American Journal of Plant Sciences
Vol.08 No.06(2017), Article ID:76489,12 pages
10.4236/ajps.2017.86095
Cadmium Effects on Enzymes of Ammonia Assimilation in Excised Etiolated Maize Leaf Segments during Greening: A Mechanistic Approach
Juliana Sarangthem, Sonal Dhamgaye, Rekha Gadre*
School of Biochemistry, Devi Ahilya University, Takshashila Campus, Khandwa road, Indore, India

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: January 5, 2017; Accepted: May 23, 2017; Published: May 26, 2017
ABSTRACT
Supply of CdCl2 in the presence of NH4NO3 to excised etiolated maize leaf segments during greening decreased the glutamine synthetase and nicotinamide adenine dinucleotide reduced (NADH) dependent glutamate synthase activities, while the ferredoxin (Fd) dependent glutamate synthase and glutamate dehydrogenase activities were increased. Inclusion of inorganic nitrogen, metabolites and the inhibitor influenced the effect of Cd on glutamine synthetase activity. The % inhibition of activity caused by Cd was higher with 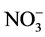 but lower with
but lower with . Glutamine, 2-oxoglutarate, glutathione and sucrose decreased the % inhibition by Cd with the more prominent effect with glutamine and sucrose. Methionine sulfoximine exerted a more prominent effect for + Cd enzyme at lower concentration. The results indicate the involvement of reciprocal effects of Cd on glutamine synthetase and glutamate dehydrogenase activities and also on NADH- and Fd-glutamate synthase activities. For the inhibitory effect of Cd on glutamine synthetase activity,
. Glutamine, 2-oxoglutarate, glutathione and sucrose decreased the % inhibition by Cd with the more prominent effect with glutamine and sucrose. Methionine sulfoximine exerted a more prominent effect for + Cd enzyme at lower concentration. The results indicate the involvement of reciprocal effects of Cd on glutamine synthetase and glutamate dehydrogenase activities and also on NADH- and Fd-glutamate synthase activities. For the inhibitory effect of Cd on glutamine synthetase activity,  , glutamine, 2-oxoglutarate, glutathione and sucrose exerted a protective effect with the sucrose being most effective.
, glutamine, 2-oxoglutarate, glutathione and sucrose exerted a protective effect with the sucrose being most effective.
Keywords:
Glutamine Synthetase, Cd Effects, Ammonia Assimilation, Zea mays, Maize Leaves

1. Introduction
The environmental pollution with heavy metals is becoming an increasing problem in day to day life. The source of heavy metals in plant is the environment in which they grow and their growth medium (soil) from where heavy metals are then taken up by roots or foliage of plants. In general, heavy metal toxicity is attributed to binding of heavy metal to enzymes, resulting in inhibition and alteration of metabolism. Cadmium (Cd) is a common environmental contaminant introduced into the soil through anthropogenic activities. It is released into the environment by power stations, heating systems, metal-working industries, waste incinerations, urban traffic, cement factories and as a by-product of phosphate fertilizers [1] . Despite of being a non-essential element, it is readily taken up and accumulated by plants and increases the potential for contamination of the food chain [2] . The degree to which higher plants are able to take up Cd depends on its concentration in the soil and its bioavailability is modulated by the presence of organic matter, pH, redox potential, temperature and concentrations of other elements [1] . The toxic effects of Cd on plant growth and metabolism are well documented [3] .
Nitrogen is one of the nutrients that most commonly limit plant growth. Higher plants take up principally nitrate from the soil and reduce it to ammonium by the plant enzymes, nitrate reductase and nitrite reductase. Since 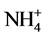 is toxic, it is rapidly assimilated into non toxic metabolites. The major route for ammonia assimilation involves sequential action of glutamine synthetase and glutamate synthase. Glutamine and glutamate produced by ammonia assimilation are donors for the biosynthesis of major nitrogen-containing compounds, including amino acids, nucleotides, chlorophylls, polyamines and alkaloids [4] . Glutamine synthetase (GS, EC 6.3.1.2) plays a central role in nitrogen metabolism of higher plants. It is responsible for the primary assimilation of ammonia in root cells, and for that of ammonia generated by nitrite reduction in chloroplasts. It also works in the assimilation of ammonia produced by nitrogen fixation in root nodules and in the reassimilation of ammonia released by photorespiration in leaf cells. In many plant species leaf cells contain two isoforms of GS, one in the cytosol (GS1) and the other in the chloroplast (GS2). Root nodules of legumes contain another type of enzyme, nodule specific GS. The accumulations of these isoforms are differentially regulated. Glutamate synthase (GOGAT) tend to control the glutamate level in the cell. The two forms of GOGAT found in higher plants use different electron donors: one is ferredoxin (Fd) specific (EC 1.4.7.1) and the other is reduced nicotinamide adenine dinucleotide (NADH) specific (EC 1.4.1.14) [5] [6] . Both the forms may coexist but found to predominate one over the other under different conditions. Thus, in rice leaves highest level of NADH-GOGAT protein and activity occurred in the youngest, non- green, unexpanded leaves but decreased with increasing leaf age and leaf expansion. In contrast, Fd-GOGAT protein and activity was highest in the full expanded, green leaf blades and lowest in the immature, non-green blades [7] . Glutamate dehydrogenase, (GDH, EC 1.4.1.2) plays a central role in linking C- and N- metabolism [8] [9] . Reductive amination catalysed by the enzyme serve as alternate route for ammonia assimilation particularly under stressful conditions [10] [11] . Cd effects on ammonia assimilation enzymes have been analysed in some of the plants, such as, oat plants [12] , roots and nodules of soyabean plants [13] , tomato seedlings [14] and Solanum nigrum leaves [15] , but the effects were found to vary depending upon the enzyme, plant tissue and its nutritional and environmental condition. In the present study, Cd effect on ammonia assimilation enzymes in excised etiolated maize leaf segments during greening were studied with an emphasis on the key enzyme, GS, to analyse the mechanistic aspects of inhibitory effects due to Cd.
is toxic, it is rapidly assimilated into non toxic metabolites. The major route for ammonia assimilation involves sequential action of glutamine synthetase and glutamate synthase. Glutamine and glutamate produced by ammonia assimilation are donors for the biosynthesis of major nitrogen-containing compounds, including amino acids, nucleotides, chlorophylls, polyamines and alkaloids [4] . Glutamine synthetase (GS, EC 6.3.1.2) plays a central role in nitrogen metabolism of higher plants. It is responsible for the primary assimilation of ammonia in root cells, and for that of ammonia generated by nitrite reduction in chloroplasts. It also works in the assimilation of ammonia produced by nitrogen fixation in root nodules and in the reassimilation of ammonia released by photorespiration in leaf cells. In many plant species leaf cells contain two isoforms of GS, one in the cytosol (GS1) and the other in the chloroplast (GS2). Root nodules of legumes contain another type of enzyme, nodule specific GS. The accumulations of these isoforms are differentially regulated. Glutamate synthase (GOGAT) tend to control the glutamate level in the cell. The two forms of GOGAT found in higher plants use different electron donors: one is ferredoxin (Fd) specific (EC 1.4.7.1) and the other is reduced nicotinamide adenine dinucleotide (NADH) specific (EC 1.4.1.14) [5] [6] . Both the forms may coexist but found to predominate one over the other under different conditions. Thus, in rice leaves highest level of NADH-GOGAT protein and activity occurred in the youngest, non- green, unexpanded leaves but decreased with increasing leaf age and leaf expansion. In contrast, Fd-GOGAT protein and activity was highest in the full expanded, green leaf blades and lowest in the immature, non-green blades [7] . Glutamate dehydrogenase, (GDH, EC 1.4.1.2) plays a central role in linking C- and N- metabolism [8] [9] . Reductive amination catalysed by the enzyme serve as alternate route for ammonia assimilation particularly under stressful conditions [10] [11] . Cd effects on ammonia assimilation enzymes have been analysed in some of the plants, such as, oat plants [12] , roots and nodules of soyabean plants [13] , tomato seedlings [14] and Solanum nigrum leaves [15] , but the effects were found to vary depending upon the enzyme, plant tissue and its nutritional and environmental condition. In the present study, Cd effect on ammonia assimilation enzymes in excised etiolated maize leaf segments during greening were studied with an emphasis on the key enzyme, GS, to analyse the mechanistic aspects of inhibitory effects due to Cd.
2. Materials and Methods
2.1. Plant material and Treatment
Seeds of Zea mays L., cv. Ganga safed-2 were surface sterilized with 0.1% HgCl2 for 1 - 2 min and then washed thoroughly with distilled water. Seedlings were raised in small plastic pots containing acid washed sand in continuous dark for 7 - 8 d at 25˚C ± 3˚C. They were watered on alternate days with half strength Hoagland’s solution, which was modified to exclude nitrogen. For various treatments, primary leaves from uniformly grown seedlings were cut into about 0.5x0.5 cm2 pieces and floated on 1/4th strength Hoagland’s solution (pH 6.0) containing the desired compounds for 24 h in continuous light of intensity about 40 W∙m−2 at 25˚C ± 2˚C. Treated material was used for enzymatic analyses.
2.2. Analytical Procedures
Glutamine synthetase was extracted from the treated material in cold using pestle and mortar in the presence of a pinch of sand and the extraction medium, 0.1 M potassium phosphate buffer pH 7.8 containing 0.4 M sucrose, 10 mM DTT, 10 mM KCl, 1 mM MgCl2 and 10 mM EDTA. The ratio of leaf material to extraction medium was 1:4 (w/v). The crude homogenate of the enzyme was desalted by running through a Sephadex G-25 column using the eluent buffer consisting of 1 M immidazole/HCl buffer, pH 7.0 and used for assay. Enzyme activity was assayed by the method described in Shapiro and Stadtman [16] . The reaction mixture contained 1.0 ml 1 M Imidazole-HCl buffer; 2.5 ml 0.12 M ATP solution; 2.0 ml 2 M L-Glutamate; 1.0 ml 1 M NH4Cl solution; 0.6 ml 1.67 M MgCl2; 2.9 ml distilled water. For assay 0.2 ml of the reaction mixture, 0.1 ml of distilled water and 0.1 ml of enzyme preparation was incubated at 37˚C for 10 minutes followed by the addition of 1.8 ml of 29 mM FeSO4 prepared in 0.3 N H2SO4 to stop the reaction and 0.15 ml of the coloring reagent, 5.3 mM ammonium molybdate prepared in 7.5 N H2SO4. The blank was prepared by reversing the sequence of the addition of enzyme and FeSO4 solution. The absorbance of blue colored solution was measured at 660 nm against blank. One unit of enzyme activity was defined as μmoles Pi formed min−1 g−1 fr. wt.
For Fd-GOGAT analysis the extraction and assay was carried out by the method of Hecht et al. [17] . NADH-GDH and NADH-GOGAT were extracted as per method described in Duke and Ham [18] . The crude extract was desalted by using Sephadex G-25 column. The activities of desalted preparations were measured spectrophotometrically by monitoring the change in absorbance at 340 nm according to the method of Duke and Ham [18] . To calculate specific activity of the enzymes, the protein content of the preparations were determined by the method of Lowry et al. [19] after precipitation with 10 % TCA followed by dissolving the ppt in 0.1 N NaOH.
2.3. Data Analysis
Data presented in the paper are average of at least four independent experiments with ± S.E. Significance of difference obtained for various treatments was tested by student’s t test and p values denoted are: *p < 0.05, **p < 0.01, ***p < 0.001.
3. Results
3.1. Cd Effects on Ammonia Assimilation Enzymes
Supply of 0.1 to 0.5 mM CdCl2 in the presence of 5 mM NH4NO3 to excised etiolated maize leaf segments during greening affected NADH-GDH activity in a concentration dependent manner being marginally reduced at 0.1 mM, remained unaffected at 0.2 mM, but increased at 0.5 mM (Table 1). The specific activity of the enzyme remained unaffected at lower Cd concentration, but increased gradually at higher concentrations. Cd exerted opposing effects on NADH-GOGAT activity being decreased with increasing concentrations of Cd (Table 1). The specific activity remained unaffected at 0.1 mM Cd, while decreased at 0.2 and 0.5 mM Cd being more significant for the later.
Supply of 0.1 to 0.5 mM CdCl2 to excised etiolated maize leaf segments during greening increased substantially the total as well as specific activity of NADH- GDH with the increase in specific activity being more pronounced than total activity (Table 2). Also Cd treatment increased both the total and specific activities of NADH-GOGAT with the more prominent effect at 0.2 mM Cd (Table 2).
Supply of 0.5 mM CdCl2 in the presence of 5 mM NH4NO3 to excised etiolated maize leaf segments during greening decreased the total and specific GS activity by 44 % and 33%, respectively (Table 3). While treatment with 0.5 mM Cd increased the total as well as specific activity of Fd-GOGAT with the effect being more prominent for the later (Table 3).
Table 1. Effect of Cd on NADH-GOGAT and NADH-GDH activities in the presence of NH4NO3 in excised greening maize leaf segments. Leaf segments from dark grown maize seedlings were treated with 1/4th strength Hoagland’s solution containing 5 mM NH4NO3 and the desired concentration of CdCl2 in continuous light for 24 h at 25 ± 2˚C. Values relative to control are given in parentheses.
Table 2. Effect of Cd on NADH-GDH and NADH-GOGAT activities in excised greening maize leaf segments. Leaf segments from dark grown maize seedlings were treated with 1/4th strength Hoagland’s solution containing the desired concentration of CdCl2 in continuous light for 24 h at 25 ± 2˚C. Values relative to control are given in parentheses.
Table 3. Effect of Cd on GS and Fd-GOGAT activities in the presence of NH4NO3 in excised greening maize leaf segments. Leaf segments from dark grown maize seedlings were treated with 1/4th strength Hoagland’s solution containing 5 mM NH4NO3 and the desired concentration of CdCl2 in continuous light for 24 h at 25˚C ± 2˚C. Values relative to control are given in parentheses.
3.2. Effects of Key Compounds on GS Inhibition by Cd
Supply of inorganic nitrogenous compounds during Cd treatment to excised etiolated maize leaf segments affected the inibitory effect on GS activity. Thus, 10 mM KNO3 decreased the enzyme activity significantly in the presence of 0.5 mM CdCl2 and thereby increasing the % inhibition prominently (Table 4). With the inclusion of 10 mM NH4Cl, GS activity was found to be reduced to same extent in the absence as well as presence of Cd, thus % inhibition remained unaffected (Table 4(a)). Supply of 5 mM NH4NO3 decreased the GS activity in Cd treated material causing more of % inhibition. When along with 5 mM NH4NO3, 10 mM KNO3 and 10 mM NH4Cl were also included during treatment, the inhibitory effect of Cd was reduced in both (Table 4).
Varying effects of metabolites, such as, glutamine, glutamate, 2-oxoglutarate (2-OG), reduced glutathione (GSH) and sucrose were observed in relation to inhibition of GS activity by cadmium. Thus, inclusion of 5 mM glutamine along with 5 mM NH4NO3, increased the GS activity in the presence of 0.5 mM CdCl2 and thereby decreased strongly the inhibitory effect of Cd (Table 5). Addition of 5 mM glutamate increased the enzyme activity in the absence of Cd to greater extent causing more of % inhibition. When 5 mM each of 2-OG, GSH and sucrose were included, the enzyme activity was increased in the absence as well as presence of Cd with the increase being more prominent for later (Table 5). The % inhibition of enzyme activity by Cd was also reduced in presence of these metabolites with more prominent effect with GSH and Sucrose.
In vitro inclusion of the competitive inhibitor of GS, methionine sulfoximine
Table 4. Effect of inorganic nitrogenous compounds on inhibition of GS activity by Cd in excised greening maize leaf segments. Leaf segments from dark grown maize seedlings were treated with 1/4th strength Hoagland’s solution containing the desired nitrogenous compound in the absence and presence of 0.5 mM CdCl2 in continuous light for 24 h at 25˚C ± 2˚C. Values relative to control are given in parentheses.
Table 5. Effect of some metabolites on GS activity in the absence and presence of Cd in excised greening maize leaf segments. Leaf segments from dark grown maize seedlings were treated with 1/4th strength Hoagland’s solution containing 5 mM NH4NO3 and the desired metabolites at conc. 5 mM each either in the absence or presence of 0.5 mM CdCl2 in continuous light for 24 h at 25˚C ± 2˚C. Values relative to control are given in parentheses.
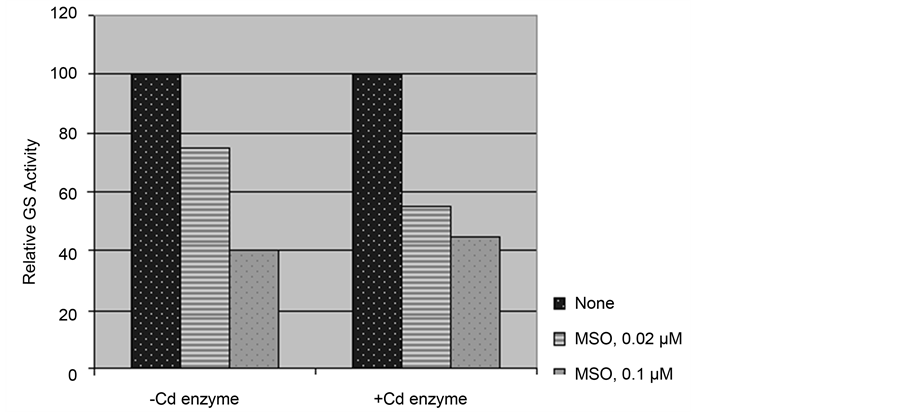
(MSO) at concentrations 0.02 and 0.1 µM during assay of enzyme activity from Cd treated (+Cd enzyme) and untreated (−Cd enzyme) material caused significant inhibition in both (Figure 1). However, more prominent inhibitory effect was observed with +Cd enzyme at lower concentration of MSO.
4. Discussion
4.1. Reciprocal Regulation of Ammonia Assimilation Enzymes
The enzymes of ammonia assimilation are glutamine synthetase, glutamate synthase and glutamate dehydrogenase. Glutamine synthetase plays a central role in nitrogen metabolism of higher plants. GS is active in a variety of plant organs and functions to assimilate ammonia generated by a number of physiological processes. Analysing the effect of metallic toxicant, Cd, on GS activity in different plants have demonstrated varying effects. Thus, decrease in GS activity has been reported in nodules and roots of soybean plants [13] , tomato seedlings [14] and leaves of Solanum nigrum at high concentration [15] while it remained unaffected in oat [12] and leaves of Solanum nigrum at low concentration [15] . The
Figure 1. Effect of in vitro inclusion of MSO on glutamine synthetase activity of the enzyme preparations obtained from greening maize leaf segments treated without Cd (−Cd enzyme and with 0.5 mM CdCl2 (+Cd enzyme). Gutamine synthetase Activity of the enzyme preparations obtained from greening maize leaf segments treated without Cd (−Cd enzyme) and with 0.5 mM Cd (+Cd enzyme) was assayed with in vitro inclusion of 0.02 and 0.1 µM MSO during assay.
results of present study demonstrate an inhibitory effect of Cd in the presence of NH4NO3 on GS activity in excised etiolated maize leaf segments during greening, while increasing NADH-GDH activity (Table 1 and Table 3). Increased NADH-GDH activity during Cd supply only is also observed (Table 2). This suggests that Cd stress increases 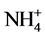 toxicity and to overcome it stress responsive GDH activity is increased. Increased GDH activity under Cd stress conditions has been reported in several systems [14] [15] [20] [21] [22] [23] . Further, NADH-GOGAT activities in the absence and presence of NH4NO3 are differently affected by Cd showing increase with former and decrease with later (Table 1 and Table 2). This indicates that NADH-GOGAT may be responsible for maintaining glutamate level under the condition of Cd toxicity in the absence of nitrogen. Increased NADH-GOGAT activity by Cd in tomato seedlings has also been reported [14] . Moreover, reciprocal effects for NADH-GOGAT activity in radish cotyledons under light and dark conditions have been reported, where it was increased by NH4NO3 in dark, but decreased in light [24] . Differential effects for Fd-GOGAT and NADH-GOGAT activities by Cd in the presence of NH4NO3 is observed showing increase for former and decrease for later (Table 1 and Table 3). Thus, it is likely that Fd-GOGAT is involved in maintaining glutamate level during Cd stress when supplied with inorganic nitrogen. However, Cd supply to tomato seedlings decreased Fd- GOGAT activity [14] .
toxicity and to overcome it stress responsive GDH activity is increased. Increased GDH activity under Cd stress conditions has been reported in several systems [14] [15] [20] [21] [22] [23] . Further, NADH-GOGAT activities in the absence and presence of NH4NO3 are differently affected by Cd showing increase with former and decrease with later (Table 1 and Table 2). This indicates that NADH-GOGAT may be responsible for maintaining glutamate level under the condition of Cd toxicity in the absence of nitrogen. Increased NADH-GOGAT activity by Cd in tomato seedlings has also been reported [14] . Moreover, reciprocal effects for NADH-GOGAT activity in radish cotyledons under light and dark conditions have been reported, where it was increased by NH4NO3 in dark, but decreased in light [24] . Differential effects for Fd-GOGAT and NADH-GOGAT activities by Cd in the presence of NH4NO3 is observed showing increase for former and decrease for later (Table 1 and Table 3). Thus, it is likely that Fd-GOGAT is involved in maintaining glutamate level during Cd stress when supplied with inorganic nitrogen. However, Cd supply to tomato seedlings decreased Fd- GOGAT activity [14] .
4.2. Role of Key Compounds on GS Inhibition by Cd
The inhibitory effect of Cd on GS activity seems to depend upon inorganic nitrogenous compound included. Thus, nitrate inclusion causes higher % inhibition of GS activity by Cd, while ammonium causes lower % inhibition and NH4NO3 causing an intermediary effect (Table 4). Hence, it is likely that Cd supply inhibits nitrate acquisition and/or assimilation severely thereby reducing ammonia assimilation. More severe inhibition of nitrate reductase activity compared to GS activity by Cd in leaves of Solanum nigrum has been demonstrated [15] . Moreover, % inhibition of activity by Cd is more with excess of nitrate compared to excess of ammonium (Table 4). Thus, Cd may also influence 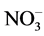 and
and 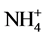 transporter activities to different extent and thereby inhibit GS activity differently. Greater sensitivity to Cd in relation to growth of nitrate-fed bean plants as compared to ammonium-fed plants has been observed [23] . Differential response of GS towards these nitrogenous compounds in light and dark has been reported in radish cotyledons [24] . In dark KNO3 inhibited it, while NH4Cl promoted it and NH4NO3 exerted intermediary effect. In light all the three sources significantly promoted it. Although the different isoforms of GS have not been analysed in the present study, possible involvement of these in differential effects of nitrogenous compounds is likely. Hence, it is worth to discuss the regulation of different isoforms of GS1 with the supply of inorganic nitrogen as reported in Arabidopsis [25] and rice roots [26] . In Arabidopsis GLN1:2 is significantly upregulated by ammonium and exhibited low affinity to ammonium, while GLN1:3 was not stimulated by ammonium but significantly inhibited by high concentration of glutamate. The high affinity form GLN1:1 accumulated during nitrogen starvation and was down regulated by ammonium excess [25] . In rice roots cytosolic GS1 isoform, OsGLN1:1 accumulated in nitrogen limited condition, while OsGLN1:2 was abundantly expressed under nitrogen sufficient conditions [26] . Further, NADH-GOGAT accumulation in root tips have been observed after ammonium treatment [27] [28] , which was identical in localization with OsGLN1:2. This indicated that the two enzymes cooperated to assimilate ammonia taken up by ammonium inducible forms of AMTs (OsAMT1:1 and OsAMT1:2) [29] .
transporter activities to different extent and thereby inhibit GS activity differently. Greater sensitivity to Cd in relation to growth of nitrate-fed bean plants as compared to ammonium-fed plants has been observed [23] . Differential response of GS towards these nitrogenous compounds in light and dark has been reported in radish cotyledons [24] . In dark KNO3 inhibited it, while NH4Cl promoted it and NH4NO3 exerted intermediary effect. In light all the three sources significantly promoted it. Although the different isoforms of GS have not been analysed in the present study, possible involvement of these in differential effects of nitrogenous compounds is likely. Hence, it is worth to discuss the regulation of different isoforms of GS1 with the supply of inorganic nitrogen as reported in Arabidopsis [25] and rice roots [26] . In Arabidopsis GLN1:2 is significantly upregulated by ammonium and exhibited low affinity to ammonium, while GLN1:3 was not stimulated by ammonium but significantly inhibited by high concentration of glutamate. The high affinity form GLN1:1 accumulated during nitrogen starvation and was down regulated by ammonium excess [25] . In rice roots cytosolic GS1 isoform, OsGLN1:1 accumulated in nitrogen limited condition, while OsGLN1:2 was abundantly expressed under nitrogen sufficient conditions [26] . Further, NADH-GOGAT accumulation in root tips have been observed after ammonium treatment [27] [28] , which was identical in localization with OsGLN1:2. This indicated that the two enzymes cooperated to assimilate ammonia taken up by ammonium inducible forms of AMTs (OsAMT1:1 and OsAMT1:2) [29] .
Inclusion of key metabolites of plant tissue during Cd treatment influences the inhibitory effect of Cd on GS activity. Glutamine, a key metabolite, may act as a regulator of overall nitrogen metabolism. It is required as a precursor of many amino acids, nucleic acids, alkaloids and polysaccharides, as well as secondary metabolites like polyamine [30] . Various amino acids (Asp, Asn, Glu, Gln) antagonizing the inductive effect of sucrose on GS has been shown in Arabidopsis [31] . In this study, Glutamine (Gln), one of the product of GS reaction exerted strong protective effect against Cd inhibition of GS activity (Table 5). Hence, it is likely that inhibitory effects of Gln/Cd are not sensed in presence of the other. So Cd may release such an effect of Gln resulting in increased GS activity in its presence. Another organic nitrogenous compound, Glu, one of the substrate of GS reaction caused stimulation of activity to lesser extent with Cd (Table 5). Further, MSO, a competitive inhibitor of GS more strongly inhibits the enzyme from Cd treated material (Figure 1) suggesting that glutamate binding to enzyme and its subsequent phosphorylation is affected by Cd. Moreover, in Medicago truncatula phosphorylation of GS1a mediated by Ca2+ independent kinases was found to increase the affinity of the enzyme for the substrate glutamate [32] .
Reduced glutathione is an important protectant against metal toxicity, may function as a precursor for phytochelatins or as an antioxidant. Decline in GSH level upon exposure to Cd in the tomato cell suspension culture [33] and maize seedlings [34] has been reported. In this study, the supply of GSH, a ubiquitous thiol being responsible for maintaining reducing environment within tissue also causes an increase in GS activity to greater extent in presence of Cd (Table 5). Thus, Cd toxicity appears to affect thiol groups of enzyme and/or reducing environment of the system. Alternatively, it may overcome Cd toxicity by mediating synthesis of heavy metal binding peptides, phytochelatins, which are involved in metal detoxification.
Importance of 2-oxoglutarate (2-OG) in higher plant ammonia assimilation has been reviewed by Lancien et al. [35] . Plant cell 2 OG level is dependent on inorganic and organic N content and their related metabolism as well as nitrate and sugar levels [36] [37] and thus could play a signaling role in coordination of C- and N- metabolism. In the present study, 2 OG, the TCA intermediate and the metabolite linking C- and N- metabolism, more prominently increases GS activity during Cd supply (Table 5). Thus, Cd stress may influence the availability of TCA intermediates and thereby affecting ATP production to inhibit GS activity. Further, Cd supply may affect the cytosolic form of GS, GS1, as 2-OG has been reported to induce specifically cytosolic GS1 in Arabidopsis [31] . Moreover, increased NADH-GDH activity during Cd stress (Table 2 and Table 3) seems to utilize more of 2OG reducing its level in the system. Other important carbon compound and also the photosynthate, sucrose, significantly increased the GS activity with a more profound effect in the presence of Cd (Table 5). Such a stimulation suggest reduced photosynthetic C- assimilation during Cd toxicity. Further, dramatic induction of chloroplastic GS2 resulted by light in Arabidopsis [31] and sucrose supply mimicked the light effect partly. Hence it is likely that GS2 isoform of the enzyme is affected to greater extent with the supply of Cd.
5. Conclusion
Supply of metallic toxicant, Cd in the presence of NH4NO3 decreased the GS and NADH-GOGAT activities, while increased the Fd GOGAT and NADH-GDH activities. Percent inhibition of GS activity caused by Cd is influenced by the inclusion of inorganic nitrogenous compounds, 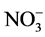 causing enhancement, but
causing enhancement, but 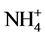 lowering it. Amongst the key metabolites tested, Gln, 2-OG, GSH and sucrose exerted a protective effect towards inhibition of GS by Cd with Gln and sucrose being more effective. The results suggest the involvement of Glu binding to the enzyme, thiol groups of the enzyme and/or disrupting C metabolism to mediate Cd effects on GS activity.
lowering it. Amongst the key metabolites tested, Gln, 2-OG, GSH and sucrose exerted a protective effect towards inhibition of GS by Cd with Gln and sucrose being more effective. The results suggest the involvement of Glu binding to the enzyme, thiol groups of the enzyme and/or disrupting C metabolism to mediate Cd effects on GS activity.
Abbreviations
GS―Glutamine synthetase; NADH―Nicotinamide adenine dinucleotide; Fd― Ferredoxin; MSO―Methionine sulfoximine; GOGAT―glutamate synthase. GDH― Glutamate dehydrogenase.
Cite this paper
Sarangthem, J., Dhamgaye, S. and Gadre, R. (2017) Cadmium Effects on Enzymes of Ammonia Assimilation in Excised Etiolated Maize Leaf Segments during Greening: A Mechanistic Approach. American Journal of Plant Sciences, 8, 1399-1410. https://doi.org/10.4236/ajps.2017.86095
References
- 1. Sanita di Toppi, L. and Gabbrielli, R. (1999) Response to Cadmium in Higher Plants. Environmental and Experimental Botany, 41, 105-130.
- 2. Prasad, M.N.V. (1995) Cadmium Toxicity and Tolerance in Vascular Plants. Environmental and Experimental Botany, 35, 525-545.
- 3. Pal, M., Horvath, E., Janda, T., Paldi, E. and Szalai (2006) Physiological Changes and Defense Mechanisms Induced by Cadmium Stress in Maize. Journal of Plant Nutrition and Soil Science, 169, 239-246.
https://doi.org/10.1002/jpln.200520573 - 4. Coruzzi, G. and Last, R. (2000) Amino Acids. In: Buchanan, B., Gruissem, W. and Jones, R., Eds., Biochemistry and Molecular Biology of Plants, American Society of Plant Biologists, Rockville, 358-410.
- 5. Oaks, A. and Hirrel, B. (1985) Nitrogen Metabolism in Roots. Annual Review of Plant Physiology, 36, 345-365.
https://doi.org/10.1146/annurev.pp.36.060185.002021 - 6. Lea, P.J., Robinson, S.A. and Stewart, G.R. (1990) The Enzymology and Metabolism of Glutamate, Glutamine and Asparagine In: Miflin, B.J. and Lea, P.J., Eds., The Biochemistry of Plants, Amino Acids and Derivatives, Vol. 16, Academic Press, New York, 121-159.
- 7. Yamaya, T., Hayakawa, T., Tanasawa, K., Kamachi, K., Mae, T. and Ojima, K. (1992) Tissue Distribution of Glutamate Synthase and Glutamine Synthetase in Rice Leaves: Occurrence of NADH-Dependent Glutamate Synthase Protein and Activity in the Unexpanded Non-Green Leaf Blades. Plant Physiology, 100, 1427-1432.
https://doi.org/10.1104/pp.100.3.1427 - 8. Miyashita, Y. and Good, A.G. (2008) NAD(H)-Dependent Glutamate Dehydrogenase Is Essential for the Survival of Arabidopsis thaliana during Dark-Induced Carbon Starvation. Journal of Experimental Botany, 59, 667-680.
https://doi.org/10.1093/jxb/erm340 - 9. Labboun, S., Therese, T.L., Roscher, A., Bedu, M., Restivo, F.M., Velanis, C.N., Skopelitis, D.S., Moshou, P.N., Roubelakis-Angelakis, K.A., Suzuki, A. and Hirel, B. (2009) Resolving the Role of Plant Glutamate Dehydrogenase I in Vivo Real Time Nuclear Magnetic Resonance Spectroscopy Experiments. Plant Cell Physiology, 50, 1761-1773.
https://doi.org/10.1093/pcp/pcp118 - 10. Srivastava, H.S. and Singh, R.P. (1987) Role and Regulation of L-Glutamate Dehydrogenase Activity in Higher Plants. Phytochemistry, 28, 597-610.
- 11. Skopelitis, D.S., Paranychianakis, N.V., Paschalidis, K.A., Pliakonis, E.D., Delis, I.D., Yakoumakis, D.L., Kouvarakis, A., Papadakis, A.K., Stephanou, E.G. and Roubelakis-Angelakisa, K.A. (2006) Abiotic Stress Generates ROS That Signal Expression of Anionic Glutamate Dehydrogenases to Form Glutamate for Proline Synthesis in Tobacco and Grapevine. Plant Cell, 18, 2767-2781.
https://doi.org/10.1105/tpc.105.038323 - 12. Astolfi, S., Zuchi, S. and Passera, C. (2004) Effect of Cadmium on the Metabolic Activity of Avena sativa Plants Grown in Soil or Hydroponic Culture. Biologia Plantarum, 48, 413-418.
https://doi.org/10.1023/B:BIOP.0000041095.50979.b0 - 13. Balestrasse, K.B., Benavides, M.P., Gallego, S.M. and Tomaro, M.L. (2003) Effect of Cadmium Stress on Nitrogen Metabolism in Nodules and Roots of Soybean Plants. Functional Plant Biology, 30, 57-64.
https://doi.org/10.1071/FP02074 - 14. Chaffei, C., Pageau, K., Suzuki, A., Gouia, H., Ghorbel, M.H. and Masclaux-Daubresse, C. (2004) Cadmium Toxicity Induced Changes in Nitrogen Management in Lycopersicon esculentum Leading to a Metabolic Safeguard through an Amino Acid Storage Strategy. Plant Cell Physiology, 45, 1681-1693.
https://doi.org/10.1093/pcp/pch192 - 15. Wang, L., Zhou, Q., Ding, L. and Sun, Y. (2008) Effect of Cadmium Toxicity on Nitrogen Metabolism in Leaves of Solanum nigrum L. as a Newly Found Cadmium Hyperaccumulator. Journal of Hazardous Material, 154, 818-825.
- 16. Shapiro, B.M. and Stadtman, E.R. (1970) Metabolism of Amino Acids and Amines. In: Tabor, H. and Tabor, C.W., Eds., Methods in Enzymology, Vol. 17, Academic Press, New York, 910-922.
- 17. Hecht, U., Oclmuller, R., Schmidt, S. and Mohr, H. (1988) Action of Light, Nitrate and Ammonium on the Levels of NADH- and Ferredoxin-Dependent Glutamate Synthases in the Cotyledons of Mustard Seedlings. Planta, 175, 130-138.
https://doi.org/10.1007/BF00402890 - 18. Duke, S.H. and Ham, G.E. (1976) The Effect of Nitrogen Addition on N2-Fixation and on Glutamate Dehydrogenase and Glutamate Synthase Activities in Nodules and Roots of Soybeans Inoculated with Various Strains of Rhizobium japonicum. Plant Cell Physiology, 17, 1037-1044.
- 19. Lowry, O.H., Rosenbrough, N.J., Farr, A.L. and Randall, R.J. (1951) Protein Measurement with the Folin Phenol Reagent. Journal of Biological Chemistry, 193, 265-275.
- 20. Jain, M. and Gadre, R. (1998a) Effect of Cadmium on Glutamate Dehydrogenase and Glutamate Synthase in Excised Greening Bean Leaf Segments. Proceedings of National Academy of Sciences, 68, 307-309.
- 21. Jain, M. and Gadre, R. (1998b) Effect of Cadmium on NADH-Glutamate Dehydrogenase and NADH-Glutamate Synthase Activities in Excised Bean Leaf Segments: Role of Glutathione. Indian Journal of Experimental Biology, 36, 625-627.
- 22. Chaffei, C., Masclaux-Daubresse, C., Gouia, H. and Ghorbel, M.H. (2006) Purification of Glutamate Dehydrogenase Isoenzymes from Control and Cadmium Treated Tomato Leaf. In: Samiullah, N., Ed., Cadmium Toxicity and Tolerance in Plants, Narosa Publishing House, New Delhi, 137-156.
- 23. Houda, G., Chiraz, C., Mohamed, D. and Habib, G.M. (2008) Differential Toxicological Response to Cadmium Stress of Bean Seedlings Grown with NO3+ or NH4+ as Nitrogen Source. International Journal of Botany, 4, 14-23.
https://doi.org/10.3923/ijb.2008.14.23 - 24. Sood, C.R., Chandra, S.V. and Singh, Y.D. (2002) Effect of Different Nitrogen Sources and Plant Growth Regulators on Glutamine Synthetase and Glutamate Synthase Activities of Radish Cotyledons. Bulgerian Journal of Plant Physiology, 28, 46-56.
- 25. Ishiyama, K., Inoune, E., Watanabe-Takahasi, A., Obaras, M., Yamaya, T. and Takahashi, H. (2004) Kinetic Properties and Ammonium-Dependent Regulation of Cytosolic Isoenzymes of Glutamine Syntetase in Arabidopsis. Journal of Biological Chemistry, 279, 16598-16605.
https://doi.org/10.1074/jbc.M313710200 - 26. Ishiyama, K., Inoue, E., Tabuchi, M., Yamaya, T. and Hideki, T. (2004b) Biochemical Background and Compartmentalized Functions of Cytosolic Glutamine Synthetase for Active Ammonium Assimiation in Rice Roots. Plant Cell Physiology, 45, 1640-1647.
https://doi.org/10.1093/pcp/pch190 - 27. Ishiyama, K., Hayakawa, T. and Yamaya, T. (1998) Expression of NADH-Dependent Glutamate Synthase Protein in the Epidermis and Exodermis of Rice Roots in Response to the Supply of Ammonium Ions. Planta, 204, 288-294.
https://doi.org/10.1007/s004250050258 - 28. Ishiyama, K., Kojima, S., Takahasi, H., Hayakawa, T. and Yamaya, T. (2003) Cell Type Distinct Accumulations of mRNA and Protein for NADH-Dependent Glutamate Synthetase in Rice Roots in Response to the Supply of NH4+. Plant Physiology Biochemistry, 41, 643-647.
- 29. Sonoda, Y., Ikeda, A., Saiki, S., Von Wiren, N., Yamaya, T. and Yamaguchi, J. (2003) Distinct Expression and Function of Three Ammonium Transporter Genes (OsAMT1; 1-1; 3) in Rice. Plant Cell Physiology, 44, 726-734.
https://doi.org/10.1093/pcp/pcg083 - 30. Bagh, K., Hiraoki, T., Thorpe, T.A. and Vogel, H.J. (2004) Nitrogen-15 NMR Studies of Nitrogen Metabolism in Picea glauca Buds. Plant Physiology and Biochemistry, 42, 803-809.
- 31. Oliveira, I.C. and Coruzzi, G.M. (1999) Carbon and Amino Acids Reciprocally Modulate the Expression of Glutamine Synthetase in Arabidopsis. Plant Physiology, 121, 301-309.
https://doi.org/10.1104/pp.121.1.301 - 32. Lima, L., Seabra, A., Melo, P., Cullimure, J. and Crvalho, H. (2006) Post-Translational Regulation of Cytosolic Glutamine Synthetase of Medicago truncatula. Journal of Experimental Botany, 57, 2751-2761.
https://doi.org/10.1093/jxb/erl036 - 33. Scheller, H.V., Huang, B., Hatch, E. and Goldsbrough, P.B. (1987) Phytochelatin Synthesis and Glutathione Levels in Response to Heavy Metals in Tomato Cells. Plant Physiology, 85, 1031-1035.
https://doi.org/10.1104/pp.85.4.1031 - 34. Rauser, L. and Ackerly, C.A. (1987) Localization of Cadmium in Granule within Differentiating and Mature Root Cells. Canadian Journal of Botany, 65, 643-646.
https://doi.org/10.1139/b87-084 - 35. Lancien, M., Gadal, P. and Hodges, M. (2000) Enzyme Redundancy and the Importance of 2-Oxoglutarate in Higher Plant Ammonium Assimilation. Plant Physiology, 123, 817-824.
https://doi.org/10.1104/pp.123.3.817 - 36. Scheible, W.R., Gonzalez-Fontes, A., Lauerer, M., Muller-Robert, B. and Stitt, M. (1997) Nitrate Acts as a Signal to Induce Organic Acid Metabolism and Repress Starch Metabolism in Tobacco. Plant Cell, 9, 789-798.
https://doi.org/10.1105/tpc.9.5.783 - 37. Morquende, R., Krapp, A., Hurry, V. and Stitt, M. (1998) Sucrose Feeding Leads to Increased Rates of Nitrate Assimilation, Increased Rates of 2-Oxoglutarate Synthesis, and Increased Synthesis of a Wide Spectrum of Amino Acids in Tobacco Leaves. Planta, 206, 394-409.
https://doi.org/10.1007/s004250050415