American Journal of Plant Sciences
Vol.4 No.1(2013), Article ID:27645,5 pages DOI:10.4236/ajps.2013.41017
Phenolic Compounds Influence Seed Dormancy of Palicourea rigida H.B.K. (Rubiaceae), a Medicinal Plant of the Brazilian Savannah*
![]()
1Departamento de Horticultura, Universidade Estadual Paulista Julio de Mesquita Filho, Botucatu, Brazil; 2National Center for Natural Products Research, School of Pharmacy, The University of Mississippi, Oxford, USA; 3Center for Water and Wetland Resources, The University of Mississippi, Abbeville, USA; 4Departamento de Biotecnologia de Plantas Medicinais, Universidade de Ribeirão Preto, Ribeirão Preto, Brazil.
Email: #apereira@unaerp.br
Received October 4th, 2012; revised November 9th, 2012; accepted December 17th, 2012
Keywords: Cerrado; Douradinha; Medicinal Plant; Conservation
ABSTRACT
Palicourea rigida H.B.K. (Rubiaceae), a medicinal species commonly known as douradinha, has wide distribution across ecosystems in Central and South America. This species exhibits seed dormancy delaying germination until optimal conditions for seedling growth and development are in place. While dormancy ensures species survival, it also presents a technical problem for developing P. rigida’s plant production program. Thus, the objective of this study was to investigate if secondary metabolites present in seeds influence the seed dormancy of P. rigida. Mature fruits were harvested from the native habitat, in the savanna region of the State of Minas Gerais during February 2009, 2010 and 2011. The content of phenolic compounds in the seed of P. rigida was measured, and the allelopathic effects were assessed using the germination of lettuces as model to detect phytotoxicity. The P. rigida seeds geminated at rates varying between 7% and 31% with a Seed Germination Index (SGI) of 0.09. Data suggest that the phenolic compounds present in the seeds may be responsible for seed dormancy.
1. Introduction
The majority of medicinal plants commercialized in Brazil are collected from wild populations [1]. This practice threats species diversity, promotes exhaustion of natural resources, while it lacks safety assurance to consumers. Conservation of medicinal plants is an important task that promotes species’ sustainability, maintains healthy ecosystems while ensures economic support for rural populations.
There are little genetic materials of medicinal species in genbanks. Many plants with great economic value for the local communities present horticultural problems associated with undesirable dormancy that have not been studied. Detailed information on seed physiology includeing type of seed dormancy is lacking. Such information is requisite for conservation initiatives to preserve medicinal plants. Palicourea rigida H.B.K. (Rubiaceae) is one of these medicinal plants native to the Brazilian savannah, also known as Cerrado that need conservation actions.
Seed dormancy prevents germination of viable seed for a certain period of time despite of favorable environmental conditions [2,3]. Breaking dormancy occur by changing the balance of growth inhibitors such as abscisic acid (ABA) and phenolic compounds to growth promoters such as gibberellins, ethylene and brassinosteroids [4]. Germination delays due to dormancy may maximize seedling survival. Thus, a wide range of dormancy mechanisms evolved in response to climate and habitats diversity of each species [5]. Forest recovery through germination of dormant seeds does not follow the speed of which habitat erosion occurs. This is happening to the Cerrado areas where P. rigida is native.
According to Rodrigues and Carvalho [6], P. rigida is part of the traditional medicine in the Cerrado areas for the treatment of kidney diseases and female genital tract’s inflammation. A number of bioactive compounds have been isolated from P. rigida including the flavonoids quercetin 3-O-β-D-glucoside, quercetin-3-O-sophoroside and isorhamnetin-3-glucoside [7], and the iridoid monoterpene loganin [8,9].
In addition to P. rigida’s pharmacological role in the traditional medicine of the Cerrado, the species plays a significant role in maintaining the savannah’s biodiversity. Unfortunately, data have shown that P. rigida is particularly vulnerable to variations in temperature. According to Siqueira [10], it could undergo extinct if global warming temperature increases in the region of 1.8˚C - 2˚C across the planet. Thus, the study evaluates seed germination, the role of phenolic compounds present in seeds of P. ridiga and their allelopathic effects.
2. Materials and Methods
2.1. Seed Collection and Storage
Mature fruits of P. rigida were collected in situ in the savannah region of the State of Minas Gerais, Brazil (latitude 17˚49'16.7''; longitude 47˚46'08.2''; altitude 940 m) during the months of February 2009, 2010 and 2011. Selected seeds (Figure 1) were stored in Kraft paper bags at 26˚C.
Seeds (collected in 2009) were germinated in three different types of substrate, namely, the commercial substrate BioplantÒ (Bioplant Agrícola, Nova Ponte, MG, Brazil), a mixture of the commercial substrate and sand (1:1, w/w) or sand alone. Germinator box containing substrate were seeded and later incubated in a MA1403/ UR germination chamber (Marconi Equipamentos para Laboratório, Piracicaba, SP, Brazil) at 27˚C with 70% relative humidity and 16 h photoperiod provided by 40 W fluorescent lamps. Daily irrigation was provided manually. To establish the first P. rigida trial on seed germination each treatment had 25 seeds per replicate, and four repetitions per treatment with 100 seeds per treatment planted in a complete randomized design. The numbers of emerged seedlings were recorded daily during a period of 6 months based on cotyledon opening [11]; the numbers were recorded as percentage of germination, following by the speed of germination index (SGI) [12]. Data was analysed by analysis of variance (ANOVA), and the mean values were compared using the Scott-Knott test at the 5% significance level. All statistical analyses were performed using SISVAR software [13].
2.2. Chemical and Mechanical Scarification
Seeds collected in 2009 were chemically scarified using sulphuric acid 98% for 5, 10, 40, 80, 120 and 160 min, then rinsed with distilled water and later planted on Bioplant substrate to determine the germination rate. The mechanical scarification procedure consisted of sanding the seeds with a stone disc (#220) connected to a high speed rotary tool (Dremel® MultiPro™; Robert Bosch GmbH, Gerlingen, Germany), followed, if appropriate, by 24 h immersion in water under constant agitation at 100 rpm. Scarified seeds were sown on Bioplant substrate and germination rates were assessed. The experimental design and statistical analyzes were similar to the description above.
2.3. Effects of Gibberellic Acid (GA3) on Germination of P. rigida
Mechanically scarified and non-scarified seeds were immersed in solutions containing 0.1, 1.0 or 10.0 mg/L of GA3. Following each of the treatments, seeds were sown on Bioplant substrate and germination rates were recorded daily. Non-treated seeds, with and without scarification, were used as controls. The experimental design and statistical analyzes were similar to item 2.1.

Figure 1. Seeds of Palicourea rigida.
2.4. Warm and Cold Stratification
Seeds of the 2009 harvest were stored at 26˚C and at 5˚C (warm and cold stratification, respectively) for 12 months. After the storage period, seeds were sown on Bioplant substrate and germination rates were recorded. The fresh harvested seeds were used as control for this trial. The experimental design and statistical analyzes were similar to item 2.1.
2.5. Chemical Analysis of P. rigida Seeds
Chemical analysis were done on three types of seed extracts: seeds harvested in 2009 submitted to warm and cold stratification, seeds collected in 2010 that were stored at 26˚C, and seeds freshly collected in 2011.
Seeds of P. rigida were dried and ground to a fine powder, and extracted with 3 mL of methanol: water (1:1, v/v) for 45 min at room temperature under sonication to extract and quantify loganin. Following the method described by Morel et al. (2011) to quantify loganin, 1 mL aliquot was removed, filtered through a filter membrane (0.45 µm) and analysed by reverse-phase high performance liquid chromatography (RP-HPLC). A Shimadzu (Kyoto, Japan) chromatograph equipped with a Supelco™ (Sigma-Aldrich, St. Louis, MO, USA) C18 column (250 × 4.6 mm i.d.; 4.6 μm) and a photodiode array detector was used. The chromatographic conditions were mobile phase—50 min linear gradient of methanol (from 10% to 50%, v/v) in water and 0.1% acetic acid; flow rate—1 mL/min; injection volume—20 µL; detection wavelength—254 nm. Analyses ran in triplicates and the presence of loganin was assessed by comparison with the retention time of a pure commercial standard purchased from Fluka, St. Louis, MO, USA.
Total phenolics were determined by heating 5 mg of dried powdered seeds with 3 mL of methanol: water (1:1, v/v) to boiling point. An aliquot (500 μL) of the cooled and filtered extract was mixed with distilled water (7 mL) and Folin-Ciocalteau reagent (500 μL) under constant stirring for 1 min, following which saturated sodium carbonate solution (1 mL) was added to the mixture and the whole diluted to 10 mL with distilled water. After 30 min at room temperature, the absorbance of the mixture was determined at 730 nm. A calibration curve using tannic acid in the range of 1 - 5 μg/mL as the reference standard was done to analyze total phenolic content.
2.6. Phytotoxicity of P. rigida Seed Extract
Extracts of P. rigida seeds collected in 2009 stored at 5˚C or 26˚C for 24 months, seeds collected in 2010 that were stored at 26˚C for 12 months and freshly collected in 2011, were powdered and extracted with water at 90˚C (water bath) for 16 min [14]. Lettuce seeds were placed in Petri dishes (9 cm diameter) lined with germination paper that had been soaked with a volume of P. rigida extract, at a concentration of 5%, 10% or 15%, equivalent to 2.5-times the weight of the germination paper. The control treatment was filter paper soaked with distilled water. Each treatment had three repetitions of 50 lettuce seeds with 150 seeds per treatment. The percentage of germination was determined 7 days after the beginning of the experiment considering only the number of normal seedlings that emerged [11].
3. Results and Discussion
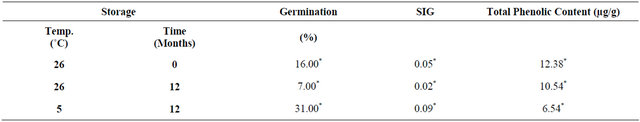
Scarified seeds by chemical and physical treatments, stratified seeds by cold and warm storage and GA3 application resulted in germination rates that varied between 16% and 23% (Figure 2(a)), and the SGI value was around 0.05. Seeds maintained their viability during a short-term by storing at 26˚C, whereas cold stratification treatment at 5˚C for 12 months was the best treatment for breaking dormancy, increasing germination to 31% and SGI to 0.09 (Table 1). Although, there was an improvement in breaking seed dormancy, the percentage of seed germinations remains low.
3.1. Chemical Analysis of P. rigida Seeds for Presence of Germination Inhibitors
Concentrations of loganin in freshly collected seeds and treated with warm and cold stratification were measured according to procedure described by Morel et al. [5]. Our
Table 1. Effect of storage temperature and timing on germination of Palicourea rigida.
*Means followed by the same letter within the column are not significantly different at 5% probability (Scott-Knott test).
 (a)
(a)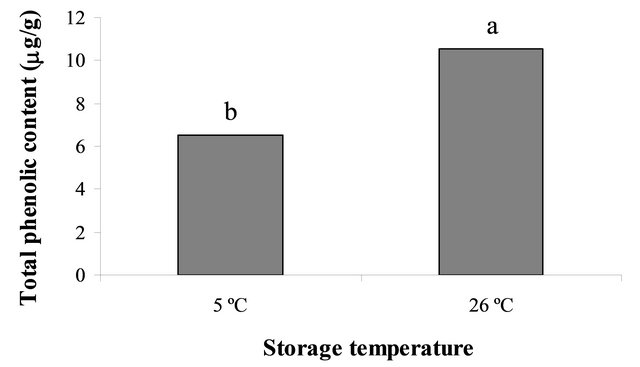 (b)
(b)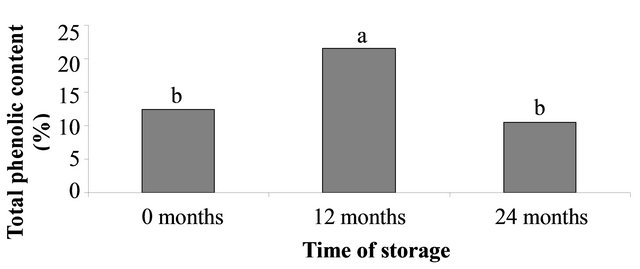 (c)
(c)
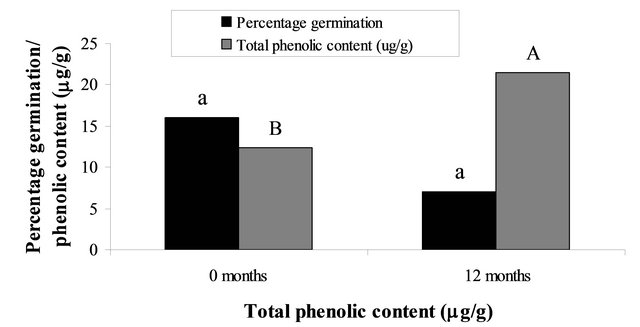
Figure 2. (a) Percentage seed germination Palicourea rigida of freshly collected seeds and after 12 months of storage at 26˚C. Total phenolic content in seeds that were freshly harvested and 12 month stored seeds; (b) Total phenolic content of seeds that had been stored at 5˚C or 26˚C for 24 months; and (c) Total phenolic content of freshly collected seeds and seeds that had been stored for 12 or 24 months at 26˚C. Within each panel, bars bearing dissimilar letters are significantly different at 5% probability (Scott-Knott test).
results reveal that loganin was not present in stratified seeds or freshly harvested (Data not shown). Shimomura et al. [15] found that iridoids present in seeds of Gardenia jasminoides, a species belonging to the Rubiaceae, were responsible for the species seed dormancy. While Pereira et al. [16] reported the alkaloids as inhibitors of germination in Coffea arabica seeds.
Phenolic compounds found in seeds are inhibitors of seed germination, reason for quantifying their content in P. rigida seeds. Data show that total phenolics present in seeds varied with time and storage temperature. It was higher in fresh seeds (21.50 mg/g) than in seeds stored at 26˚C for 12 months (12.38 mg/g). In seeds stored at 5˚C after 24 months the content was significantly lower (6.54 mg/g; P < 0.05) than that of seeds stored at 26˚C for the same length of time (Figure 2(b)). According to Willemsen and Rice [17], cold stratification influences the phenolic content of seeds, which regulates auxin synthesis in the embryo tissues, thus influencing the rate of seed germination.
Our results also indicate that total phenolic concentration in P. rigida seeds maintained at 26˚C in storage for 12 months was 2.04-fold higher than the seeds stored for 24 months and 1.74-fold higher than that of fresh seeds (Figure 2(c)). It appears that the total phenolic content increases after seed dispersal and degrades with time. Considering the latest classification of seed dormancy proposed by Baskin and Baskin [2] that includes physiological dormancy caused by chemical inhibitors, these results indicate that P. rigida seed dormancy may be the result of phenolic compounds found in the seeds. The increased concentration of phenolics following seed dispersal suggests that such compounds may be responsible for prolonging germination in seed banks.
3.2. Allelopathic Effects of P. rigida Seed Extract
L. sativa seeds imbibed on distilled water showed 74.78% ± 11.83% germination which the treatment used as positive control to determine the inhibition effects of P. rigida seeds extracts. Results of Table 2 show that application of P. rigida seeds extracts at 5% or 10% on L.
Table 2. Percentage of germination inhibition of Latuca sativa seeds treated with Palicourea rigida extracts of seeds stored under different temperatures and time of storage periods.
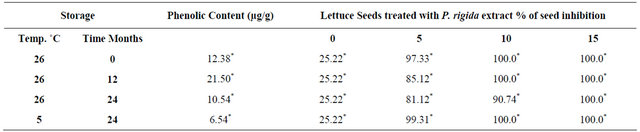
*Same lowercase and uppercase in the column are not significantly different at 5% probability (Scott-Knott test).
sativa seeds considerably reduced seed germination. Using P. rigida seeds extracts at 15% extract, seed inhibittion was complete According to Ali et al. [18], phenolics are the most common class of allelopathic compounds. These constituents are likely responsible for the allelopathic activity of seeds of P. rigida. Phenolic compounds reduce germination by inhibiting the activities of peroxidases that take part in the neutralisation of reactive oxygen species and in the oxidation of other phenolics, processes that are essential for breaking the hard seed coat and facilitating the emergence of the seedling [19].
4. Conclusion
The presence of phenolic compounds in seeds of P. rigida may be the actives responsible for the species deep physiological dormancy and seed viability preservation during short-term storage at 26˚C, whereas storage at 5˚C for 12 months may induced phenolic compounds degradation with dormancy release and the increase the percentage of germination and the SGI value.
REFERENCES
- J. G. Melo, E. L. C. Amorin and U. P. Albuquerque, “Native Medicinal Plants Commercialized in Brazil—Priorities for Conservation,” Environment Monitoring and Assessment, Vol. 156, No. 1-4, 2009, pp. 567-580. doi:10.1007/s10661-008-0506-0
- J. M. Baskin and C. C. Baskin, “A Classification System for Seed Dormancy,” Seed Science Research, Vol. 14, No. 1, 2004, pp. 1-16. doi:10.1079/SSR2003150
- C. C. Baskin and J. M. Baskin, “Seed Dormancy in Trees of Climax Tropical Vegetation Types,” Tropical Ecology, Vol. 46, No. 1, 2005, pp. 17-28.
- B. Kucera, M. A. Cohn and G. Leubner-Metzger, “Plant Hormone Interactions during Seed Dormancy Release and Germination,” Seed Science Research, Vol. 15, No. 4, 2005, pp. 281-307. doi:10.1079/SSR2005218
- W. E. Finch-Savage and G. Leubner-Metzger, “Seed Dormancy and the Control of Germination,” New Physiologist, Vol. 171, No. 3, 2006, pp. 501-523. doi:10.1111/j.1469-8137.2006.01787.x
- V. E. G. Rodrigues and D. A. Carvalho, “Etnobotanical Survey of Medicinal Plants in the Dominion of Meadows in the Region of the Alto Rio Grande—Minas Gerais,” Ciência e Agrotecnologia, Vol. 25, No. 2, 2001, pp. 102- 123.
- E. A. Rosa, B. C. Silva, F. M. Silva, C. M. A. Tanaka, R. M. Peralta, C. M. A. Oliveira, L Kato, H. D. Ferreira and C. C. Silva, “Flavonoids and Antioxidant Activity in Palicourea rigida Kunth, Rubiaceae,” Revista Brasileira de Farmacognosia, Vol. 20, No. 4, 2010, pp. 484-488. doi:10.1590/S0102-695X2010000400004
- S. Lopes, G. L. Von Poser, V. A Kerber, F. M. Farias, E. L. Konrath, P. Moreno, M. E. Sobral, J. A. S. Zuanazzi and A. T. Henriques, “Taxonomic Significance of Alkaloids and Iridoid Glucosides in the Tribe Psychotrieae (Rubiaceae),” Biochemical Systematics and Ecology, Vol. 32, No. 12, 2004, pp. 1187-1195. doi:10.1016/j.bse.2004.04.015
- L. J. F. Morel, D. M. Baratto, P. S. Pereira, S. H. T. Contini, H. G. Momm, B. W. Bertoni, S. C. França and A. M. S. Pereira, “ Loganin Production in Palicourea rigida H.B.K. (Rubiaceae) from Populations Native to Brazilian Cerrado,” Journal of Medicinal Plants Research, Vol. 5, No. 12, 2011, pp. 2559-2565.
- M. F. Siqueira, “A Terra Mais Quente,” Pesquisa Fapesp, Vol. 96, 2005, pp. 34-37.
- Brasil, Ministério da Agricultura, Pecuária e Abastecimento, “Regras para Análise de Sementes/Ministério da Agricultura, Pecuária e Abastecimento. Secretaria de Defesa Agropecuária,” Mapa/ACS, Brasília, 2009, 399 Pages.
- J. D. Maguire, “Speed of Germination Aid in Selection and Evaluation for Seeding Emergence and Vigor,” Crop Science, Vol. 2, No. 2, 1962, pp. 176-177. doi:10.2135/cropsci1962.0011183X000200020033x
- D. F. Ferreira, “Sisvar 5. 1-Análises estatíSticas Por Meio do Sisvar para Windows,” Universidade Federal de Lavras, Lavras, 2005. http://www.dex.ufla.br/~danielff/softwares.htm
- M. M. Ramirez-Rodrigues, M. L Plaza, A. A. M. O Balaban and M. R. Marshall, “Physicochemical and Phytochemical Properties of Cold and Hot Water Extraction from Hibiscus sabdariffa,” Journal of Food Science, Vol. 76, No. 3, 2011, pp. 428-435. doi:10.1111/j.1750-3841.2011.02091.x
- H. Shimomura, Y. Sashida, H. Nakata, A. Yamamoto, Y. Kawakubo and J. Kawasaki, “Germination and Growth Inhibitors in Fruits of Gardenia jasminoides,” Plant Cell Physiology, Vol. 24, No. 1, 1983, pp. 123-126.
- C. E. Pereira, E. V. R. V Pinho, D. F Oliveira and A. L. P Kikuti, “Germination Inhibitors Determination in the Spermoderm of Coffee (Coffea Arabica L.) Seeds,” Revista Brasileira de Sementes, Vo. 24, No. 1, 2002, pp. 306-311.
- R. W. Willemsen and E. L. Rice, “Mechanism of Seed Dormancy in Ambrosia artemisiifolia,” American Journal of Botany, Vol. 59, No. 3, 1972, pp. 248-257. doi:10.2307/2441425
- Z. H. Ali, Q. Wang, X. Ruan, C. D. Pan and D. A. Jiang, “Phenolics and Plant Allelopathy,” Molecules, Vol. 15, No. 12, 2010, pp. 8933-8952. doi:10.3390/molecules15128933
- L. Kong, F. Wang, J. Si, B. Feng and S. Li, “WaterSoluble Phenolic Compounds in the Coat Control Germination and Peroxidase Reactivation in Triticum aestivum Seeds,” Plant Growth Regulators, Vol. 56, No. 3, 2008, pp. 275-283. doi:10.1007/s10725-008-9307-2
NOTES
*This work was partially funded by a grant of Fundacao de Amparo a Pesquisa do Estado de Sao Paulo (FAPESP) 2007/58503-7.
#Corresponding author.

