American Journal of Plant Sciences
Vol.5 No.1(2014), Article ID:42065,6 pages DOI:10.4236/ajps.2014.51017
The Application of K Phosphites to Seed Tubers Enhanced Emergence, Early Growth and Mycorrhizal Colonization in Potato (Solanum tuberosum)
1Laboratorio de Fisiología Vegetal, Unidad Integrada Balcarce (FCA-UNMdP/INTA), Balcarce, Argentina; 2Laboratorio de Microbiología de Suelos, Unidad Integrada Balcarce (FCA-UNMdP/INTA), Balcarce, Argentina; 3INBIOTEC-CONICET, Mar del Plata, Argentina; 4Instituto de Investigaciones Biológicas, CONICET-UNMdP, Mar del Plata, Argentina; 5McCain Argentina SA, Balcarce, Argentina; 6Corporate Agronomy McCain Foods Ltd., Balcarce, Argentina.
Email: *gdosio@mdp.edu.ar
Copyright © 2014 Cecilia Tambascio et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2014 are reserved for SCIRP and the owner of the intellectual property Cecilia Tambascio et al. All Copyright © 2014 are guarded by law and by SCIRP as a guardian.
Received November 15th, 2013; revised December 15th, 2013; accepted January 4th, 2014
KEYWORDS
Seed Tubers; Leaf Area; Dry Matter; Cultivar Kennebec; Cultivar Shepody
ABSTRACT
Rapid emergence and a vigorous growth prevent the seed tubers from infections by soil microbes and allow a rapid interception of solar radiation. In this work, the effect of the potassium phosphites (KPhi) applied to seed tubers of two potato cultivars on crop emergence and early growth was studied. Two experiments were performed under greenhouse and field conditions. Emergence of plants, leaf area, dry matter and the number of primary stems were measured in both experiments. Furthermore, mycorrhizal colonization was also measured on roots under field conditions. The application of KPhi reduced the period between planting and emergence, and increased leaf area and dry matter. The ratio between dry matter of aerials and underground organs was not affected by KPhi. Indigenous mycorrhizal colonization increased after KPhi application to seed tubers. These results confirm the benefit of the application of KPhi to seed tubers on early plant growth and suggest that their application in crop production would be advantageous.
1. Introduction
A rapid emergence of potato plants allows a rapid increase of intercepted radiation and crop growth preventing infections or damage of seed tubers caused by soil microbes and insects [1]. A vigorous sprout growth before emergence is also helpful to overcome soil mechanical resistance [2]. Phosphites (Phi) applied alone, or combined with Ca, K, Al, Mn, Mg, Zn or S, could protect plants against diseases [3]. They could exert both direct (inhibition of mycelium growth, alterations in membrane metabolism, and reduction in zoospore production [4,5]) and/or indirect (activation of plant defense responses [6,7]) mode of actions against diseases. The use of Phi to manage foliar potato diseases is broadly extended in developing countries [8] and positive effects of the application of KPhi to seed tubers on plant emergence and the number of stems have been already reported in Argentina [9].
Some attempts indicate that introduction of arbuscular mycorrhizal fungi (AMF) could be a strategy to optimized micro-tuber production and thus improve potato growth [10,11]. However, results about Phi’s effects on mycorrhizal colonization in roots have been conflicting [12,13] mainly due to a probable inhibitory effect of Phi on high-affinity phosphate transporters of AMF expression [14]. Moreover, although potato is an important staple food and horticultural crop in Argentina and worldwide [15], to our knowledge there is no information regarding the effect of the Phi application to the seed tubers on early potato growth and mycorrhizae formation. Then, the aim of this work was to study the effect of the application of KPhi to seed tubers on the early growth and mycorrizae formation of potato plants.
2. Materials and Methods
A greenhouse experiment (Exp. 1) was carried out at the McCain Experimental Station, Balcarce, Argentina (37˚50'S; 58˚11'W, 115 m altitude). Certified seed tubers from the commercial cultivars Shepody and Kennebec were planted in 60 cylindrical plastic pots with 5885 cm3 capacity on 8 July 2008. The substrate was a vermiculite (5%), humus (20%), peat (40%) and organic matter (35%) mixture. Air temperature was maintained above 15˚C (daily mean) with the assistance of two heating units strategically located within the greenhouse.
A field experiment (Exp. 2) was carried out during the 2009/10 growing season at the INTA-Balcarce Experimental Station, Argentina (37˚45'S; 58˚18'O, 130 m altitude). Certified seed tubers from the commercial cultivars Shepody and Kennebec, were planted manually on 21 October 2009 in a typical Argiudol soil, in plots consisting of 4 rows, 5 m long, 0.85 m between rows. Plant density was 5.8 plants m−2.
Both experiments were conducted without water or nutrition deficit. Weeds and insects were adequately controlled by a combination of chemical and/or mechanical practices.
The following treatments were applied to seed tubers immediately after cutting with a manual spray pack (Knapsack Style Manual Sprayer, 15,000 cm3) using a pressure of 0.3 to 0.4 MPa:
1) KPhi at 3000 cm3.ha-1 (Afital K®, AgroEmcodi, S.A.);
2) Control (C): 3000 cm3.ha-1 of water.
A plant was considered emerged when the first leaf was fully expanded. Measurements were performed when the first plot achieved 100% (Exp. 1) or 90% (Exp. 2) of emerged plants. Emergence was measured in 15 or 50 plants, in Exp. 1 and Exp. 2, respectively.
Measurements of leaf area, dry matter and number of primary stems were performed on 9 plants per treatment, 52 (Exp. 1) and 60 days after planting (Exp. 2).
Leaf area was determined as the sum of all green leaflets of each leaf of the plant. Leaf area in Exp. 1 was assessed with an area meter (Li3000, Li Cor Inc., Lincoln, NE, USA), and in Exp. 2 with a linear adjustment obtained from the length and width of each leaflet in Exp. 1 for each cultivar (P ≤ 0.0001). Dry matter per plant was determined as the sum of dry matter of leaves, stems, roots and tubers, dried in a stove at 60˚C until constant weight. Leaf area and dry matter were expressed per primary stem. The ratio below to above ground dry matter was calculated as the quotient between the sum of the dry roots and tubers, and the sum of the dry stem and leaves. A stem was considered as primary when it appeared directly from below ground. In Exp 2, roots were separated from the soil by dry sieving (2 mm) and stained with a trypan blue (0.05%) in distilled water-acid lactic-glycerol (1:1:1) solution according to the modification of the Phillips and Hayman method [16], in which the phenol reagent has been omitted. Arbuscular mycorrhizal colonization (AMC) and the proportion of arbuscules (A) on potato roots were quantified by the Trouvelot et al. method [17].
A completely randomized design with 3 and 4 replications was used for Exp. 1 and Exp. 2, respectively. The experimental unit was 5 pots, or 1 plot (17 m2 surface), in Exp. 1 and Exp. 2, respectively. Results were analyzed for significance by the analysis of variance, and means were compared by Tukey’s test at P ≤ 0.05 level of significance [18].
3. Results and Discussion
3.1. Emergence
The application of KPhi to seed tubers increased emergence of plants (%) in both cultivars and experiments (P ≤ 0.05, Figure 1).
In both experiments, the effect was greater in cultivar Kennebec than in Shepody (48% vs. 12%, average Exp. 1 and Exp. 2, Kennebec and Shepody, respectively). An interaction cultivar × treatment was detected in Exp. 1 (P = 0.0008). Working under greenhouse conditions with the same cultivars Lobato et al. [9] also found a benefit in the emergence of plants after application of KPhi or CaPhi to seed tubers. The effect of KPhi could be related to carbohydrate partition where stem growth was favored in detriment of root growth, and thus accelerating emergence.
3.2. Leaf Area
Leaf area increased with the application of KPhi to seed tubers in all tested situations with the only exception of cultivar Shepody in Exp. 2 (P ≤ 0.05, Figure 2). In Exp. 1, leaf area of KPhi treated plants was 15% and 6% higher than C plants, for cultivars Shepody (P ≤ 0.05) and Kennebec (P ≤ 0.05), respectively (Figure 2(a)). In Exp. 2, an interaction cultivar × treatment was detected (P = 0.003) and greatest difference was found in cultivar Kennebec (75% higher in KPhi than C, P ≤ 0.05, Figure 2(b)).
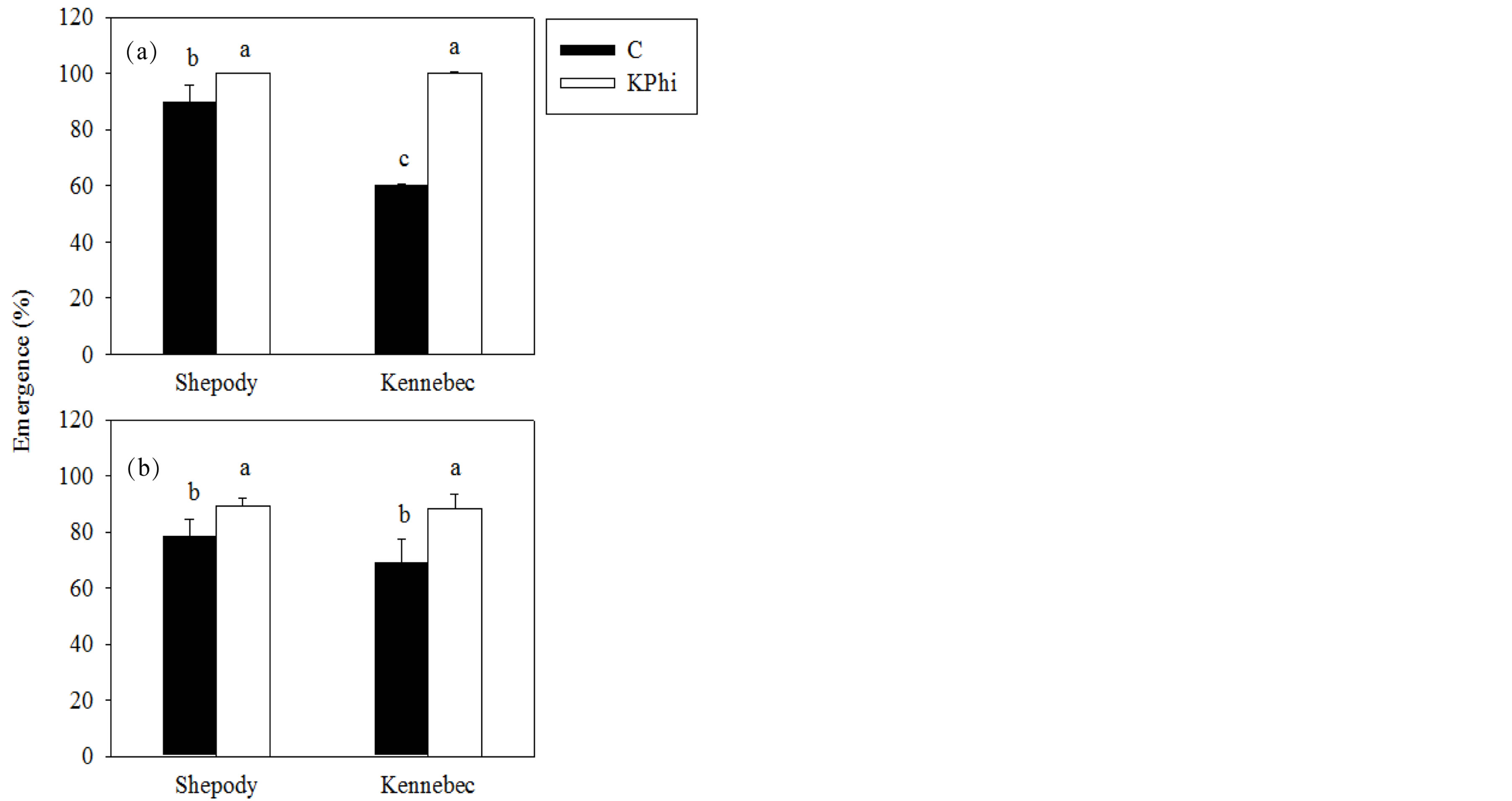
Figure 1. Emergence (%) in Exp. 1 (a) and Exp. 2 (b), in cultivars Shepody and Kennebec. Treatments were: KPhi (white) or C (black). Vertical lines on bars represent the standard error. Different letters indicate significant differences among treatments and/or cultivars (P ≤ 0.05).
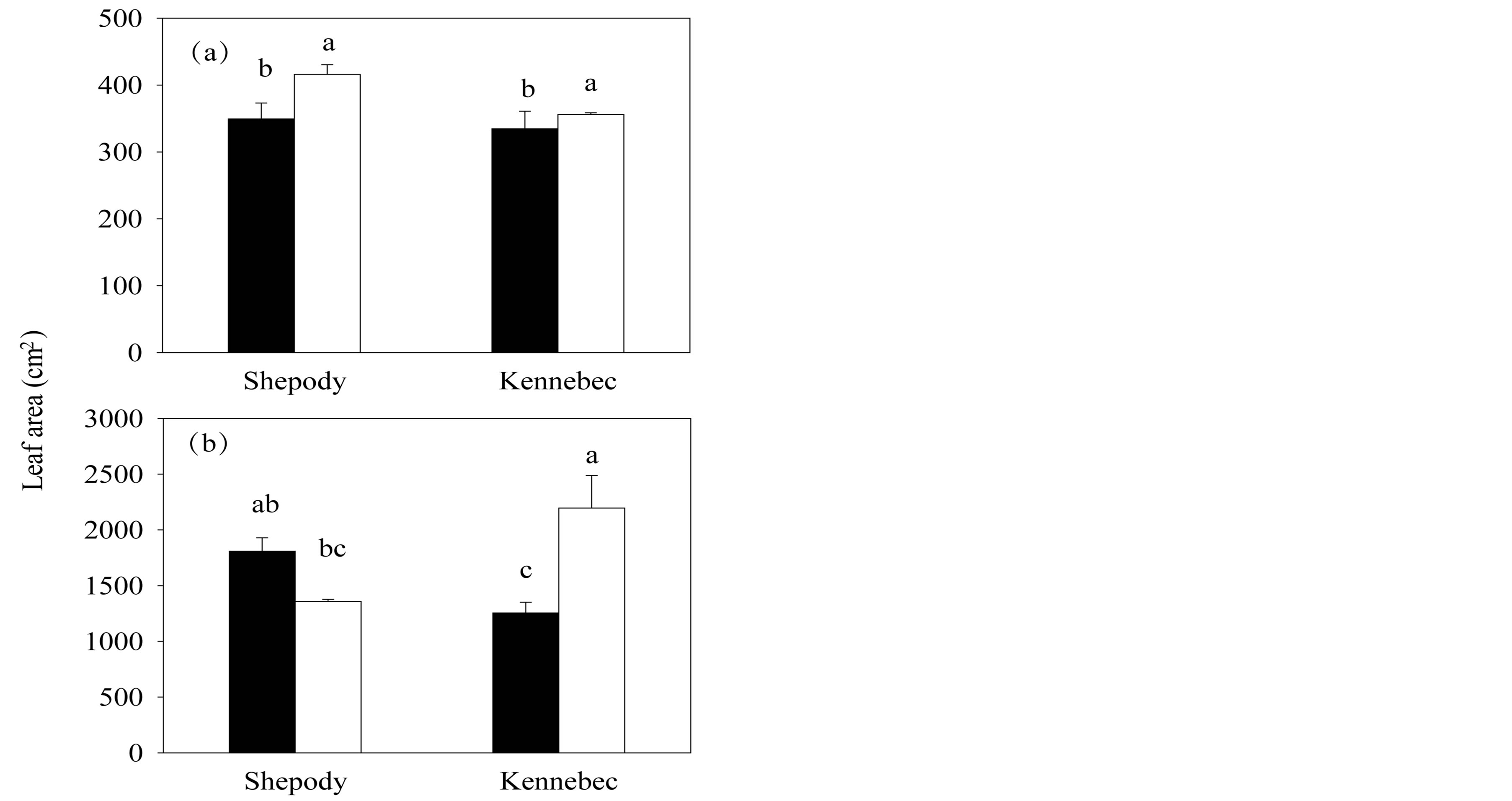
Figure 2. Leaf area per primary stem in Exp. 1 (a) and Exp. 2 (b), in cultivars Shepody and Kennebec. Same treatments as Figure 1. Vertical lines on bars represent the standard error. Different letters indicate significant differences among treatments and/or cultivars (P ≤ 0.05).
3.3. Dry Matter
Total dry matter increased around 25% after application of KPhi in most cases with the exception of cultivar Shepody in Exp. 2 (P < 0.05, Table 1). Differences in cultivar Kennebec were mostly the consequence of higher dry matter accumulation in leaves (13% and 35%, Exp. 1 and Exp. 2, respectively), stems (46% and 18%) and roots (16% and 50%), while in Shepody during Exp. 1 an increase in dry matter was only observed in leaves (19%) and roots (52%) (P ≤ 0.05, Table 1). An interaction cultivar x treatment was detected in Exp. 2 for this variable (P = 0.0006). As seen, plants from KPhi treatment which presented higher leaf area also produced higher dry matter than control plants, probably as a consequence of a greater intercepted radiation. McDonald et al. [19] also reported a benefit of KPhi on growth of potatoes plants related to an effect on physiological functions. In our study, differences in total dry matter never included differences in dry matter of tubers. This was probably the consequence of an early evaluation which could prevent to trigger the enhance effect. An evidence of this could be observed in Exp. 1, where even not significant the application of KPhi increased dry matter of tubers, 31% and 60% in Shepody and Kennebec, respectively (P = 0.2727, Table 1).
3.4. Ratio Below to Aboveground Dry Matter
The application of KPhi to seed tubers did not affect the ratio below to aboveground dry matter in both cultivars and experiments (P > 0.05, Table 1). In Exp. 1, a significant effect of the cultivar was observed for this variable and cultivar Shepody showed a ratio 59% higher than Kennebec (P = 0.005). Partially in opposition to our results, Thao et al. [20] reported a decreased in this ratio in hydroponic spinach culture treated with KPhi applied to foliage and where Pi (inorganic phosphorus) was scarce in the soil. Although plants with Pi deficiency usually increase more the root system than aerial organs, the opposite was observed after KPhi application in tomato [14] and Arabidopsis [21]. In our work, the root weight was mostly increased as a consequence of the application of KPhi to seed tubers.
3.5. Number of Primary Stems
Depending on the cultivar and/or the growing conditions, the application of KPhi to seed tubers increased, decreased or did not affect the number of primary stems (Table 1). In both experiments, an interaction cultivar x treatment was detected (P ≤ 0.05). Under certain conditions or cultivars, carbohydrates could be prioritized to differentiation and growth of primary stems instead of roots growth. Differences in partitioning of dry weight and carbon translocation to different organs could be modified by environmental conditions as high temperatures or photoperiod [22]. A different number of
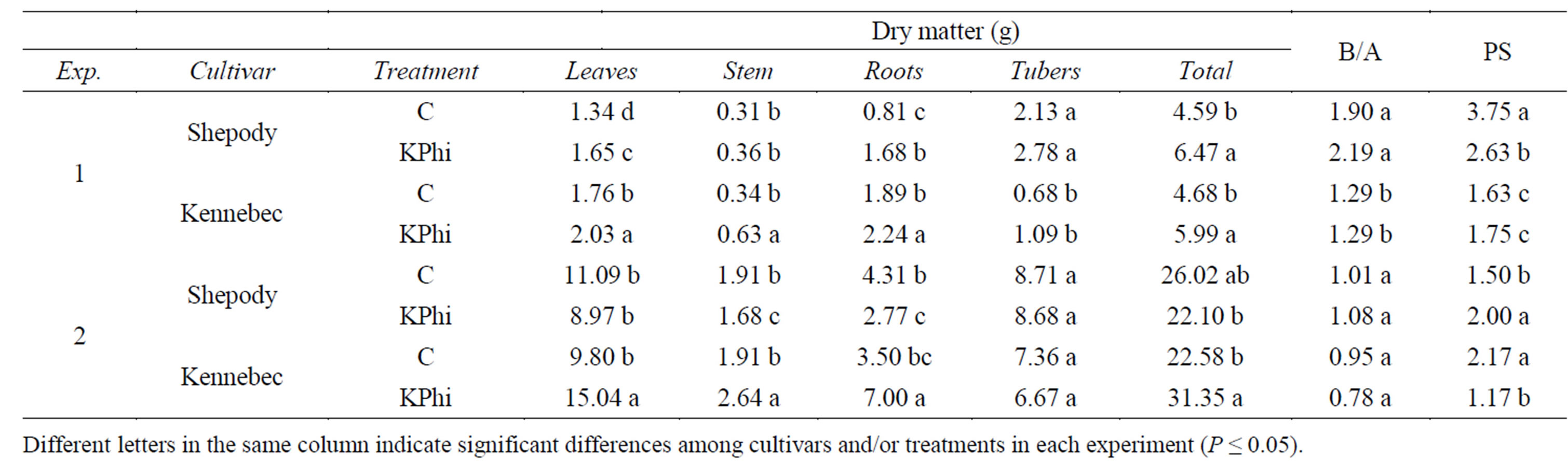
Table 1. Total dry matter and partition to leaves, stem, roots and tubers per primary stem, ratio below to above ground dry matter (B/A) and number of primary stems (PS) measured in Exp. 1 (Greenhouse) and Exp. 2 (Field), in cultivars Shepody and Kennebec. See treatments applied to seed tubers at Figure 1.
stems between experiments as was observed in Shepody, could be related to the size of seed tubers as reported in literature [23,24]. Genotypic differences could explain that in Kennebec this effect was not found.
3.6. Mycorrhizal Colonization
The application of KPhi to seed tubers increased arbuscular mycorrhizal colonization and the proportion of arbuscules of the roots in both cultivars. Differences of more than 300% were observed in both variables (P ≤ 0.05, Figure 3). In opposition to previous reports, in our work, the KPhi application to seed tubers not only did not inhibit [25] or did not affect [12,13] mycorrhiza formation, but also stimulated it. Mycorrhizal colonization was lower than expected, however, within values found in literature [10,11]. These results are, to our knowledge, the first contributions on the degree of indigenous mycorrhizal colonization of potato in Argentina and its relation to the application of Phi to seed tubers.
4. Conclusion
The application of KPhi to seed tubers enhanced emergence of plants, leaf area and early dry matter in most studied situations. These results could be partially the consequence of a stimulated arbuscular mycorrhizal colonization after KPhi application to seed tubers. After these results, we have scientific evidence to propose the application of KPhi to seed tubers in crop production.
Funding
This work was supported by Universidad Nacional de Mar del Plata, Instituto Nacional de Tecnología Agropecuaria, Agencia Nacional de Promoción Científica y Tecnológica and McCain Argentina SA. Thanks are also due to McCain Corporate Agriculture for partial funding this research.
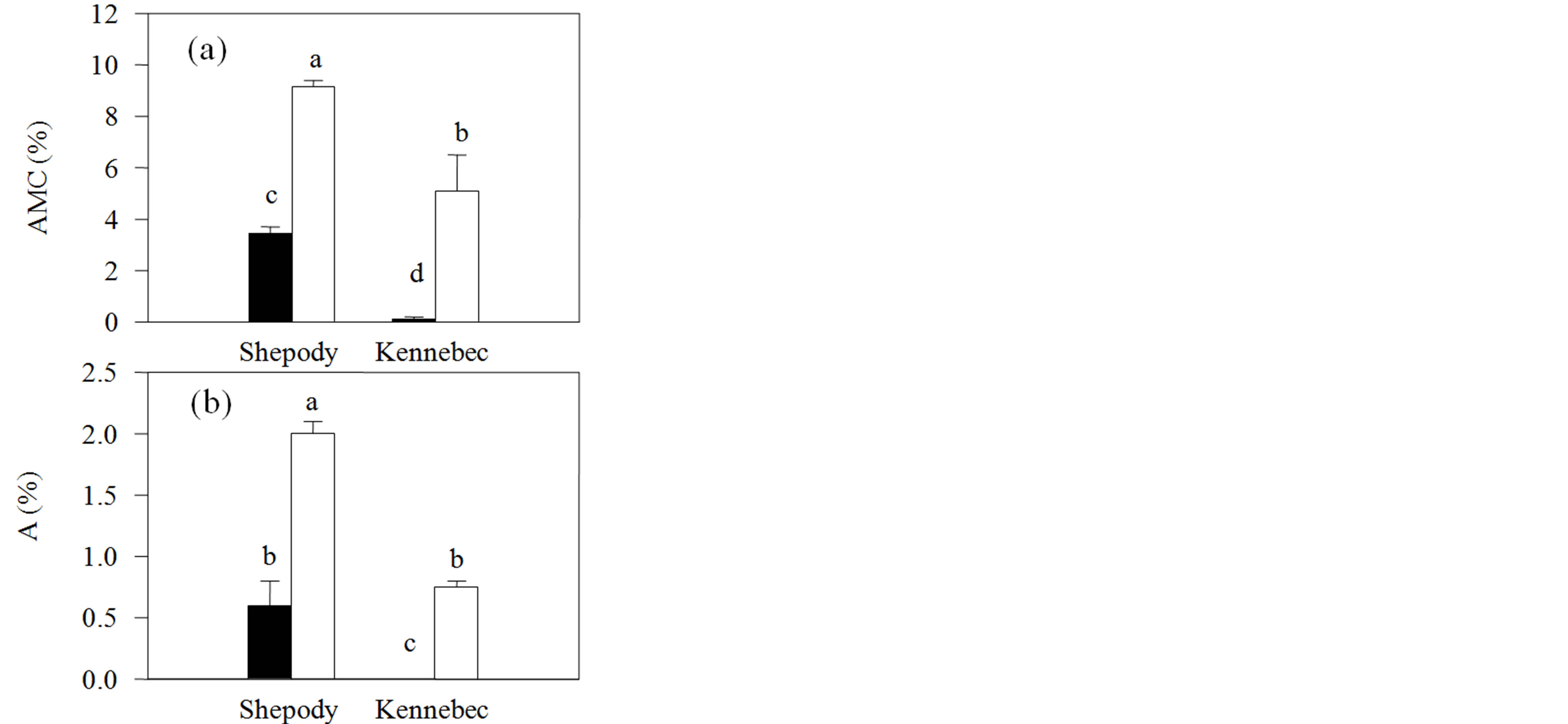
Figure 3. Indigenous mycorrhizal colonization of roots in Exp. 2 (Field) in cultivars Shepody and Kennebec. (a) Intensity of arbuscular mycorrhizal colonization (AMC); (b) Proportion of arbuscules (a). Same treatments as Figure 1. Vertical lines on bars represent the standard error. Different letters indicate significant differences among treatments and/or cultivars (P ≤ 0.05).
Acknowledgements
Part of these results was taken from the CT thesis of Magister Scientiae. AA, FC, MCL and GD are members of the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), the research council of Argentina. Silvio Giuliano and Carlos Antonelli helped with the field experiment.
REFERENCES
- L. Mustonem, “Yield Formation and Quality Characteristics of Early Potatoes during a Short Growing Period,” Agricultural and Food Science, Vol. 13, No. 4, 2004, pp. 390-398. http://dx.doi.org/10.2137/1239099043633314
- A. G. Taylor and C. W. Ten Broeck, “Seedling Emergence Forces of Vegetable Crops,” Horticultural Science, Vol. 23, No. 2, 1988, pp. 367-369.
- C. J. Lovatt and R. L. Mikkelsen, “Phosphite Fertilizers: What Are They? Can You Use Them? What Can They Do?” Better Crops, Vol. 90, No. 4, 2006, pp. 11-13.
- D. Guest and B. Grant, “The Complex Action of Phosphonates as Antifungal Agents,” Biological Reviews, Vol. 66, No. 2, 1991, pp. 159-187. http://dx.doi.org/10.1111/j.1469-185X.1991.tb01139.x
- M. C. Lobato, F. P. Olivieri, G. R. Daleo and A. B. Andreu, “Antimicrobial Activity of Phosphites against Different Potato Pathogens,” Journal of Plant Diseases and Protection, Vol. 117, No. 3, 2010, pp. 102-109.
- R. Smillie, B. R.Grant and D. Guest, “The Mode of Action of Phosphite: Evidence for Both Direct and Indirect Modes of Action on Three Phytophthora spp. in Plants,” Phytopathology, Vol. 79, No. 9, 1989, pp. 921-926. http://dx.doi.org/10.1094/Phyto-79-921
- M. C. Lobato, M. F. Machinandiarena, C. Tambascio, G. A. A. Dosio, D. O. Caldiz, G. R. Daleo, A. B. Andreu and F. P. Olivieri, “Effect of Foliar Applications of Phosphite on Post-Harvest Potato Tubers,” European Journal of Plant Pathology, Vol. 130, No. 2, 2011, pp. 155-163. http://dx.doi.org/10.1007/s10658-011-9741-2
- P. Kromann, W. G. Pérez, A. Taipe, E. Schulte-Geldermann, B. P. Sharma, J. L. Andrade-Piedra and G. A. Forbes, “Use of Phosphonate to Manage Foliar Potato Late Blight in Developing Countries,” Plant Disease, Vol. 96, No. 7, 2012, pp. 1008-1015. http://dx.doi.org/10.1094/PDIS-12-11-1029-RE
- M. C. Lobato, F. P. Olivieri, E. A. González Altamiranda, E. A. Wolski, G. R. Daleo, D. O. Caldiz and A. B. Andreu, “Phosphite Compounds Reduce Disease Severity in Potato Seed Tubers and Foliage,” European Journal of Plant Pathology, Vol. 122, No. 3, 2008, pp. 349-358. http://dx.doi.org/10.1007/s10658-008-9299-9
- D. D. Douds, G. Nagahashi, C. Reider and P. R. Hepperly, “Inoculation with Arbuscularmycorrhizal Fungi Increases the Yield of Potatoes in a High P Soil,” Biological Agriculture and Horticulture, Vol. 25, No. 1, 2007, pp. 67-78.
- Y. Cheng, D. Bay, L. Sun, F. Feldmann and G. Feng, “Utilization of Arbuscularmycorrhizal Fungi during Production of Micropropagated Potato Solanumtuberosum,” In: F. Feldmann and Y. B. J. Kapulnik, Eds., Mycorrhiza Works, Deutsche Phytomedizinische Gesellschaft, Braunschweig, 2008, pp. 165-178.
- K. Howard, B. Dell and G. E. Hardy, “Phosphite and Mycorrhizal Formation in Seedlings of Three Australian Myrtaceae,” Australian Journal of Botany, Vol. 48, No. 6, 2000, pp. 725-729. http://dx.doi.org/10.1071/BT00007
- N. Sukarno, F. A. Smith, E. S. Scott, G. P. Jones and S. E. Smith, “The Effect of Fungicides on Vesicular-Arbuscular Mycorrhizal Symbiosis. III. The Influence of VA Mycorrhiza on Phytotoxic Effects Following Application of Fosetyl-Al and Hosphonate,” New Phytologist, Vol. 139, No. 2, 1998, pp.321-330. http://dx.doi.org/10.1046/j.1469-8137.1998.00204.x
- D. K. Varadarajan, A. S. Karthikeyan, P. D. Matilda and K. G. Raghothama, “Phosphite, an Analog of Phosphate, Suppresses the Coordinated Expression of Genes under Phosphate Starvation,” Plant Physiology, Vol. 129, No. 3, 2002, pp. 1232-1240. http://dx.doi.org/10.1104/pp.010835
- G. Kessel, M. Huarte, F. Lucca, M. Santini, C. Rijzebol, P. Raatjes, J. Rovers, J. Den Boer and H. Schepers, “Opportunities for Potato Late Blight DSS’s in Argentina,” Proceedings Twelfth Euro Blight Workshop Arras, France, 2010. http://www.euroblight.net/Workshop/2010Arras/Proceedings/Page75_78_Kessel_web.pdf
- J. M. Phillips and D. S. Hayman, “Improved Procedures for Clearing Roots and Staining Parasitic and Vesicular Arbuscular-Mycorrhizal Fungi for Rapid Assessment of Infection,” Transactions of the British Mycological Society, Vol. 55, No. 1, 1970, pp. 158-161. http://dx.doi.org/10.1016/S0007-1536(70)80110-3
- A. Trouvelot, J. L. Kough and V. Gianinazzi-Pearson, “Mesure du Taux de Mycorhization VA d’un Systemeradiculaire,” In: V. Gianinazzi-Pearson and S. Gianinazzi, Eds., Physiological and Genetical Aspects of Mycorrhizae, Proceedings of the 1st European Symposium on Mycorrhizae, INRA, Paris, 1986, pp. 217-221.
- SAS Institute Inc. “SAS/STAT® 9.2. User’s Guide,” 2nd Edition, SAS Institute Inc., Cary, 2009.
- A. E. McDonald, B. R. Grant and W. C. Plaxton, “Phosphite (Phosphorous Acid): Its Relevance in the Environment and Agriculture, and Influence on the Plant Phosphate Starvation Response,” Journal of Plant Nutrition, Vol. 24, No. 10, 2001, pp. 1505-1519. http://dx.doi.org/10.1081/PLN-100106017
- H. T. B. Thao, T. Yamakawa, A. K. Myint and P. S. Sarr, “Effects of Phosphite, a Reduced form of Phosphate, on the Growth and Phosphorus Nutrition of Spinach (Spinaciaoleracea L.)”, Japanese Society of Soil Science and Plant Nutrition, Vol. 54, No. 5, 2008, pp. 761-768. http://dx.doi.org/10.1111/j.1747-0765.2008.00290.x
- C. A. Ticconi, C. A. Delatorre and S. Abel, “Attenuation of Phosphate Starvation Responses by Phosphite in Arabidopsis,” Plant Physiology, Vol. 127, No. 3, 2001, pp. 963-972. http://dx.doi.org/10.1104/pp.010396
- S. Wolf, A. Marani and J. Rudich, “Effects of Temperature and Photoperiod on Assimilate Partitioning in Potato Plants,” Annals of Botany, Vol. 66, No. 5, 1990, pp. 513- 520.
- W. Bohl, S. Love and A. Thompson, “Effect of Seed Piece Removal on Yield and Agronomic Characteristics of Russet Burbank Potatoes,” American Journal of Potato Research, Vol. 78, No. 6, 2001, pp. 397-402. http://dx.doi.org/10.1007/BF02896370
- L. Güllüoglu and H. Arioglu, “Effects of Seed Size and In-Row Spacing on Growth and Yield of Early Potato in a Mediterranean-Type Environment in Turkey,” African Journal of Agricultural Research, Vol. 4, No. 5, 2009, pp. 535-541.
- N. P. Seymour, J. P. Thompson and M. L. Fiske, “Phytotoxicity of Fosetyl-Al and Phosphonic Acid to Maize during Production of Vescicular-Arbuscular Mycorrhizal Inoculum,” Plant Disease, Vol. 78, No. 5, 1994, pp. 441- 446. http://dx.doi.org/10.1094/PD-78-0441
NOTES
*Corresponding author.

