Open Journal of Anesthesiology
Vol.3 No.3(2013), Article ID:31725,6 pages DOI:10.4236/ojanes.2013.33045
The Effect of Intraperitoneal Ropivacaine for Post-Operative Pain Management in Patients Undergoing Laparoscopic Cholecystectomy: A Prospective Double-Blind Randomized Control Study
![]()
Department of Anaesthesia, KG’s MU, Lucknow, India.
Email: dr.sulekha2008@rediffmail.com
Copyright © 2013 Dinesh Singh et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received January 26th, 2013; revised March 29th, 2013; accepted April 15th, 2013
Keywords: Intraperitoneal; Bupivacaine; Cholecystectomy; Anaesthetic
ABSTRACT
Objective: To compare the effect of intraperitoneal ropivacaine with placebo for post-operative pain management in patients undergoing laparoscopic cholecystectomy. Material and Methods: All patients were pre-medicated with glycopyrrolate 0.2 mg, ondansetron 4 mg and ranitidine 150 mg intravenously half an hour prior to induction of anesthesia. All patients were given standard general anaesthesia with propofol (2 - 2.5 mg/kg), fentanyl 2 µg/kg, and succinylcholine (2 mg/kg) to facilitate tracheal intubation. Anesthesia was maintained with 60% N2O in oxygen with 0.5% to 1% Halothane. Group A: Patients received 20 ml of 0.9% normal saline as placebo (n = 25). Group B: Patients received 20 ml of 0.5% ropivacaine (n = 25). Results: The age and sex distribution of both the groups was similar. The heart rate, systolic & diastolic blood pressure, mean blood pressure and mean trend of SpO2 in both groups remained similar over the periods. The mean VAS varied considerably within (between time) and between the groups (treatment) and was especially comparatively higher in Group A at initial hours 15 min to 30 min and at end hours 12 - 24 hrs as compared with Group B. On an average, the frequent dosing of rescue analgesia and mean No. of rescue analgesia doses were higher in Group A followed by Group B. In both groups, the treatment related adverse events were mostly emetic symptoms and shoulder pain with the highest being in Group A. Conclusion: We conclude that intraperitoneal instillation of local anaesthetic is an easy, cheap, and non-invasive method which provides good analgesia in the immediate postoperative period after laparoscopic surgery.
1. Introduction
Pain after laparoscopy results from the stretching of the intra-abdominal cavity (Joris et al. 1995) [1], peritoneal inflammation and phrenic nerve irritation caused by residual carbon dioxide in the peritoneal cavity. Pain can prolong hospital stay and lead to increased morbidity. Intra-peritoneal injections of local anaesthetic have been proposed to minimize postoperative pain after laparoscopic surgery (Zmora et al. 2000) [2]. Several studies have shown that visceral pain is the major component. Local anaesthetics have been administered into the peritoneal cavity during minimally invasive procedures, such as laparoscopic cholecystectomy and gynaecological laparoscopy for sterilization and diagnosis [3] (A. Helvacioglu et al. 1992), in addition to open abdominal procedures, such as total abdominal hysterectomy. Administration of intra-peritoneal local anaesthetic (LA), either during or after surgery, is used by many as a method of reducing post-operative pain. Although a number of these studies have reported a significant reduction in postoperative pain after the use of intra-peritoneal analgesia, others have reported no benefit. Most of these initial studies have used small doses of bupivacaine or of lidocaine. The main advantage of using local anaesthetics is that they do not have the adverse effects of opioids, which may delay recovery and discharge from hospital. These effects include postoperative nausea, sedation, impairment of return of gastrointestinal mortality, and pruritus. In addition, time to return of bowel function in the postoperative period may be reduced when the use of opioids is obviated by administering local anaesthetics. Intra-peritoneal ropivacaine nebulization was also used for pain relief after laparoscopic cholecystectomy [4] (M. Bucciero et al. 2011). It was found to be effective in reducing shoulder tip pain. Intraperitoneal ropivacaine injected during laparoscopic cholecystectomy significantly decreased post-operative pain when compared with injection of intra-peritoneal placebo [5] (Labaille et al. 2002). Local infiltration of 1% ropivacaine combined with preincisional low dose I.V ketamine reduces postoperative pain after laparoscopic cholecystectomy [6] (G. Pappas-Gogos et al., 2008).
In this study, we designed a prospective double-blind randomized control study to compare the effect of intraperitoneal ropivacaine and saline as placebo for postoperative pain management in patients undergoing laparoscopic cholecystectomy.
2. Material and Methods
After getting approval from ethical Committee of King George’s Medical University, UP, Lucknow, for research on human subject, written informed consent was taken from each patients selected for this study. Patients aged 20 - 50 years of either sex belonging to ASA physical status I or II planned for laparoscopic cholecystectomy were included in this prospective, randomized, double blind, placebo-controlled study.
All patients were pre-medicated with glycopyrrolate 0.2 mg, ondansetron 4 mg and ranitidine 150 mg intravenously half an hour prior to induction of anesthesia. All patients were given standard general anaesthesia with propofol (2 - 2.5 mg/kg), fentanyl 2 µg/kg, and succinylcholine (2 mg/kg) to facilitate tracheal intubation. Anesthesia was maintained with 60% N2O in oxygen with 0.5% to 1% Halothane. Muscle relaxation was achieved with intermittent vecuronium bromide. Ventilation (tidal volume 8 - 10 ml/kg) was adjusted to maintain end-tidal carbon dioxide between 34 and 40 mm Hg. Patients were placed in 15˚ - 20˚ reverse Trendelenburg’s position with left-side down tilting position. During laparoscopy, intra-abdominal pressure was limited to 10 - 12 mmHg. The CO2 was carefully evacuated at the end of surgery by manual compression of the abdomen with open trocars. At the end of surgery, using a computer generated table of random numbers patients were randomized into one of the three groups.
Group A: Patients received 20 ml of 0.9% normal saline as placebo (n = 25).
Group B: Patients received 20 ml of 0.5% Ropivacaine (n = 25).
Drug solution was prepared by an anaesthesiologist who had not participated in the study, and drug was filled in pre-coded 20 ml syringes. Surgeon and the anaesthesiologist in the post-anaesthesia care unit were unaware of the treatment to which each patient was randomized.
The drug was injected intra-peritoneally before the removal of trocar at the end of the surgery, in Trendelenburg’s position to facilitate dispersion of drug solution in sub hepatic region. Local anaesthetic or placebo solutions were given as follows: the surgeon sprayed 10 mL of solution into the hepato-diaphragmatic space, 5 mL in the area of the gallbladder, and 5 mL into the space between liver and kidney. During the operation non-invasive blood pressure, heart rate, etco2 and peripheral oxygen saturation were recorded regularly. Surgical wounds were not infiltrated with local anaesthetic solution.
Before induction of anaesthesia, the patients were instructed how to use a 100 mm visual analogue scale (VAS; with end point to be labelled “no pain” and “worst possible pain”). The degree of postoperative pain was assessed using the VAS at 15 min, 30 min, 1 hr, 2, 4, 8, 12 and 24 hours post-operatively. Those patients had VAS > 40, were administered a bolus of Diclofenac aqueous (75 mg) as rescue analgesia. Ondansetron (4 mg i.v.) was administered on complaint of nausea and vomiting. Time to first analgesic requirement, total analgesic consumption in the first 24 hours postoperatively and occurrence of adverse events was also recorded.
Analysis
Continuous data were summarized as Mean ± SD while discrete (categorical) in %. The primary outcome measures (heart rate, systolic BP, diastolic BP, MBP and SpO2, EtCo2, and VAS) of three groups over the periods (time) were compared by repeated measures two factor (Groups and Periods) analysis of variance (ANOVA) using general linear models (GLM) followed by Tukey’s post hoc test after ascertaining the normality by Shapiro-Wilk test and the homogeneity of variance by Levene’s test. Groups were also compared by unpaired t-test. The discrete (categorical) variables were compared by chi-square (χ2) test. A two-sided (α = 2) p < 0.05 was considered statistically significant. All analyses were performed on STATISTICA (Windows Version 6.0).
3. Results
The age of Group A and Group B ranged from 20 - 50 yrs and 20 - 50 yrs, respectively with mean (±SD) 39.80 ± 9.07 yrs and 38.96 ± 9.56 yrs, respectively. In both the groups, the %age of females was higher than males and mostly with ASA grade 1 (Table 1).
The heart rate (HR) in both the groups remained similar over the periods with slightly being higher in Group A at intraoperative periods as compared to Group B. For each time, comparing the mean HR between the groupsTukey’s test revealed significantly higher HR of Group A at intraoperative periods and 30 min of postoperative periods as compared to Group B. SBP and DBP were also remained similar over the periods with slightly being higher in Group B at postoperative periods as compared to Group A The mean blood pressure (MBP) in both the groups remained similar over the periods in both Group A and Group B. The mean MBP did not differed significantly (p > 0.05) between Group A and Group B at all periods i.e. found to be statistically the same (Table 2).
The mean trend of SpO2 in both the groups remained
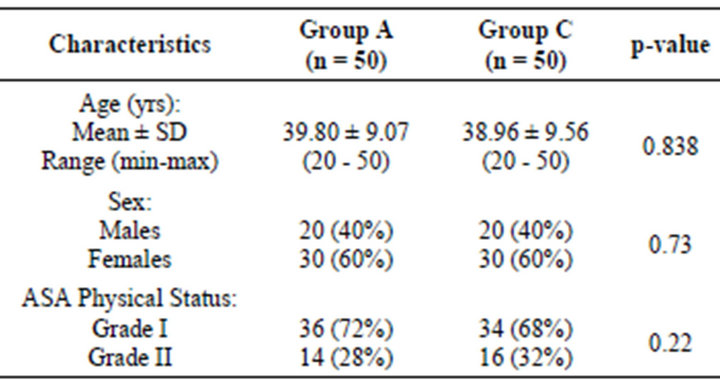
Table 1. Basic characteristics of three groups.
similar over the periods with slightly being higher in Group B at all periods as compared to Group A (Table 3). For each time, comparing the mean EtCo2 between the groups, Tukey’s test revealed insignificant (p > 0.05) difference in EtCo2 between the groups at all periods i.e. found to be statistically the same (Figure 1).
The mean VAS in both the groups varied considerably within (between time) and between the groups (treatment) especially comparatively higher in Group A at initial hours 15 min to 30 min and at end hours 12 - 24 hrs as compared to Group B. Comparing the mean VAS scores of both the groups over the periods, unpaired t-test revealed significant effect of both groups (treatments) (p < 0.001) and time (periods) (p < 0.001) on pain. The interaction (groups × time) effect of both on pain was also found to be significant (p < 0.001). For each time, comparing the mean VAS between the groups, Tukey’s test revealed significantly (p < 0.001) higher VAS score in Group A as compared to Group B C at 15 min, 30 min, 12 hrs and 24 hrs (Figure 2).
On an average, the frequent dosing of rescue analgesia was higher in Group A than Group B. Further, the requirement in subjects was also higher in Group A (Table 4).
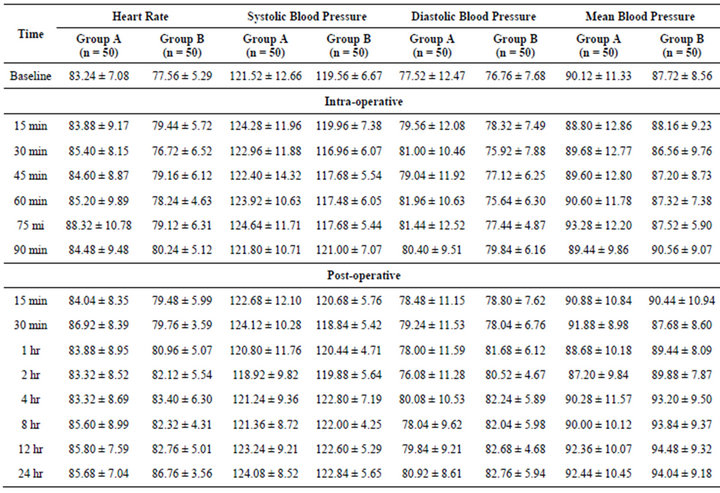
Table 2. Comparison of heart rate and blood pressure among the three groups.
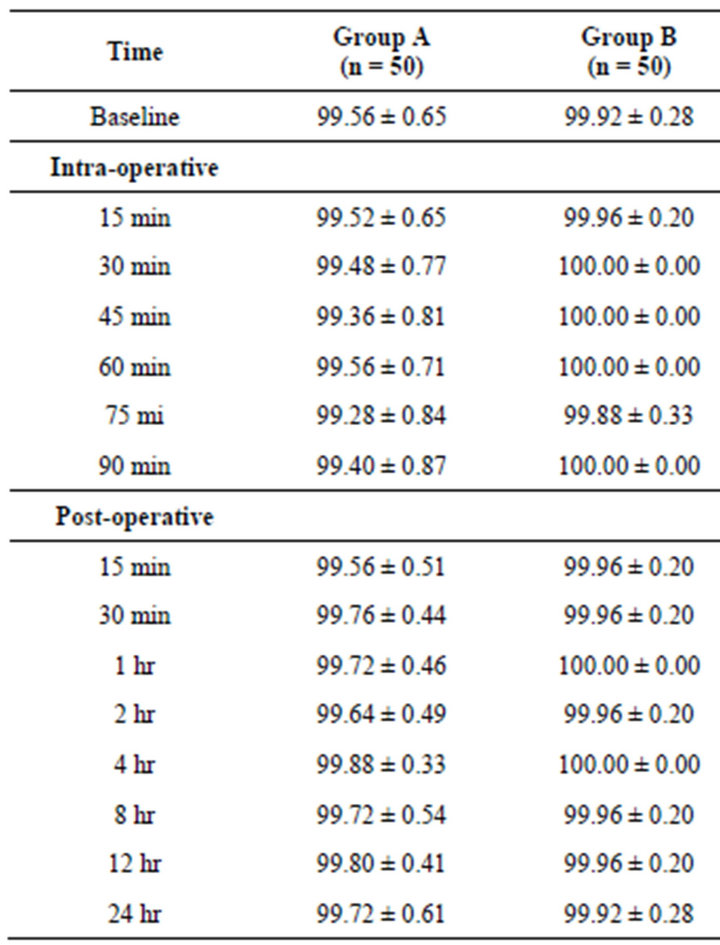
Table 3. Comparison of SpO2 among the three groups.
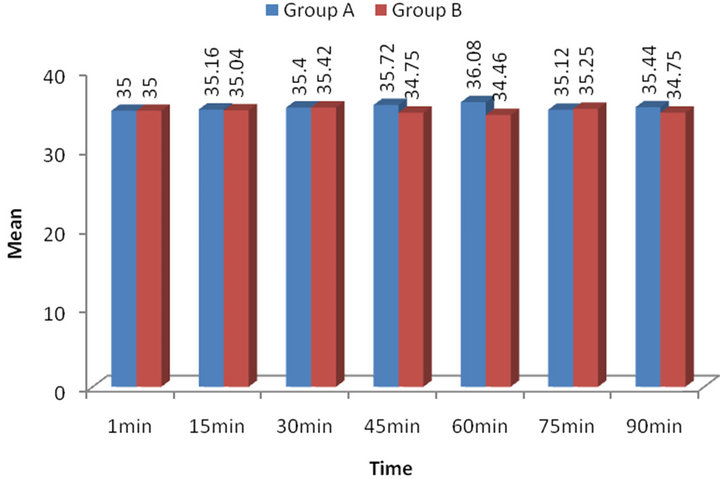
Figure 1. Intra-operative EtCo2 levels.
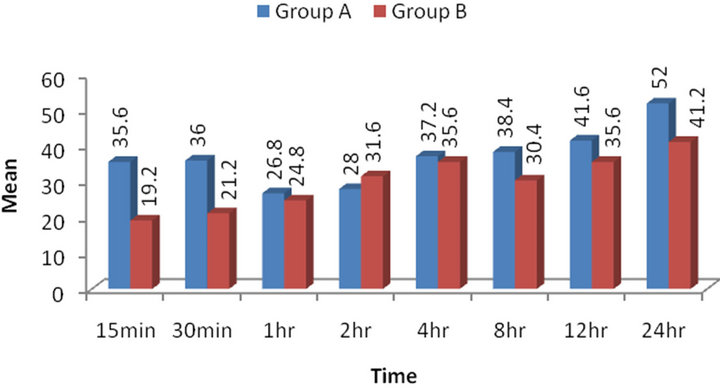
Figure 2. Postoperative pain levels (VAS scores).
The mean no. of rescue analgesia doses of Group A (3.84 ± 0.75) was comparatively higher than Group B (2.72 ± 0.46). Comparing the mean no. of rescue analgesia doses between the groups, unpaired t-test revealed significantly different no. of rescue analgesia doses both the groups (p < 0.001). Further, Tukey test revealed significantly (p < 0.001) higher mean no. of rescue analgesia doses in Group A as compared to Group B (Table not shown).
In both the three groups, the treatment related adverse events were mostly emetic symptoms and shoulder pain with highest being in Group A. However, hypotension, bradycardia and sedation were not seen. The frequency of emetic symptoms (χ2 = 12.32, p = 0.002) and shoulder pain (χ2 = 29.55, p < 0.0001) were significantly higher in Group A as compared to Group B (Table 5).
4. Discussion
Postoperative pain is multifactorial in origin, and therefore, multimodal therapy may be needed to optimize pain relief. After laparoscopic cholecystectomy, patients complain of pain from the incision of the skin (somatic pain), of visceral pain, and of shoulder pain from diaphragm stimulation. Incisional pain is defined as superficial pain, wound pain, or pain located in the abdominal wall. Visceral pain is defined as pain inside the abdomen, which may be deep, dull, and more difficult to localize, and may
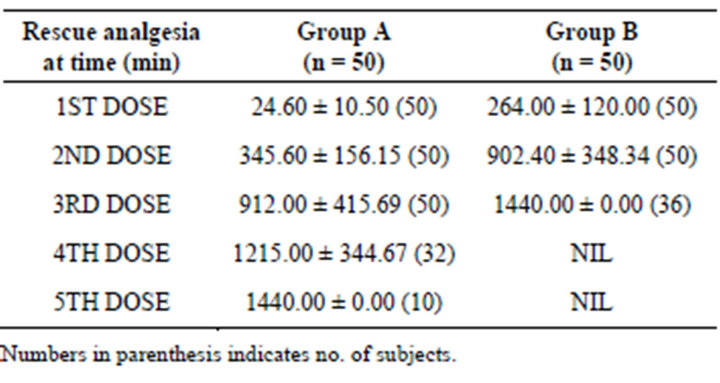
Table 4. Comparison of Rescue analgesia time among groups.
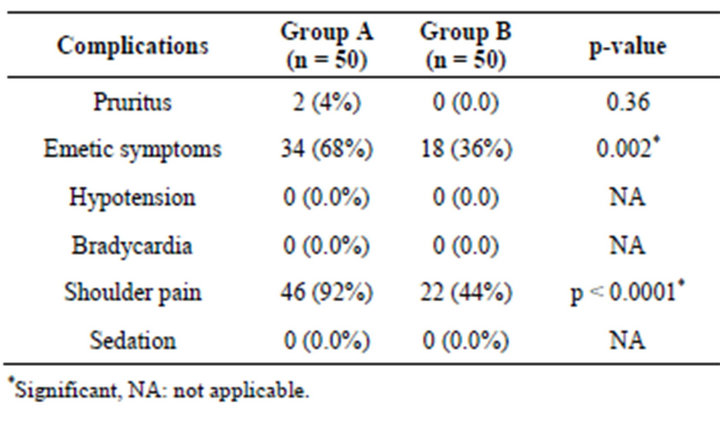
Table 5. Adverse events summary of three groups.
resemble biliary colic. Shoulder pain is defined as a sensation of pain in the shoulder. Rapid distension of the peritoneum by CO2 insufflation results in tearing of blood vessels, traction of nerves, and release of inflammatory mediators producing visceral pain; inflammation or local irritation around the gallbladder bed, liver, diaphragm or peritoneum, or both, secondary to gallbladder removal and abdominal muscle distension add to tissue injury and produce visceral pain. The exact mechanism of shoulder pain after laparoscopic surgery still remains unclear. Shoulder pain results from peritoneal insufflation especially when an exaggerated Trendelenburg position is used. Most authors believe it is an irritation of the phrenic nerve causing referred pain of C4 projected to the shoulder.
Improved postoperative pain management using opioidsparing regimens may facilitate a high success rate of out patient laparoscopic cholecystectomy [7] (Bisgaard et al., 1999). Various techniques have been investigated to reduce shoulder pain. Local anaesthetic techniques are part of the multimodal approach to postoperative pain management [8] (Alkhamesi et al., 2007). The main advantage of using local anaesthetics is that they do not have the adverse effects of opioids, which may delay recovery and dischargefrom hospital. These effects include postoperative nausea, sedation [9] (Papagiannopoulou et al., 2003), impairment of return of gastrointestinal motility, and pruritus. In addition, time to return of bowel function in the postoperative period may be reduced when the use of opioids is obviated by administering local anaesthetics.
The present study showed that visceral pain accounts for most of the discomfort experienced in the early postoperative period after laparoscopic cholecystectomy in Placebo group. Visceral pain developed after laparoscopic cholecystectomy was not affected by mobilization. However, coughing increased its intensity. This can be explained by that, mobilization requires contraction of the abdominal muscles, and does not involve movement of the intra-abdominal viscera. On the other hand, cough produces an abrupt displacement of the liver, and consequently results in stimulation of the inflamed cholecystectomy wound. Also, our results showed that incisional pain is less intense than visceral pain and worsened only by coughing not by mobilization. This can be explained by that cough not mobilization causes intense abdominal muscle contraction. The postoperative pain induced by laparoscopic cholecystectomy has a considerable visceral component (owing to surgical handling and diaphragmatic irritation by dissolved carbondioxide). At times the visceral component is such that it results in shoulder pain, similar in location and type to the pain that occurs with biliary colic. Incisional pain is less intense than visceral pain, owing to the small abdominal incisions made in the abdominal wall for the trocars and the limited damage to the abdominal wall.
Our study demonstrates the intra-peritoneal instillation of ropivacaine reduces pain after LC significantly. It shows reduced incidence of both shoulder pain and postoperative nausea and vomiting. The total analgesic consumption was reduced significantly as compared to placebo. VAS scores were lower in group B (ropivacaine) than group A (placebo) during the overall estimated time and as well as the interval times of estimation. During the study, none of the patients were excluded from the study because of uncontrolled pain or undesirable surgical outcomes such as delayed bowel movements and patient intolerance.
Pain is a highly personal experience which is whatever the experiencing person expresses and exist whenever the person appeals. The ambiguity of pain lies in that it is a subjective sensation or emotion and thorough objective observation of such is difficult. Because VAS scores are estimated by patients the accurate measurement is limited and objective estimation of pain could be deleterious. In the study of [10] Gupta et al. (2002), intermittent injections of 0.5% ropivacaine through a catheter reduced early postoperative pain after laparoscopic cholecystectomy. In our study the same concentration of intraperitoneal ropivacaine reduced pain significantly after LC. In the study of Labaille et al. (2002) [5], thirty seven ASA physical status I or II patients received in double-blinded fashion 20 mL of 0.9% saline solution(placebo), ropivacaine 0.25% (Rop 0.25%), or ropivacaine 0.75% (Rop 0.75%) immediately after trocar placementand at the end of surgery. They observed visceral pain at rest, during cough, and on movement and total consumption of morphine were significantly smaller in Groups Rop 0.25% and Rop 0.75% when compared with Placebo. Similar findings were also reported in other studies [11-14] (Goldstein et al., 2000; Jian-Zhu et al., 2009; Ljiljana et al., 2011; Mohamed et al., 2006). Similarly, in our study intraperitoneal ropivaccaine reduced the pain after laparoscopic surgery as compared to placebo.
The findings of the present study reveal that intraperitoneal ropivacaine administered after surgery pre-empts postoperative pain relative to an untreated placebo controlled group. This is similar to the study by Pasqualucci A et al. (1996) [15]. Kucuk et al. (2007) [16] compared the effect of intraperitoneal ropivacaine (150 mg) in patients undergoing a laparoscopic cholecystectomy. They found that for preventing postoperative pain 150 mg ropivacaine proved to be significantly more effective than placebo group. Almost similar findings were seen in the present study.
5. Conclusion
We conclude that intraperitoneal instillation of local anaesthetic is an easy, cheap, and non-invasive method which provides good analgesia in the immediate postoperative period after laparoscopic surgery.
REFERENCES
- J. Joris, E. Thiry, P. Paris, J. Weerts and M. Lamy, “Pain after Laparoscopic Cholecystectomy: Characteristics and Effect of Intraperitoneal Bupivacaine,” Anesthesia & Analgesia, Vol. 81, No. 2, 1995, pp. 379-384.
- O. Zmora, O. Srolik-Dollberg, B. Bar-Zakai, et al., “Intraperitoneal Bupivacaine Does Not Attenuate Pain Following Laparoscopic Cholecystecromy,” Journal of the Society of Laparoendoscopic Surgeons, Vol. 4, No. 4, 2000, pp. 301-304.
- A. Helvacioglu and R. Weis, “Operative Laparoscopy and Postoperative Pain Relief,” Fertility and Sterility, Vol. 57, No. 3, 1992, pp. 548-552.
- M. Bucciero, P. M. Ingelmo, R. Fumagalli, E. Noll, A. Garbagnati, M. Somaini, G. P. Joshi, G. Vitale, V. Giardini and P. Diemunsch, “Intraperitoneal Ropivacaine Nebulization for Pain Management after Laparoscopic Cholecystectomy: A Comparison with Intraperitoneal Instillation,” Anesthesia & Analgesia, Vol. 113, No. 5, 2011, pp. 1266-1271. doi:10.1213/ANE.0b013e31822d447f
- T. Labaille, X. Mazoitl, X. Paqueron, D. Franco and D. Benhamou, “The Clinical Efficacy and Pharmacokinetics of Intraperitoneal Ropivacaine for Laparoscopic Cholecystectomy,” Anesthesia & Analgesia, Vol. 94, No. 1, 2002, pp. 100-105.
- G. Pappas-Gogos, K. E. Tsimogiannis, N. Zikos, K. Nikas, A. Manataki and E. C. Tsimoyiannis, “Preincisional and Intraperitoneal Ropivacaine plus Normal Saline Infusion for Postoperative Pain Relief after Laparoscopic Cholecystectomy: A Randomized Double-Blind Controlled Trial,” Surgical Endoscopy, Vol. 22, No. 9, 2008, pp. 2036-2045. doi:10.1007/s00464-008-9762-x
- T. Bisgaard and B. Klarskov, “Multiregional Local Anaesthetic Infiltration during Laparoscopic Cholecystectomy in Patients Receiving Prophylactic Multimodal Analgesea: A Randomezed, Double Blinded, Placebo Controlled Study,” Anesthesia & Analgesia, Vol. 89, No. 4, 1999, pp. 1017-1024.
- N. A. Alkhamesi, D. H. Peck, D. Lomax and A. W. Darzi, “Intra-Peritoneal Aerosolization of Bupivacaine Reduces Post-Operative Pain in Laparoscopic Surgery: A Randomized Prospective Controlled Double-Blinded Clinical Trial,” Surgical Endoscopy, Vol. 21, No. 4, 2007, pp. 602-6. doi:10.1007/s00464-006-9087-6
- P. Papagiannopoulou, H. Argiriadou, M. Georgiou, B. Papaziogas, E. Sfyra and F. Kanakoudis, “Preincisional Local Infiltration of Levobupivacaine vs Ropivacaine for Pain Control after Laparoscopic Cholecystectomy,” Surgical Endoscopy and Other Interventional Techniques, Vol. 17, No. 12, 2003, pp. 1961-1964. doi:10.1007/s00464-002-9256-1
- A. Gupta, S. E. Thorn, K. Axelsson, L. G. Larsson, G. Agren, B. Holmstrom, et al., “Postoperative Pain Relief Using Intermittent Injections of 0.5% Ropivacaine through a Catheter after Laparoscopic Cholecystectomy,” Anesthesia & Analgesia, Vol. 95, No. 2, 2002, pp. 450-456.
- A. Goldstein, P. Grimault, A. Henique, M. Keller, A. Fortin and E. Darai, “Preventing Postoperative Pain by Local Anesthetic Instillation after Laparoscopic Gynecologic Surgery: A Placebo Controlled Comparison of Bupivacaine and Ropivacaine,” Anesthesia & Analgesia, Vol. 91, No. 2, 2000, pp. 403-407.
- J.-Z. Fu, J. Li and Z.-L. Yu, “Effect of Implanting Fibrin Sealant with Ropivacaine on Pain after Laparoscopic Cholecystectomy,” World Journal of Gastroenterology, Vol. 15, No. 46, 2009, pp. 5851-5854. doi:10.3748/wjg.15.5851
- L. Gvozdenović, V. Pajtić, D. Ivanov, R. Cvijanović, S. Gavrilović, Z. Gojković and S. Milić, “Acute Postoperative Pain Relief, by Intraperitoneal Application of Local Anesthetics, during Laparoscopic Cholecystectomy,” Journal of Health Sciences, Vol. 1, No. 2, 2011.
- M. R. Hemida, D. N. Abbas, H. A. Hosny and S. A. Al-Korashy, “Analgesic Efficacy of Intraperitoneal Ropivacaine or Combined with Intravenous Paracetamol for Laparoscopic Cholecystectomy,” Tanta Medical Sciences Journal, Vol. 1, No. 4, 2006, pp. 140-149.
- A. Pasqualucci, V. De Angelis, R. Contardo, et al., “Preemptive Analgesia: Intraperitoneal Local Anesthetic in Laparoscopic Cholecystectomy,” Anesthesiology, Vol. 85, No. 1, 1996, pp. 11-20. doi:10.1097/00000542-199607000-00003
- C. Kucuk, N. Kadiogullari, O. Canoler and S. Savli, “A Placebo Controlled Comparison of Bupivacaine and Ropivacaine Instillation for Preventing Postoperative Pain after Laparoscopic Cholecystectomy,” Surgery Today, Vol. 37, No. 5, 2007, pp. 396-400. doi:10.1007/s00595-006-3408-1

