Food and Nutrition Sciences
Vol. 3 No. 3 (2012) , Article ID: 17975 , 4 pages DOI:10.4236/fns.2012.33057
Garbanzo Diet Lowers Cholesterol in Hamsters
![]()
1Western Regional Research Center, United States Department of Agriculture, Agricultural Research Service (USDA-ARS), Albany, USA; 2University of California, Davis, USA.
Email: Talwinder.Kahlon@ars.usda.gov
Received September 21st, 2011; revised November 17th, 2011; accepted November 25th, 2011
Keywords: Hamster; Lipoproteins; Cholesterol; HDL; LDL; Garbanzo; Bengal Gram; Lentils
ABSTRACT
The present study was conducted to investigate the effect garbanzo containing diet on cholesterol in hamster fed cholesterol containing high fat diet. It was hypothesized that garbanzo diet would lower cholesterol in hamsters, based on previous observation of the bile acid binding potential of garbanzo. Garbanzo (Cicer arietinum), Bengal gram (Cicer arietinum), lentils (Lens culinaris), soy protein isolate (SPI) or casein (control) diets were fed to hamsters for three weeks. Initial and final animal weights, feed intakes and plasma triglycerides values were similar among all the treatments. Garbanzo containing diet significantly lowered total plasma cholesterol (TC) compared with casein control. There was 17% reduction in low density lipoprotein cholesterol (LDL-C) in hamsters fed the garbanzo diet; this difference was not significant due to high variability in within treatment values. Plasma cholesterol values with lentils diet were similar those with the control diet. Liver lipid and liver cholesterol values with lentils diet were higher than all the other treatments. Data suggest that garbanzo diet has the potential to lower the risk of atherosclerosis and improve human health.
1. Introduction
The population of the Asian countries has a notably low risk of coronary vascular disease (CVD), presumably due to their lower intake of animal protein and higher intake of various beans, which are being introduced to the Western World mainly by migrants. Daily per capita consumption of all bean products in Asia is estimated to be 110 g, whereas in the USA it is about 9 g. Beans are high in carbohydrates, fiber, minerals and protein, and are especially rich in lysine but limited in some sulfur amino acids [1,2]. Bengal gram (smaller size, yellow to black color, wrinkled, Desi Chana, Asian variety of garbanzo) a protein rich bean which forms the staple diet of people of low socioeconomic status in South East Asia, was found to have a marked hypocholesterolemic effect in cholesterol and cholic acid fed rats [3]. Both its protein and fat fractions were found to cause this effect. Increased excretion of cholesterol as bile acids and neutral sterols and decreased synthesis in liver are the probable mechanisms of its action. We have previously observed significant in vitro bile acid binding by garbanzo [4]. It was hypothesized that garbanzo diet would lower cholesterol in hamster, a rodent model for cholesterol studies due to its response similar to humans for diet and drugs [5-7]. Significant reductions in serum total cholesterol (TC) and low density lipoprotein cholesterol (LDL-C) with a garbanzo containing diet have been reported [8].
In USA about 11% of the population is of Hispanic origin and they consume 33% of all bean products [9]. Beans consumed are pinto, kidney, black and navy as cooked dry beans or canned (cooked, baked or refried). In Western countries, kidney bean, lentils and garbanzo are used in salads, soups and other food products. Bean consumption of four times or more per week compared with less than once a week has been associated with a 22% lower risk of CVD [10]. Rats and hamsters fed soy protein isolate diet had significantly lower TC compared with those fed a casein diet [11,12]. Meta-analysis of 38 controlled clinical trials revealed that 47 g per day intake of soy protein isolate (SPI) was associated with decrease of TC 9%; LDL-C 13%; and triglycerides (TG) 11% [13]. The changes in TC and LDL-C concentrations were directly related to the initial serum cholesterol levels. This three week hamster feeding study was conducted to evaluate cholesterol-lowering potential of diets containing garbanzo, Bengal gram, lentils, soy protein isolate (SPI) or casein.
1.1. Materials and Methods
Male, 25-day-old weanling golden Syrian hamsters (Charles River Laboratories, Wilmington, MA) were housed individually in wire-bottomed cages in a controlled environment (20˚C - 22˚C, 60% relative humidity, 12-hr light and dark cycle) and fed ad-libitum Rat Laboratory Chow 5001 M (Purina, Richmond, IN) for one week. Animals were then weighed and assigned to one of five treatments by selective randomization (blocked by weight, one animal per treatment from each block, 10 animals per treatment). Total feed consumption was measured, fresh feed was provided twice weekly, and animals were weighed once a week during the 21-day feeding period. All the procedures described were approved by the Animal Care and Use Committee of the Western Regional Research Center, USDA, Albany, CA, and conformed to the principles specified by the Committee on Care and Use of Laboratory Animals (1985).
Treatment diets (Table 1) were formulated to contain 10% total dietary fiber (TDF), 15% fat, 3.5% nitrogen, and 0.4% cholesterol. The control diet contained casein as protein source and cellulose as dietary fiber with the same level of TDF, fat and cholesterol. Garbanzo (Cicer arietinum), Bengal gram (Cicer arietinum) and lentils (Lens culinaris) were obtained from local vendors. Soy protein isolate, casein and other diet ingredients were obtained from Dyets Inc. (Dyets Inc., Bethlehem, PA). Diet ingredients were analyzed for total dietary fiber by method 991.16 [14], nitrogen by method 990.03 [15] with a Vario Macro Elemental Analyser (Elementar Analysen systeme GmbH, Hanau, Germany), crude fat with hexane, isopropanol (3:2) by the accelerated solvent extractor (ASE 200 Dionex Corp., Sunnyvale, CA), ash by method 942.05 [15] and for moisture by method 935.29 [15].
After two weeks of feeding the treatment diets, total feces were collected for four consecutive days and analyzed for dry matter at 50˚C under vacuum for 24 hr by Method 934.01 [15]. Fecal samples were analyzed for crude fat by accelerated solvent extractor using hexane isopropanol (3:2) as solvent, extracted samples were evaporated under nitrogen to determine lipid weight. Lipid was dissolved in chloroform methanol (5:2), for determination of total neutral sterols. Aliquots (50 μL) were dried under nitrogen, solubilized with Triton X-100 [16], and analyzed for total neutral sterols by the same enzy-
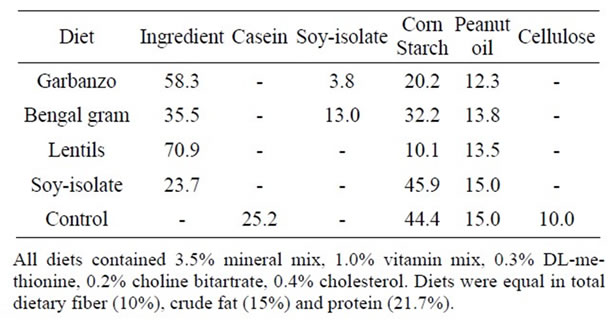
Table 1. Composition of diets (% dwb)a.
matic colorimetric procedure as that used for total plasma cholesterol (TC).
At the end of the 21-day feeding period, all animals were fasted for 16 hr and anesthetized with isoflurane for tissue sample collection. Blood was drawn by cardiac puncture into plastic tubes containing anticoagulant (ethylenediamine tetraacetic acid dipotassium salt, 0.8 mg/mL of blood) and centrifuged at 1500× g for 30 min at 4˚C to obtain plasma. Livers were excised, rinsed, blotted, weighed, and kept on dry ice. Liver and plasma aliquots were stored at –70˚C until analysis.
Plasma, liver, and feces samples were analyzed by an enzymatic colorimetric procedure for cholesterol and triglycerides (diagnostic kits 2350-400H and TR 22421, respectively; Thermo Fisher Scientific., Pittsburgh, PA). Values were determined with standard curves obtained by running several concentrations of standards provided with the respective kits.
Fresh plasma samples were pooled (two animals per pool) by using an equal volume of plasma from each animal. A protease inhibitor, epsilon-amino caproic acid (ICN Biomedicals, Costa Mesa, CA), 1.3 mg/mL of plasma, and an antimicrobial agent, garamycin 50 mg 1 mL (Schering, Kenilworth, NJ), 10 μL/mL of plasma, were added to stabilize the plasma. Lipoproteins were fractionated by density gradient ultracentrifugation [17]. After the background density of 1 mL of plasma was adjusted to 1.019 g/mL with 5 mL of NaCl solution (1.0214 g/mL), plasma was centrifuged in an ultracentrifuge (Optima L-60, Beckman, Palo Alto, CA) at 40 K for 18 hr at 17˚C in a rotor (model 50.3, Beckman). The top 1 mL (<1.019 g/mL) was removed as the VLDL fraction, and another 1 mL was removed as background. The subnatant (4 mL) density was adjusted to 1.067 g/mL with 2 mL of NaCl solution (1.1562 g/mL) and centrifuged similarly for 24 hr. The top 1 mL (1.019 - 1.063 g/mL) was removed as the low density lipoprotein, 2nd 1 mL was removed as background. The subnatant (4 mL) contained the high density lipoprotein (HDL) fraction. With each ultracentrifugation, two salt solution tubes with similar density were run, and the densities of their fractions were monitored with a density meter (model DMA-48, Anton Paar, Richmond, VA). Lipoprotein fractions were analyzed for cholesterol by the procedure described for plasma.
Each liver was freeze dried in Labconco Freeze Dry System (Labconco Corp. Kansas City, MO) for 72 hr. Lipids were extracted from 0.5 - 0.7 g sample of dry ground liver using hexane isopropanol (3:2) accelerated solvent extractor. Lipid weight was determined after evaporating the extracted lipid solvent under nitrogen at 37˚C. Lipid was dissolved in 10 mL of chloroform and methanol, 86:14, for cholesterol analysis. Liver total cholesterol was determined in aliquots (30 µL) of extract after evaporation under nitrogen and solubilization with Triton X-100 [16]; the enzymatic kit used was the same as that used with plasma.
1.2. Statistical Analyses
Values were determined in triplicate, and analysis of variance and Duncan’s new multiple range tests [18] were conducted. A value of P ≤ 0.05 was considered the criterion of significance.
2. Results and Discussion
The initial weight (56.2 ± 1.3 g), final weight (88.8 ± 2.1 g), weight gain (32.6 ± 1.8 g) and feed intake for 21d (156 ± 6 g) values were similar in hamsters fed all the treatment diets. Hamster fed garbanzo containing diet resulted in significantly (P ≤ 0.05) lower total plasma cholesterol (TC) than those fed the casein (control) or lentils containing diets (Table 2). Hamsters fed Bengal gram or soy protein isolate diets resulted in significantly lower TC values than those fed lentils containing diet. Diets containing Bengal gram or soy protein isolate (SPI) resulted 6% - 7% lower TC than the control treatment, but this difference was not significant. Pittaway et al. [8] reported significant reduction in free living individuals fed garbanzo diet compared with those fed wheat based diet of similar fiber content. Mathur et al. [3] reported TC reduction with Bengal gram diet in rats with elevated cholesterol. Anderson et al. [13] reported meta-analysis of 38 studies observing TC reduction with SPI diets. Non-significant reductions in TC with Bengal gram and SPI diets in study reported herein was possibly due to lower elevation of TC with the control diet. The reductions in TC values have been reported to be proportional
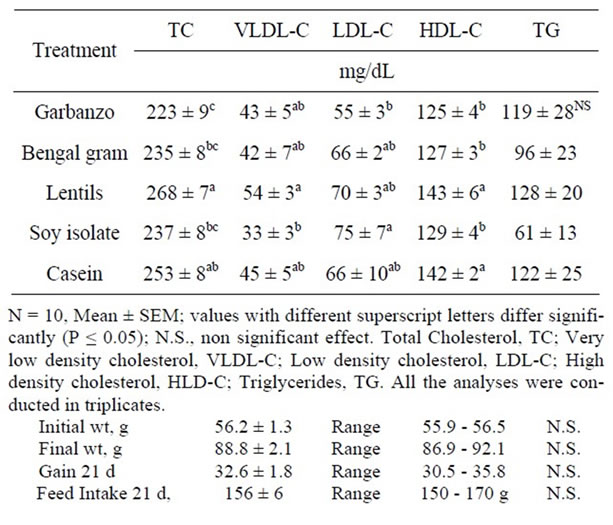
Table 2. Plasma cholesterol and triglycerides of hamsters fed garbanzo, Bengal gram, lentils, soy isolate and casein diets for three weeks.
to elevated initial level of cholesterol [11-13]. Very low density lipoprotein cholesterol (VLDL-C) values for SPI diet fed hamsters were significantly lower than those fed lentils diet. Low density lipoprotein cholesterol (LDL-C) values for garbanzo diet fed animals were significantly lower than those fed SPI diet. High density lipoprotein cholesterol (HDL-C) was significantly lowered with garbanzo, Bengal gram and SPI diets compared with those fed Lentils or control diet. With garbanzo diet there were 17% reduction in LDL-C and TC and HDL-C were lowered by 12%. In general when the TC is lowered there is some reduction in HDL-C, higher reduction in LDL-C is desirable. Triglyceride values for hamsters fed SPI and Bengal gram diet were 50% and 21% lower than those fed the control diet. Two-fold variability between treatments in TG did not result in significantly differences between treatments due to high variability within treatment values. Liver weights in animals fed SPI diet were significantly lower than those fed control, garbanzo or lentil containing diets (Table 3). Liver lipid and total liver cholesterol values were significantly lower in hamsters fed garbanzo, Bengal gram, SPI or control diet compared with those fed lentils diet. Higher liver lipid and cholesterol data suggest that lentils diet may not have cholesterol lowering potential. Fat digestibility for hamsters fed SPI diet was significantly lower than those fed all the other treatment diets (Table 4). Cholesterol digestibility in hamsters fed lentils diet was significantly lower than those fed garbanzo or Bengal gram diets. Neutral sterol excretion in hamsters fed lentils diet was significantly higher than those fed garbanzo, Bengal gram, SPI containing diets. With lentil containing diet lower cholesterol digestibility and higher sterol excretion is desirable, however higher plasma and liver cholesterol values suggest that lentils do not have cholesterol lowering potential.
3. Conclusion
Total plasma cholesterol was significantly and LDL-C
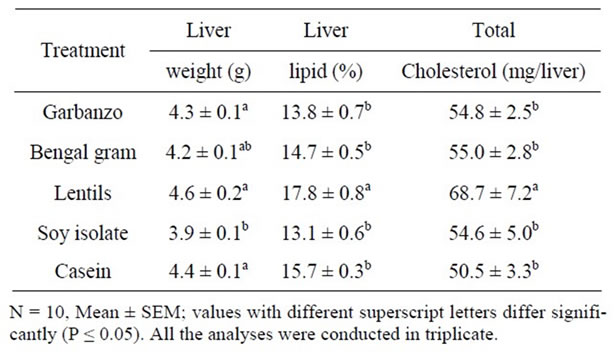
Table 3. Liver weight, lipids and cholesterol of hamsters fed garbanzo, Bengal gram, lentils, soy isolate and casein diets for three weeks.

Table 4. Digestibility of diet lipids in hamsters fed garbanzo, Bengal gram, lentils, soy isolate and casein diets for three weeks.
(–17%) lowered in hamsters fed diet containing garbanzo. Data lends credibility to in vitro study that reported significantly higher bile acid binding by garbanzo [4]. Data suggest that under the conditions of this experiment garbanzo diet showed promise in lowering the risk of atherosclerosis and improve human health.
4. Acknowledgements
The expert statistical assistance of Linda C. Whitehand, Consulting Statistician, WRRC, USDA-ARS, Albany, Ca, is greatly appreciated.
REFERENCES
- Y. Bahnassey, K. Khan and R. Harrold, “Fortification of Spaghetti with Edible Legumes. I. Physico-Chemical, Antinutritional, Amino Acid and Mineral Composition,” Cereal Chemistry, Vol. 63, No. 3, 1988, pp. 210-215.
- J. K. Chavan, S. S. Kadam and D. K. Salunkhe, “Biochemistry and Technology of Garbanzo (Cicer arietinnum L.) Seeds,” C R C Critical Reviews in Food Science and Nutrition, Vol. 25, No. 2, 1989, pp. 107-158.
- K. S. Mathur, S. S. Singhal and R. D. Sharma, “Effect of Bengal Gram on Experimentally Induced High Levels of Cholesterol in Tissues and Serum in Albino Rats,” Journal of Nutrition, Vol. 84, 1964, pp. 201-204.
- T. S. Kahlon and Q. Shao, “In Vitro Binding of Bile Acids by Soy Bean (Glycine max), Black Eye Bean (Vigna unguiculata), Garbanzo (Cicer arietinum) and Lima Bean (Phaseolus lunatus),” Food Chemistry, Vol. 86, No. 3, 2004, pp. 435-440. doi:10.1016/j.foodchem.2003.09.018
- D. K. Spady and J. M. Dietschy, “Dietary Saturated Triacylglycerols Suppress Hepatic Low Density Lipoprotein Receptor Activity in the Hamster,” Proceedings of National Academy of Sciences USA, Vol. 82, 1985, pp. 4526-4530. doi:10.1073/pnas.82.13.4526
- T. S. Kahlon, R. M. Saunders, R. N. Sayre, F. I. Chow, Chiu and A. A. Betschart, “Cholesterol-Lowering Effects of Rice Bran and Rice Bran Oil Fractions in Hypercholesterolemic Hamsters,” Cereal Chemistry, Vol. 69, No. 5, 1992, pp. 485-489.
- K. Valeille, J. F. Zou, M. Parquet, G. Amsler, D. Gripois, A. Quignard-Boulange and J.-C. Martin, “The Natural Concentration of the Conjugated Linoleic Acid, cis-9, trans-11, in Milk Fat Has Antiatherogenic Effects in Hyperlipidemic Hamsters,” Journal of Nutrition, Vol. 136, No. 5, 2006, pp. 1305-1310.
- J. K. Pittaway, K. D. K. Ahuja, K, Iain, I. K. Robertson and M. J. Ball, “Effects of a Controlled Diet Supplemented with Garbanzos on Serum Lipids, Glucose Tolerance, Satiety and Bowel Function,” Journal of American College of Nutrition, Vol. 26, No. 4, 2007, pp. 334-340.
- G. Lucer, B. Lin, J. Allshouse and L. S. Kantor, “Factors Affecting Dry Bean Consumption in the United States. USDA, Vegetables and Specialties,” A&SVGS-280, April 2000.
- L. A. Bazzano, J. He, L. G. Ogden, C. Loria, S. Vupputur, L. Myers and P. K. Whelton, “Legume Consumption and Risk of Coronary Heart Disease in US Men and Women: NHANES I Epidemiologic Followup Study,” Archives of Internal Medicine, Vol. 161, No. 21, 2001, pp. 2573-2578. doi:10.1001/archinte.161.21.2573
- J. Balmerand and D. B. Zilversmit, “Effect of Dietary Roughage on Cholesterol Absorption, Cholesterol TurnOver and Steroid Excretion in the Rat,” Journal of Nutrition, Vol. 104, 1974, pp. 1319-1328.
- C.-Y. Lin, C.-Y. Tsai and S.-H. Lin, “Effects of Soy Components on Blood and Liver Lipids in Rats Fed High-Cholesterol Diets,” World Journal Gastroenterology, Vol. 11, No. 35, 2005, pp. 5549-5552.
- J. W. Anderson, B. M. Johnstone and M. E. Cook-Newell, “Meta-Analysis of the Effects of Soy Protein Intake on Serum Lipids,” New England Journal of Medicine, Vol. 333, No. 5, 1995, pp. 276-282. doi:10.1056/NEJM199508033330502
- AOAC, “Official Methods of Analysis of the Association of Official Analytical Chemists,” 18th Edition, Arlington, The Association, 2005.
- AOAC, “Official Methods of Analysis of the Association of Official Analytical Chemists,” 15th Edition, Arlington, The Association, 1990.
- S. E. Carlson and S. Goldfarb, “A Sensitive Enzymatic Method for the Determination of Free and Esterified Tissue Cholesterol,” Clinica Chimica Acta, Vol. 79, No. 3, 1977, pp. 575-582. doi:10.1016/0009-8981(77)90178-4
- R. J. Havel, H. A. Eder and J. H. Bragdon, “The Distribution and Chemical Composition of Ultracentrifugally Separated Lipoproteins in Human Serum,” Journal of Clinical Investigations, Vol. 34, No. 9, 1955, pp. 1345- 1353. doi:10.1172/JCI103182
- R. G. D. Steel and J. H. Torrie, “Principles and Procedures of Statistics,” McGraw-Hill, New York, 1960.

