver.
1. Introduction
Diabetes mellitus known as an independent forecaster of high risk for Coronary Heart Disease (CHD) and well demonstrated that mortality from CDH in diabetic patients is two to four times higher than nondiabetics. Vascular disease cases approximately 70% - 80% of deaths in diabetic patients. Although control of glucose is essential in diabetic patients, this provides only nominal advantage with respect to CDH prevention [1]. Hence a useful treatment for diabetic patients would be a drug that controls both glycemic levels and prevents the development of arteriosclerosis.
In addition to drug therapy, certainly nutritional factors can reduce serum cholesterol and triglyceride levels. In this respect walnuts have been shown to reduce cholesterol and triglyceride levels significantly. Walnuts have lot of unsaturated fatty acids which favorably affected CVD risk [2].
There are many genes which have important role in lipid metabolism. In this respect, Liver x receptor α (LXRα) and sterol-regulatory element binding protein-1c (SREBP-1c) in the liver play vital roles in the regulation of lipogenic genes. LXRα regulates the expression of SREBP-1c [3]. Besides these genes Peroxisome Proliferator Activated Receptor α (PPARα) is part of the intracellular receptor superfamily of transcription factors which plays an important role in triglyceride metabolism. PPARα agonists Reduce plasma triglyceride levels, increase high-density lipoprotein (HDL) cholesterol levels and also increased LDL receptor expression [4]. The aim of this study was to investigate the effects of walnut on lipid profiles, as well as PPARα, and SREBP-1c protein expression in diabetic rat liver.
2. Materials and Methods
2.1. Experimental Design
Adults’ male Wistar rats weighing 195 - 205 g were used in this experiment. Animals were maintained under standard environmental conditions at 23˚C ± 1˚C, relative humidity 55% ± 5% and 12 h light/dark cycle. They also had free access to water and a standard laboratory diet. After two weeks of acclimation, animals were randomly divided into 3 groups (n = 6); Group 1: Received chow only (control), Group 2: Diabetic rats + chow, Group 3: Diabetic rats + chow supplemented with 4% of whole walnuts. After 4 weeks rats were fasted overnight, blood samples were collected from the heart of each rat under anesthesia. Serum was isolated by centrifugation for 15 min at 3000 rpm and stored at –4˚C until analysis was completed. The liver was quickly removed, washed with sterile phosphate-buffered saline (PBS), and immediately frozen in liquid nitrogen and stored at –70˚C until use. All of the experimental procedure was approved by the ethics committee of Islamic Azad University (Borūjerd, Iran). Lipid profiles were determined enzimatically. LDL-c and VLDL-c levels were calculated according to the Friedewald formula [5] and Atherogenic Index (AI):
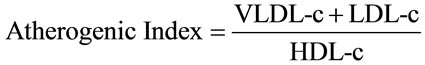
2.2. Preparation of Diabetic Rats
Diabetes was induced in non-fasted rats by a single intra peritoneal injection of 70 mg/kg body weight streptozotocin (Sigma, USA). STZ was extemporaneously dissolved in 10mM citrate buffer, pH 4.5. After one week, animals with fasting blood glucose levels more than 300 mg/dl were considered diabetic. Six rats were used in each group and each animal was used once only in all experiments [6].
2.3. Western Blotting
For protein analysis 40 - 50 mg of liver samples were homogenized in 700 µl of RIPA buffer which contained 1 µM PMSF and 1% Protein Inhibitor Cocktail (Santa Cruz). The samples were centrifuged (14,000 rpm for 15 minutes at 4˚C (and the supernatant was obtained and protein concentrations were calculated using Bradford method (Bio-Rad Laboratories, Muenchen, Germany). For SREBP-1c and PPARα proteins expression, 100 µg of proteins were run onto SDS-PAGE gel (10%) and transferred to PVDF membrane (Roche Applied Science). The membrane was blocked with 5% skim milk in Tris buffered saline containing Tween-20 (TBS-T) (Roche Applied Science) for 2 hours at room temperature, and then washed 3 times in TBS-T (three times, 15 minutes). The membrane was probed with rabbit anti-SREBP-1 antibody (1:300 dilutions, Santa Cruz), rabbit anti-PPARα antibody (1:300 dilution, Santa Cruz), and rabbit polyclonal β-actin antibody (1:2500 dilutions, Novus Biological) for 1.5 hours. The secondary antibodies were also incubated for 1.5 hours (1:10,000 dilutions, Roche Applied Science). The membranes were then washed and exposed to ECL western blotting detection reagents (Roche Applied Science), and band density was determined. Data are expressed as the ratio of SREBP-1 and PPARα to β-actin protein expression [7].
2.4. Statistical Analysis
The data from the experiments were analyzed using oneway analysis of variance with ANOVA followed by Tukey. The differences between groups were considered significant when P < 0.05 and results are shown as means ± SD.
3. Results and Discussion
3.1. Lipid Profile
Table 1 shows TC, HDL-c and TG in rats which treated differently. Amount of TC (P < 0.001), TG (P < 0.001), VLDL-c (P < 0.001), and LDL-c (P < 0.001) and Atherogenic Index (P < 0.001) significantly increased in diabetic group compared with control. On the other hand, in walnut group TC (P < 0.05), TG (P < 0.001), VLDL-c (P < 0.001), LDL-c (P < 0.01) and Atherogenic Index (P < 0.001) significantly decreased and HDL-c significantly increased compared (P < 0.01) with diabetic rat. Body weight significantly reduced in diabetic and treatment group (P < 0.01 and P < 0.05 respectively), while change between diabetic and walnut group was not significant and also the change between chow and walnut group was not significant (Table 2).
A cardio-protective dietary fat profile is suggested for diabetes type 2 treatment. Walnuts are exceptional since they have a great balance of n-6 and n-3 polyunsaturated fatty acids (PUFA), linoleic acid (LA), and α-linolenic acid (ALA). Hence consumption of this herb seems to lower the progress of coronary heart disease [8].
According to the US Department of Agriculture National Nutrient Database, 100 g of walnuts contain approximately 9 g of ALA and 38 g of LA [9]. In addition to high amount of PUFA, walnuts also contain vitamin E, folate, and melatonin and antioxidant polyphenolics [10, 11].
Ros et al. and Zhao et al. have shown that diets counting the LA and the ALA such as walnuts significantly lower total cholesterol, LDL, and TG [12]. Furthermore the study of Lavedrine et al. has shown that serum HDLc and apo A1 rise by walnut. Many studies demonstrate that there are negative correlations between HDL-c and cardiovascular morbidity [12,13].
In this study walnut significantly reduced TC by 8%, TG by 31%, LDL-C by 43.3%, VLDL-C by 31%. It also

Table 1. Lipid profile and Atherogenic Index in diabetic, walnut and chow groups.
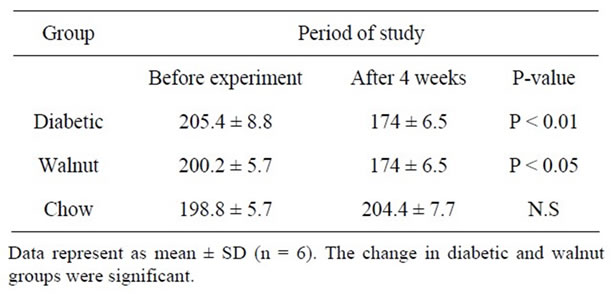
Table 2. Body weight in diabetic, walnut and chow groups.
increased HDL-C by 34.9% in comparison with diabetic rats.
Zavvarreza et al. study has shown that the administration of walnut oil extract in hypercholesterolemic rats, significantly reduced TG, total cholesterol and LDL-c levels. Moreover, koohsoltani, et al. [14]. reported that walnut pulp, considerably reduced TG, total cholesterol and LDL-c in normolipidemic and hyperlipidemic people.
3.2. Protein Levels
Liver SREBP-1c protein significantly increased in diabetic rats (P < 0.05). In comparison with diabetic rats, SREBP-1c protein had significant reduction in walnut group (P < 0.005) (Figure 1). A significant rise (P < 0.05) in PPARα levels was observed in walnut group in comparison with diabetic rats (Figure 2).
Two classes of transcriptional control systems controlling several genes in lipids and glucose homeostasis are the peroxisome proliferator-activated receptors (PPAR alpha, beta and gama), which are members of the nuclear
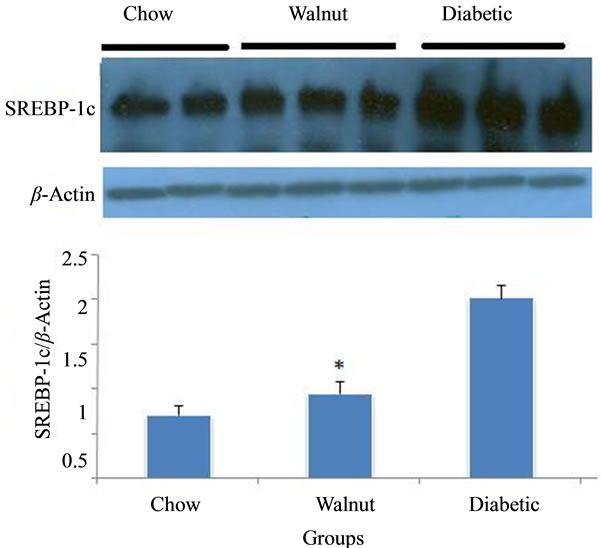
Figure 1. The average band density ratio of liver sterolregulatory element binding protein-1 (SREBP-1) in chow, walnut and diabetic groups (n = 6). β-actin was used as an internal control. Liver SREBP-1c protein significantly reduced in walnut group (diabetic rats + chow + 4% of whole walnuts) compared with diabetic rats (diabetic rats + chow) (P < 0.05).

Figure 2. The average band density ratio of liver Peroxisome Proliferator Activated Receptor α (PPARα) in chow, walnut and diabetic groups (n = 6). β-actin was used as an internal control. Liver PPARα significantly increased in walnut group (diabetic rats + chow + 4% of whole walnuts) compared with diabetic rats (diabetic rats + chow) (P < 0.05).
hormone receptor superfamily, and the sterol-regulatory element-binding proteins (SREBP-1a and -1c, and -2), a subgroup of basic-helix-loop-helix-leuzine zipper (BHLHLZ) transcription factors. PPARs have a crucial role in the regulation of mediatory metabolism, both in adipose and liver tissue. SREBPs transcriptionally activated a cascade of enzymes which is required for endogenous synthesis of triglyceride, cholesterol, FA and phospholipid [15]. In liver, transgenic expression of SREBP-1c entirely stimulates fatty acid synthesis [15,16].
In this study the expression of SREBP1c up-regulated in diabetic rat liver compared with control, while walnut significantly suppressed the expression of SREBP1c protein in this group.
Feeding rats by a diet supplemented with walnut, probably because of omega-3 PUFA content, suppressed SREBP-1c expression in liver. Moreover Ou J. et al. [17]. reported that unsaturated fatty acids blocked SREBP1c gene transcription by antagonizing the activation of LXR.
PUFAs by inhibition of LXRα binding to liver X receptor response elements (LXREs) suppressed SREBP- 1c expression which as a result led to significant decrease the in expression of lipogenic genes [18].
Omega-3 PUFA supplement in mouse diet improved hepatic steatosis, insulin sensitivity, and lowered fasting free fatty acid concentrations. It lowered triglyceride levels in serum as well [19].
On the other hand, PPARα has important role in lipid and glucose metabolism, which it is due to regulating the expression of proteins, involved in the β-oxidation and the transport of free fatty acids (FFAs) [20].
PPARα is expressed at high levels in liver and is a molecular target of long chain fatty acids, eicosanoids and fibrates. PPARα agonists lowered plasma lipid levels, decreased lipid accumulation in liver, stabilized glucose and insulin levels, and consequently reduced the risk of type 2 diabetes [20]. In this study walnut significantly increased PPARα protein.
Fibrates known as PPARα-activator has been used in the treatment of dyslipidemia. In dyslipidemic patients, these drugs by lowering triglyceride, LDL-c and by increasing HDL-c cholesterol levels improved the plasma lipid profile [21].
PUFA which is known as a source of PPARα ligands inhibited lipogenesis by antagonizing the activation of LXR [20].
4. Conclusion
Walnut is an inexpensive and safe treatment, which significantly reduces lipid profile and raises HDL-c. These effects perhaps are due to the reduction of SREBP-1c expression and the rise of PPARα expression in diabetic rat.
5. Acknowledgements
This study was funded by Borūjerd Azad University. We would like to thank Professor Ahmad Gholamhoseinian from Afzalipour School of Medicine, Kerman, Iran, for his advice and for help in measuring lipid profile. We would also like to thank Ayat Kaeidi from Razi Herbal Medicines Research Center, Lorestan University of Medical Sciences, Khoramabad, Iran, for preparation of β- actin antibody. We would also like to thank Jahanbanoo shahryari for her advice.
REFERENCES
- J. Stamler, O. Vaccaro, J. Neaton and D. Wentworth, “For the Multiple Risk Factor Intervention Trial Research Group: Diabetes, Other Risk Factors, and 12-Year Cardiovascular Mortality for Men Screened in the Multiple Risk Factor Intervention Trial,” Diabetes Care, Vol. 16, No. 2, 1993, pp. 434-444. doi:10.2337/diacare.16.2.434
- J. Sabate, E. G. Fraser, K. Burke, S. F. Knutsen, H. Bennett and K. D. Lindsted, “Effects of Walnut on Serum Lipid Levels and Blood Pressure in Normal Men,” New England Journal of Medicine, Vol. 328, No. 9, 1993, pp. 605-607. doi:10.1056/NEJM199303043280902
- P. A. Edwards, H. R. Kast and A. M. Anisfeld, “Bareing It all: The Adoption of LXR and FXR and Their Roles in Lipid Homeostasis,” Journal of Lipid Research, Vol. 43, No. 1, 2002, pp. 2-12.
- Z. Huang, X. Zhou, A. C. Nicholson, J. A. M. Gotto, D. P. Hajjar and J. Han, “Activation of Peroxisome Proliferator-Activated Receptor α in Mice Induces Expression of the Hepatic Low-Density Lipoprotein Receptor,” British Journal of Pharmacology, Vol. 155, No. 4, 2008, pp. 596-605. doi:10.1038/bjp.2008.331
- W. Friedewald and K. Leseg and D. Fredrickson, “Estimation of the Concentration of Low Density Lipoprotein Cholesterol in Plasma without Use of Preparative Centrifuge,” Clinical Chemistry, Vol. 18, 1972, pp. 499-502.
- M. A. Montanaro, A. M. Bernasconi, M. S. Gonzalez, O. J. Rimoldi and R. R. Brenner, “Effects of Fenofibrate and Insulin on the Biosynthesis of Unsaturated Fatty Acids in Streptozotocin Diabetic Rats,” Prostaglandins, Leukotrienes and Essential Fatty Acids, Vol. 73, No. 5, 2005, pp. 369-378. doi:10.1016/j.plefa.2005.06.004
- S. J. Thornton, E. Wong, D. S. Lee and K. M. Wasan, “Effect of Dietary Fat on Hepatic Liver X Receptor Expression in P-Glycoprotein Deficient Mice: Implications for Cholesterol Metabolism,” Lipids in Health and Disease, Vol. 7, 2008, p. 21.
- A. P. Simopoulos, “The Importance of the Ratio of Omega-6/Omega-3 Essential Fatty Acids,” Biomed Pharmacother, Vol. 56, No. 8, 2002, pp. 365-379. doi:10.1016/S0753-3322(02)00253-6
- C. Crews, P. Hough, J. Godward, P. Brereton, M. Lees, S. Guiet and W. Winkelmann, “Study of the Main Constituents of Some Authentic Walnut Oils,” Journal of Agriculture and Food Chemistry, Vol. 53, No. 12, 2005, pp. 4853-4860. doi:10.1021/jf0478354
- T. Fukuda, H. Ito and T. Yoshida, “Antioxidative Polyphenols from Walnuts (Juglans regia L),” Phytochemistry, Vol. 63, No. 7, 2003, pp. 795-801. doi:10.1016/S0031-9422(03)00333-9
- R. J. Reiter, L. C. Manchester and D. X. Tan, “Melatonin in Walnuts: Influence on Levels of Melatonin and Total Antioxidant Capacity of Blood,” Nutrition, Vol. 21, No. 9, 2005, pp. 920-924. doi:10.1016/j.nut.2005.02.005
- R. Mushtaq and Z. T. Khan, “Effect of Walnut on Lipid Profile in Obese Female in Different Ethnic Groups of Quetta, Pakistan,” Pakistan Journal of Nutrition, Vol. 8, No. 10, 2009, pp. 1617-1622. doi:10.3923/pjn.2009.1617.1622
- R. J. K. Srivastava, S. He and R. S. Newton, “Differential Regulation of Human Apolipoprotein AI and High-Density Lipoprotein by Fenofibrate in HapoAI and HapoAICIII-AIV Transgenic Mice,” Biochimica et Biophysica Acta, Vol. 1811, No. 2, 2011, pp. 76-83.
- Y. Koohsoltani, Y. A. Esfahani, M. Shayesteh and S. Datgiri, “Effect of Pulp Walnut Administration on Blood Lipids in Normo Lipidemic and Hyper Lipidemic People,” Journal of Tabriz University of Medical Sciences, Vol. 62, 2004, pp. 55-60.
- S. M. Ulven, K. T. Dalen, J. Gustafsson and H. I. Nebb, “LXR Is Crucial in Lipid Metabolism,” Prostaglandins, Leukotrienes and Essential Fatty Acids, Vol. 73, No. 1, 2005, pp. 59-63. doi:10.1016/j.plefa.2005.04.009
- I. Shimomura, H. Shimano, B. S. Korn, Y. Bashmakov and J. D. Horton. “Nuclear Sterol Regulatory ElementBinding Proteins Activate Genes Responsible for the Entire Program of Unsaturated Fatty Acid Biosynthesis in Transgenic Mouse Liver,” Journal of Biological Chemistry, Vol. 273, 1998, pp. 35299-35306. doi:10.1074/jbc.273.52.35299
- J. Ou, H. Tu, B. Shan, A. Luk, R. A. De Bose-Boyd, Y. Bashmakov, J. L. Goldstein and M. S. Brown, “Unsaturated Fatty Acids Inhibit Transcription of the Sterol Regulatory Element-Binding Protein-1c (SREBP-1c) Gene by Antagonizing Ligand-Dependent Activation of the LXR,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 98, No. 11, 2001, pp. 6027-6032. doi:10.1073/pnas.111138698
- N. Zaima, T. Sugawara, D. Goto and T. Hirata, “Trans Geometric Isomers of EPA Decrease LXRa-Induced Cellular Triacylglycerol via Suppression of SREBP-1c and PGC-1b,” Journal of Lipid Research, Vol. 47, No. 12, 2006, pp. 2712-2717. doi:10.1194/jlr.M600273-JLR200
- R. Kallwitz, A. M. Lachlan and S. J. Cotler, “Role of Peroxisome Proliferators-Activated Receptors in the Pathogenesis and Treatment of Nonalcoholic Fatty Liver Disease,” World Journal of Gastroenterology, Vol. 14, No. 1, 2008, pp. 22-28.
- H. Shu, B. Wong, G. Zhou, Y. Li, J. Berger, J. W. Woods, S. D. Wright and T. Q. Cai, “Activation of PPAR Alpha or Gamma Reduces Secretion of Matrix Metalloproteinase 9 but Not Interleukin 8 from Human Monocytic THP-1 Cells,” Biochemical and Biophysical Research Communications, Vol. 267, No. 1, 2000, pp. 345-349. doi:10.1006/bbrc.1999.1968
- A. Keech, R. J. Simes and P. Barter, “Effects of LongTerm Fenofibrate Therapy on Cardiovascular Events in 9795 People with Type 2 Diabetes Mellitus (the FIELD Study): Randomized Controlled Trial,” Lancet, Vol. 366, No. 9500, 2005, pp. 1849-1861. doi:10.1016/S0140-6736(05)67667-2
NOTES
*Corresponding author.

