American Journal of Plant Sciences
Vol.3 No.5(2012), Article ID:19551,4 pages DOI:10.4236/ajps.2012.35083
A New Record on Flowering in Harar (Terminalia chebula Retz.) Seedling
![]()
1Dr. Y.S. Parmar University of Horticulture and Forestry, Regional Horticultural and Forestry Research Station, Hamirpur, India; 2Dr. Y.S. Parmar University of Horticulture and Forestry, Regional Horticultural Research Station, Kangra, India.
Email: kamal_64in@yahoo.com
Received May 19th, 2011; revised July 15th, 2011; accepted October 11th, 2011
Keywords: Terminalia chebula; Early Flowering; Seedling
ABSTRACT
Harar (Terminalia chebula), a large deciduous tree belongs to family combretaceae. It grows naturally in greater part of India up to 1500 m elevation. Due to several alkaloids present in fruit, it is used as laxative, purgative and astringent for curing a number of ailments. Keeping in view its medicinal and tanning properties, the authors have been working for the last two decades on various aspects like propagation and development of promising strains of harar. Grafting/budding techniques have been standardized to produce true to type precocious plants which bear flower in two to three years. However, flowering has been observed in three months old seedling, which can be ascribed to biochemical and/or cellular changes. Early flowering is a rare incidence in tree seedlings which otherwise could be very useful for breeding and early evaluation of fruit species.
1. Introduction
Harar scientifically known as Terminalia chebula belongs to Kingdom Plantae, Division Magnoliophyta, Class Magnoliopsida, Order Myrtales and Family Combretaceae. It is a large deciduous tree up to 1.5 - 2.5 m diameter with round crown and spreading branches. The long ovate leaves are acute, in opposite pairs about 10 to 20 cm long. The flowers are dull white in spikes found at the end of the branches. The fruit scientifically known as drupe is hard and generally yellowish green in colour. Harar grows naturally in the sub-Himalayan tract from Sutlej eastwards ascending up to 1500 m elevation and also in deciduous forests of peninsular India [1] and Madhya Pradesh [2]. In Himachal Pradesh, Harar is found up to 1100 m elevation in Sirmour, Hamirpur, Mandi, Bilaspur, Kangra, and Una districts [3]. It can grow in different environmental conditions. Soil supporting Harar vary widely in depth and composition. The mean maximum temperature in its habitat varies from 37˚C to 48˚C, absolute minimum temperature from 1˚C to 15˚C and annual rainfall from 750 to 3250 mm [4]. Terminalia chebula is a rich source of tannin (27.3% - 40.0%) which varies with genotype and geographical location [5]. The Tannins are of pyrogallol (hydrolyzable) type. The chief constituents of tannin are chebulic acid, chebulagic acid, corrilagin and gallic acid. Besides these, fructose, amino acids, succinic acid, betasitosterol, resin and purgative principles of anthroquinone and sennoside nature are also present [6]. Owing to several alkaloids present in fruit, it is used for curing a number of ailments in Ayurvedic and Tibetan systems of medicines. Harar fruit is considered laxative, purgative and astringent along with antibacterial, antifungal and antiviral properties. It has also demonstrated therapeutic activity against Herpes simplex virus (HSV) both in vitro and in vivo [7] and is also useful in sexual transmitted diseases and AIDS [8].
2. Materials and Methods
Ripened fruits of Harar were collected from wild growing trees in the month of December. The fruits were depulped and endocarps were separated and dried in open sun. The endocarps were stored in plastic containers until following March. Kernels were extracted after breaking endocarps manually. Kernels soaked in two per cent Bavistin solution for ten minutes were allowed to germinate in petriplates lined with germination paper. In total, 12,000 kernels were used for germination studies. The petriplates were kept in seed germinator at 30˚C ± 2˚C on 15th April 2010. Germination started after four days i.e. on 19th April and completed on 24th April. The germinated kernels were pricked in polybags of size 9" × 4" for further growth and development. The plants were observed regularly. Growth as well as development parameters viz., height, number of branches, spike length and number of flowers were recorded after three months.
3. Results and Discussion
In spite of similar growing conditions provided to all the plants, none of the seedlings except one (0.008 percent) produced flowers at the age of three months (Figure 1). The data recorded with regard to growth and development parameters of this unique seedling are presented in Table 1. The height of the seedling was just 14.4 cms whereas three numbers of branches were recorded. The spike length was 3.1 cm, however, 57 number of flowers were counted. The authors have been working for the last twenty years on Harar for development of improved cultivars and standardization of propagation techniques through seed and vegetative means. First ever grafted/

Figure 1. Flowering in three months old Terminalia chebula seedling.
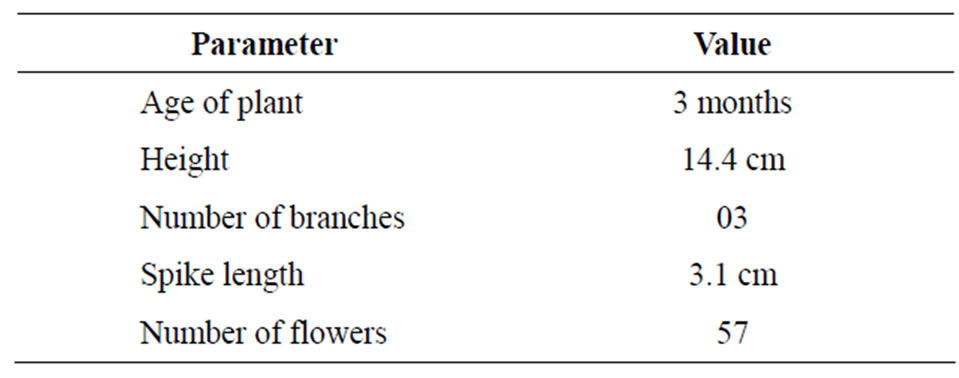
Table 1. Age, growth and development parameters of Harar seedling.
budded plant of Harar has been produced by the authors [9] for quality and early fruiting as fruit is the main part of the plant from medicinal point of view. The budded/ grafted plants start bearing fruits at the age of three years. In present case, the seedling plant of Harar produced flowers at the age of just three months which is a unique phenomenon. This is the first ever reported seedling plant of Harar came in flowering at such a young age otherwise plants of same origin bear flowers after 10 - 12 years of age. Over the years, more than 30,000 Harar plants have been produced by the authors from seeds yet it is the first instance when flowering observed in just three months old seedling.
Flowering in this seedling of Harar may be due to biochemical and cellular changes responsible for induction of flowering in plants. Untimely floral primordial form and develop at the shoot apex as a result of biochemical and cellular changes leading to the production of the floral organs. The transition of flowering may involve a complex system of interacting factors that include, among others Carbohydrates, Gibberellins and Cytokinins [10]. Cone formation can be promoted in juvenile plants of several gymnosperm families by GA3 [11]. In addition to GAs, other growth hormones can either inhibit or promote flowering [12]. One commercially important example is striking flowering in pine apple (Ananas comosus) by ethylene and ethylene releasing substances, a response that appears to be restricted to members of the pineapple family (Bromeliaceae). Cytokinins may be a component of floral stimulus, although it is insufficient by itself to bring about flowering. Other potential compounds include the polyamines. Putrescence, the major polyamine is a component of the floral stimulus in Sinapsis alba [13]. In addition to receiving signal from the leaves, the shoot apex may accumulate flowering signals from the roots. The floral stimulus may also have multiple components; the limiting component may vary among species. Moreover, exposure to a high level of one component such as particular gibberellins may compensate for the lack of a limiting component and induce flowering.
Earliness of flowering is heritable [14]. Flowering control is exercised by single dominant gene [15]. Single major gene for precocious flowering in Dalbergia sissoo has been reported [16]. Genetic control is exerted over the first step in the process, not over many interacting processes that culminate in flower development. Aq7 treatment and selection induced 30 - 60 percent flowering at 55 - 58 weeks age in different families suggesting that Aq7 and the gene or genes controlling earliness of flowering regulate the same initial step in flower development in jack pine seedlings [17,18].
The multiple delays in onset of flowering in trees pose a substantial obstacle to research and breeding [19]. The generation of inbred lines is a key step in crop improvement programmes because it allows the production of true breeding varieties and reproducible hybrids [18]. Flowering is very rare in seedlings of trees as in present case. However, it could be very useful for breeding and early evaluation of trees regarding floral biology and fruiting behaviour. This is a rare plant on which studies with regard to flowering can be conducted to ascertain the biochemical or cellular changes responsible for flowering in Terminalia chebula.
4. Acknowledgements
The authors are thankful to Sh. Mohinder Singh Chauhan, Artist cum Photographer for photography.
REFERENCES
- D. Brandis, “Indian Trees,” Bishen Singh Mahendra Pal Singh, Dehradun, 1906.
- H. H. Haines, “Descriptive List of Trees, Shrub and Economic Herbs of the Southern Circle: Central Provinces,” Pioneer Press, Allahabad, 1916.
- N. S. Chauhan, “Medicinal and Aromatic Plants of Himachal Pradesh,” Indus Publishing Company, Delhi, 1999.
- R. S. Troup, “The Silviculture of Indian Trees,” International Book Distributors, Dehra Dun, 1921.
- M. Thakur, R. C. Rana and S. Thakur, “Physiochemical Evaluation of Terminalia chebula Fruits,” Journal of Non Timber Forest Products, Vol. 15, 2008, pp. 37-42.
- R. R. Chattopadhyay and S. K. Bhattacharya, “Terminalia chebula: An Update,” Pharmacognosy Reviews, Vol. 1, No. 1, 2007, pp. 151-156.
- M. Kurowa, K. Nagasaka, T. Hirabayashi, S. Uyama, H. Sato, T. Kagiyama, S. Kodata, H. Ohyama, T. Hzumi and T. Namba, “Efficacy of Traditional Herbal Medicines in Combination with Acyclovir against Herpes Simplex Virus-1 Infection in Vitro and in Vivo,” Antiviral Research, Vol. 27, No. 1-2, 1995, pp. 19-37. doi:10.1016/0166-3542(94)00076-K
- K. Vermani and S. Garg, “Herbal Medicines for Sexually Transmitted Diseases and AIDS,” Journal of Ethnopharmacology, No. 80, 2002, pp. 49-66.
- K. Sharma, S. Thakur, S. D. Badiyala and N. K. Sharma, “First Report on the Propagation of Terminalia chebula Retz. through Patch Budding,” Indian Forester, Vol. 121, No. 8, 1995, pp. 760-761.
- G. Bernier, “The Control of Floral Evocation and Morphogenesis,” Annual Review of Plant Physiology and Plant Molecular Biology, Vol. 39, 1988, pp. 175-219. doi:10.1146/annurev.pp.39.060188.001135
- R. P. Pharis and R. W. King, “Gibberellins and Reproductive Development in Seed Plants,” Annual Review of Plant Physiology, Vol. 36, 1985, pp. 517-568. doi:10.1146/annurev.pp.36.060185.002505
- D. Vince-Prue, “Photoperiod and Hormones,” In: R. P. Pharis and D. S. Reid, Eds., Encyclopedia of Plant Physiology, New Series, Vol. 11, No. 3, 1985, pp. 308-364.
- A. Havelange, P. Lejeune, G. Bernier, R. Kaur-Sawhney and A. W. Galston, “Putrescine Export from Leaves in Relation to Floral Transition in Sinapsis alba,” Physiology Plantarum, Vol. 96, No. 1, 1996, pp. 59-65. doi:10.1111/j.1399-3054.1996.tb00183.x
- L. C. Johnson and W. B. Critchfield, “The Production of Functional Pollen and Ovules by Pine Seedlings Less than l-Year-Old,” Forest Science, Vol. 24, 1978, pp. 467- 468.
- A. H. Teich and M. J. Holst, “Genetic Control of ConeClusters and Precocious Flowering in Pinus sylvestris,” Canadian Journal of Botany, Vol. 47, No. 7, 1969, pp. 1081-1084. doi:10.1139/b69-154
- R. K. Vakshasya and M. S. Rawat, “Mutation for Precocious Flowering in Dalbergia sissoo,” Silvae Genetica, Vol. 35, 1986, pp. 247-248.
- R. A. Cecich, “Applied Gibberellin Aq7 Increases Ovulate Strobili Production in Accelerated Growth Jack Pine Seedlings,” Canadian Journal of Forest Research, Vol. 11, No. 3, 1981, pp. 580-585. doi:10.1139/x81-079
- R. A. Cecich, H. Kang and W. Chalupka, “Regulation of Early Flowering in Pinus banksiana,” Tree Physiology, Vol. 14, 1994, pp. 275-284.
- H. Zhang, D. E. Harry, C. Ma, C. Hsu, C.-Yu. Yuceer, V. Vikram, O. Shevchenko, E. Etherington and S. H. Strauss, “Precocious Flowering in Trees: The FLOWERING LOCUST Gene as a Research and Breeding Tool in Populus,” Journal of Experimental Botany, Vol. 61, No. 10, 2010, pp. 2549-2560. doi:10.1093/jxb/erq092

