Open Journal of Veterinary Medicine
Vol.2 No.4(2012), Article ID:25616,4 pages DOI:10.4236/ojvm.2012.24042
The Cellular Populations of Normal Camel (Camelus dromedaries) Milk
1Animal Quarantine, Ministry of Agriculture, Al Bat’ha, Saudi Arabia
2Department of Microbiology, College of Science, King Saud University, Riyadh, Saudi Arabia
3Immunology Unit, Regional diagnostic laboratory, Ministry of Health, Dammam, Saudi Arabia
4Department of Microbiology and Parasitology, College of Veterinary Medicine, King Faisal, Al Ahsaa, Saudi Arabia
Email: *alluwaimi@kfu.edu.sa
Received September 15, 2012; revised October 15, 2012; accepted October 22, 2012
Keywords: Camel; Mammary Glands; Wc1, Cd8; Lymphocyte Trafficking; Lactation
ABSTRACT
Overwhelming demand on the camel milk has considered camel mammary glands as an important organ. However, poor research of the camel mammary glands immune system is considered major obstacle in improving the camel welfare. The cellular population of camel milk at the mid-lactation was exploited using overlapping reactive antibodies. The CD markers and adhesion molecules, CD3+, CD8+, WC + 1+, CD62L, CD11b/c (MAC-1) and the LPAM-1 were studied with flow cytometer. The high expression of CD3+, CD8+, WC+1+ and LPAM-1 was detected in all of the examined samples. The CD62L, CD11b/c expression were not detected consistently. The cross reacted antibodies with camel CD markers have revealed interesting overview of the nature of the cellular activities in the camel mammary glands at the lactation period. The level of CD8+ cells is in parallel with the findings at the cattle mammary glands. The high level of WC + 1+ gd cells in camel milk, despite the stage of the lactation and age, could indicate their significant role in the immunity of the camel mammary glands. The expression of the LPAM-1 on the lymphocytes has provided further support to the notion that the lymphocytes trafficking to the camel mammary glands could be of mucosal nature.
1. Introduction
Dromedary camel (Camelus dromedarius) is one of the highly valuable domestic animals in Saudi Arabia and most of the Middle East countries. Camel is multipurpose animal that can be used for meat, milk, and wool production. In addition to the previous traditional commodities, modern applications in the dairy industry lead to the development of camel dairy farms that are capable of producing camel milk on the commercial level. Camel milk and meat are considered an important source of proteins for wide range of population [1]. It was estimated that world camel milk market worth 10 billion dollars [2].
The camel mammary glands immune system is not disclosed in detail due to the poor level of research in this aspect. One of the major obstacles in this field is scarceness of the camel specific immune reagents especially the antibodies. One of the practical aspects that have enabled the researchers so far in the study of the camel immune system was the utilization of the cross reactive property of certain human and other animal species monoclonal antibodies (mAbs). In wide scale approach, the cross reactive efficiency of 377 commercially available mAbs from different companies was tested against cells from 17 different species [3]. Despite the wide species overlapping reactivity among most of the tested species, the cross reaction with CD markers ofllama was limited to some of the markers like CD9, CD11a, CD14, CD29, CD44 [3]. Furthermore, in another approach 490 mAbs against the CD markers of cattle goat, sheep, llama, pig, and human were tested against wide range of species including camel (Camelus dromedaries and bactrianus). Nevertheless, results confirmed further the limitation in the overlapping reactivity with the camel CD markers [4].
In an attempt to define the nature of adhesion molecules and certain CD markers of normal camel mammary glands at different lactation periods, recently Immuno peroxidase technique with aid of different commercially available mAbs was conducted [5]. The detection of the expression with anti-human CD20+, CD62L, vascular cell adhesion protein-1 (VCAM-1) CD44+ and anti-rat TCR-αβ mAbs, failed despite their expression in the original species that the antibodies were raised against. The expression of CD4+and CD11a/CD18 [leukocyte function antigen-1 (LFA-1)] with anti-bovine CD4+ and anti-rat CD11a/CD18 were not detected at all. However, the expression of the workshop cluster + 1+ (WC + 1+), CD8+ and mucosal address in cell-adhesion molecule-1 (MAdCAM-1) was detected in all of the selected tissues of the mammary glands with the anti-bovine WC + 1+ and CD8+ and anti-human MAdCAM-1 [5].
This study was conducted in the aim of examining the cross reactive efficiency of the above studied antibodies [5] and to examine further the overlapping reactivity of new antibodies to define the nature of the CD markers and adhesion molecules in the camel milk at mid-lactation period.
The difficulties in finding an efficient cross reacting antibodies with camel CD markers left no alternative other than blind selection of clones with possibility of cross reaction property. However, the non-reacted antibodies in the previous studies were helpful guide in avoiding their selection.
2. Materials and Methods
2.1. Milk Samples
The 220 - 250 ml composite fresh milk samples were collected from six apparently healthy camels at approximately mid-lactation period.
2.2. Preparation of the Milk Cells for the Flow Cytometry
The milk cells pellet washed three times with PBS and then the cells viability and their numbers were adjusted up to 10.000 cells/ml.
Seven Falcon tubes were labeled according to the primary monoclonal antibodies used (mAbs) (Table 1). In each of the six tubes, 100 μl of milk cells were mixed with 5 μl of each of the primary antibodies, anti-CD3+, CD8+, WC + 1+, CD62L, CD11b/c (macrophage-1 antigen [MAC-1])and the Peyer’s patches adhesion molecule-1 (LPAM-1), also known as integrin α4β7 labeled
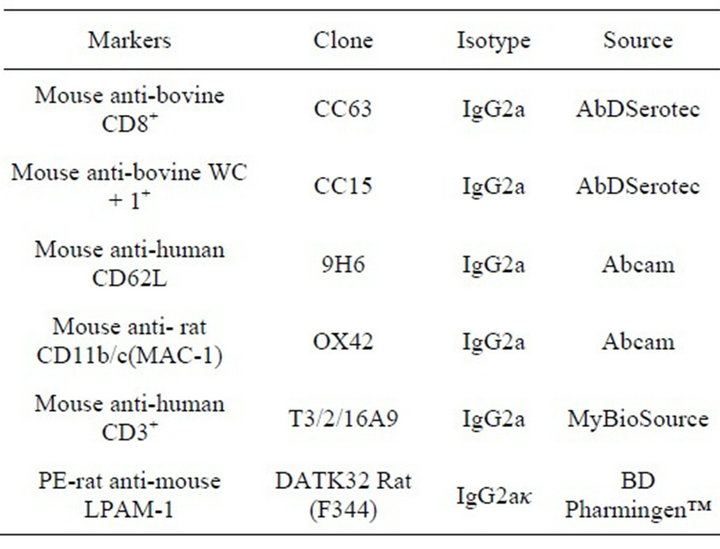
Table 1. The primary monoclonal antibodies.
with peroxidase (PE). The tube #7 used as negative control to define the cut off and the autoflourescense (Table 1) and then they incubated for 30 min. The cells then washed with BD cell wash for5 min at 1500 rpm to get rid of unbound antibodies and 5 μl of goat anti-mouse IgG2a conjugated with FITC (Southern Biotech, Birmingham, USA) as secondary antibody was added to each tube except the cells labeled with anti-LPAM-1-PE. All the tubes except the LPAM-1-PE tube were then incubated for 20 min. After the incubation, the cells were washed with cell wash (BD., San Jose, USA) to remove the unbound secondary antibodies of the stained cells, centrifuged , decanted and then resuspended in 1 ml of cell wash (BD., San Jose, USA).
2.3. Running the Samples
The flow cytometry was performed to analyze the milk cells using BD FACSCnto™ II flow cytometer (BD., San Jose, USA). Voltage settings and color compensations were performed according to the manufacturer’s directions using 7 color setup beads. The tubes were run in the flow cytometer, six tubes for each sample, one sample after another and all the data were analyzed using FACS Diva software (BD., San Jose, USA) in referral to the gated population in the lymphocytes area.
Data for the lymphocyte subsets (50,000 events/sample) were presented as the percentage of the total lymphocytes expressing each of the subset markers.
3. Results
The flow cytometry analysis of the expression of the CD3+, CD8+, CD62L, WC + 1+, CD11b/c and LPAM-1 indicated variable level of CD3+, CD8+, WC + 1+ and LPAM-1 expression (Table 2). However, the expression of CD62L and the CD11b/c was considered invalid due to the high variability and fluctuation.
4. Discussion
Camel mammary glands considered an important organ due to the overwhelming demand on camel milk [2]. The scarce information about the camel mammary glands
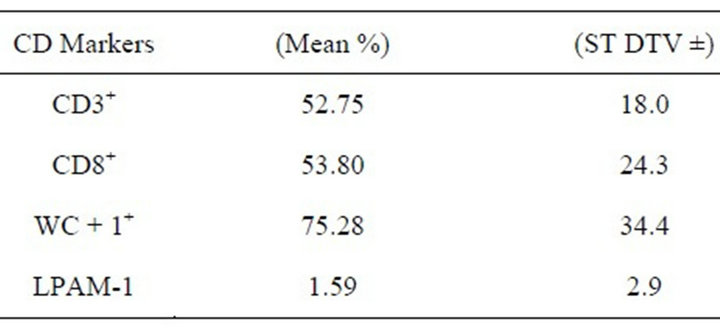
Table 2. The mean percentage of the CD markers and adhesion molecules expression by the milk cells that were gated by the CD3+marker.
immune system and poor research in this field are considered major obstacles in improving the camel welfare. Moreover, lack of efficient immune reagent present further difficulties in studying camel immune system.
In this study, the cross reactive efficiency of a battery of mAbs of different species to camel CD markers was examined. The overlapping reactivity with the CD3+, CD8+, WC + 1+, and LPAM-1 of camel milk cells were shown to be efficient. However, the reaction to CD62L and CD11b/c were not consistent. In the previous study the expression of CD8+and WC+1+was detected in the camel mammary glands at mid and late lactation period [5].
The adhesion molecules (AM) and CD markers are considered important facet of the immune activities in the mammary glands. Hence, variations of the expressed AM and the CD markers indicate their role in the movement of different leukocytes into the mammary gland [6].
The CD8+ which is major marker of the T-cytotoxic cells were detected in high level in the camel mammary glands during the lactation period [5]. In the bovine mammary glands, CD8+ T-lymphocytes also prevail during the lactation period [6].The role of the CD8+ cells that express αβTCR in the mammary gland is not fully understood. It was speculated that these cells are critical cytotoxic T-cells [7]. These cells may act as immunosuppressive cells during the periparturent period [8]. CD8+ T-lymphocytes that express gdTCR are a major source of Interferon-g.
WC + 1+ subset cells were also detected in high level of the camel milk. The gd cells coexpress the WC + 1+ which is transmembrane glycoprotein of the scavenger receptor cysteine rich (SRCR), a family that is usually expressed on the gd T cells [9,10]. The WC + 1+ coreceptors that are expressed on the gd TCR are identified with respect to their CD8+, CD2+, and WC + 1+ expression and their cytokine profile [11-13]. The γδ cells that are CD8CD2+ but WC + 1− have anti-inflammatory properties, while γδ cells that lack CD8 and CD2, but express WC + 1+ have proinflammatory properties. The level of the γδ cells usually decrease during the lactation period in cattle [14]. However, it was noticed that the level of the WC + 1+ γδ cells in the camel mammary glands during late lactation was high [5]. It appears that these subset cells could be one of the major cells in the camel mammary glands, which play important role in health and disease. In contrast to cattle, the γδ cells level could be less subjected to the effect of age factor and stage of lactation in camel. Nevertheless, further research in this aspect is essential to endorse this hypothesis.
Although the LPAM-1 expression was low, it is of high significance. The immperoxidase study of different camel mammary glands tissues indicated the expression of the mucosal address in cell-adhesion molecule-1 (MAdCAM-1), which is a ligand of theLPAM-1 [5]. Interestingly, the lymphocytes trafficking to the tissues of mammary glands that express MAdCAM-1 are of mucosal origin as in the monogastric animals. However, trafficking to the tissues that lack MAdCAM-1 expression is of peripheral nature like in most of the ruminant [15]. Hence, the expression of the LPAM-1 on the camel lymphocytes most probably endorse the notion that lymphocytes trafficking to the camel mammary glands is of mucosal nature despite it is ruminant.
In conclusion, the cross reacted antibodies with camel CD markers have revealed interesting overview of the nature of the cellular activities in the camel mammary glands at the lactation period. The expression of the LPAM-1 on the lymphocytes has provided further support to the notion that the lymphocytes trafficking to the camel mammary glands is of mucosal nature as it was proposed previously [5].
REFERENCES
- M. Breulmann, B. Böer, U. Wernery, R. Wernery, H. El Shaer, G. Alhadrami, D Gallacher, J. Peacock, A. S. Chaudhary, G. Brown and J. Norton, “The Camel from Tradition to Modern Times,” The United Nations Educational, Scientific and Cultural Organization Office, Doha, 2007.
- Food and Agriculture Organization, “Milking the Camel,” 2008. http://www.fao.org/AG/AGAINFO/themes/en/dairy/camel.html
- A. Saalmüller, J. K. Lunney, C. Daubenberger, W. Davis, U. Fischer, T. W. Göbel, P. Griebel, E. Hollemweguer, T. L. R. Meister, H. S. K. Sestak, P. Sopp, F. Steinbach, X.-W. Wu and B. Aasted, “Summary of the Animal Homologue Section of HLDA8,” Cellular Immunology, Vol. 236, No. 1-2, 2005 pp. 51-58.
- A. A. Mosaad, A. R. Elbagory, A. Khalid, M. W. Waters, A. Tibary, M. J. Hamilton and W. Davis, “Identification of Monoclonal Antibody Reactions for Use in the Study of the Immune Response to Infectious Agents in Camel and Water Buffalo,” Journal of Camel Practice and Research, Vol. 13, No. 2, 2006, pp. 91-101.
- K. T. Al-Mohammed Salem, S. Y. Al Ramadan and A. M. Alluwaimi, “Adhesion Molecules and the Cellular Population of the Normal Camel (Camelus dromedaries) Mammary Glands,” The Open Veterinary Science Journal, Vol. 6, 2012, pp. 15-22.
- K. Asai, K. Kai, H. Rikiishi, S. Sugawara, Y. Maruyama, T. Yamaguchi, M. Ohta and K. Kumagai, “Variation in CD4+ T Lymphocyte Subpopulations in Bovine Mammary Gland Secretions during Lactating and Non-Lactating Periods,” Veterinary Immunology and Immunopathology, Vol. 65, No. 1, 1998, pp. 51-61. doi:10.1016/S0165-2427(98)00176-7
- G. R. Leitner, O. Eligulashvily, S. Krifucks and A. Saran, “Immune Cell Differentiation in Mammary Gland Tissues and Milk of Cows Chronically Infected with Staphylococcus Aureus,” Journal of Veterinary Medicine B, Vol. 50, No. 1, 2003, pp. 45-52. doi:10.1046/j.1439-0450.2003.00602.x
- M. J. Paape, K. Shaver-Weaver, A. V. Capuco, K. V. Vanoostveldt and C. Burvenich, “Immune Surveillance of Mammary Tissue by Phagocytic Cells,” In: J. A. Mol and R. A. Clegg, Eds., Biology of the Mammary Gland, Advances in Experimental Medicine and Biology, Vol. 480, 1990, pp. 259-278. doi:10.1007/0-306-46832-8_31
- C. Chen, C. T. A. Herzig, J. C. Telfer and C. L. Baldwin, “Antigenic Basis of Diversity in the T Cell Co-Receptor WC1 Family,” Molecular Immunology, Vol. 46, No. 13, 2009, pp. 2565-2575. doi:10.1016/j.molimm.2009.05.010
- A. N. Rogers, D. G. Vanburen, E. E. Hedblom, M. E. Tilahun, J. C. Telfer and C. L. Baldwin, “gdT Cell Function Varies with the Expressed WC1Coreceptor,” Journal of Immunology, Vol. 174, No. 6, 2005, pp. 3386-3393.
- Y. H. Chiena and M. Bonneville, “Gamma Delta T Cell Receptors,” Cellular and Molecular Life Science, Vol. 63, No. 18, 2006, pp. 2089-2094. doi:10.1007/s00018-006-6020-z
- N. D. Machugh, J. K. Mburu, M. J. Carolt, C. R. Wyatt, A. Ordenjj and W. C. Davis, “Identification of Two Distinct Subsets of Bovine Cells with Unique Cell Surface Phenotype and Tissue Distribution,” Immunology, Vol. 92, No. 3, 1997, pp. 340-345. doi:10.1046/j.1365-2567.1997.00350.x
- E. Wilson, J. F. Hedges, E. C. Butcher, M. M. Briskin and A. Jutila, “Bovine gd T Cell Subsets Express Distinct Patterns of Chemokine Responsiveness and Adhesion Molecules: A Mechanism for Tissue-Specific cd T Cell Subset Accumulation,” Journal ofImmunology, Vol. 169, No. 9, 2002, pp. 4970-4975.
- C. Van Kampen, B. A. Mallard and B. N. Wilkie, “Adhesion Molecules and Lymphocyte Subsets in Milk and Blood of Periparturient Holstein Cows,” Veterinary Immunology and Immunopathology, Vol. 69, No. 1, 1999, pp. 23-32. doi:10.1016/S0165-2427(99)00034-3
- M. E. Kehrli and J. A. Harp, “Immunity in the Mammary Gland,” Veterinary Clinic of North America: Food Animal Practice, Vol. 17, No. 3, 2001, pp. 495-516.
NOTES
*Corresponding author.

