Paper Menu >>
Journal Menu >>
 J. Electromagnetic Analysis & Applications, 2010, 2: 205-243 doi:10.4236/jemaa.2010.24029 Published Online April 2010 (http://www.SciRP.org/journal/jemaa) Copyright © 2010 SciRes. JEMAA 205 Intra-Atomic Electric Field Radial Potentials in Step-Like Presentation Levan Chkhartishvili, Tamar Berberashvili Department of Physics, Georgian Technical University, Tbilisi, Georgia. Email: chkharti2003@yahoo.com Received December 27th, 2009; revised February 8th, 2010; accepted February 15th, 2010. ABSTRACT Within the frames of semiclassical approach, intra-atomic electric field potentials are parameterized in form of radial step-like functions. Corresponding parameters for 80 chemical elements are tabulated by fitting of the semiclassical energy levels of atomic electrons to their first principle values. In substance binding energy and electronic structure calculations, superposition of the semiclassically parameterized constituent-atomic potentials can serve as a good ini- tial approximation of its inner potential: the estimated errors of the determined structural and energy parameters make up a few percent. Keywords: Electric Field Potential, Atoms, Step-Like Radial Functions 1. Introduction Because the electron mass is negligible in comparison with masses of atomic nuclei, substances, i.e. atoms and polyatomic bound systems – molecules or condensed matters – can be considered as one-electron systems in almost stationary self-consistent electric field generated by nuclei fixed at their equilibrium positions and space- averaged electron charge density. For this reason, elec- tronic structure, which includes both electron energy spectrum and electron density space distribution, deter- mines practically all principal physical properties of a substance. From its part, theoretical prediction of the substance electronic structure should be primarily based on the inner electric field potential, so that appropriate choice of the initial potential for such kind calculations greatly increases their accuracy. When isolated atoms associate forming molecular or condensed forms of substance only part of electrons (called as valence electrons) redistributes. And what is more, corresponding changes in the electron density dis- tribution are so weak that usually a simple superposition of the free atom’s radial potentials centered at the corre- sponding sites of the atomic structure serves as a good initial approximation of the inner potential in any polya- tomic system. At the worst, initial inner electric field potentials can be presented by superposition of the atom- ic-like radial potentials with different centers. Thus, in this line the key problem consists in construction of the effective atomic potentials in relevant functional form. Relatively recently, with that end in view we have proposed piece-wise analytical and, in particular, step- like radial atomic potentials obtained within initial quasi- classical, i.e., semiclassical approximation. They have been successfully used in binding energy and electronic structure calculations carried out for some polyatomic systems like the sodium diatomic molecule and crystals [1], boron-containing diatomic molecules [2,3], and mainly for boron nitride molecular, crystalline, and nano- structures [3-16]. In addition, semiclassical interatomic boron-boron pair potentials have explained some ground- state parameters of the boron nanotubes [17-19], as well as main features of the isotopic effects in boron-rich sol- ids [20-23]. But, above cited studies exploited semiclassical poten- tials only of certain, namely, some light atoms, whereas full-scale calculations performed for any wide class of materials need a quantity of appropriate effective atomic potentials. Present work aims to build up semiclassical atomic potentials for the stable chemical elements in most convenient form of radial step-like functions. The paper is organized as follows. At first, sense of the semiclassicality for the substance-electron-system is cla- rified. Then, a semiclassical parameterization scheme is introduced for charge distributions in atoms and atomic potentials as well. Next section presents results and brief discussion of the performed numerical calculations based on fitting of the semiclassical electron-energy spectra with these obtained from first principles. And finally, accuracies of the constructed step-like radial atomic po-  Intra-Atomic Electric Field Radial Potentials in Step-Like Presentation Copyright © 2010 SciRes. JEMAA 206 tentials are estimated for energy and expansion parame- ters of a material. 2. Substance as a Semiclassical Electron System Beginning from Bohr’s fundamental work [24] semi- classically describing electronic spectrum of the one- electron hydrogen-like atom with Coulomb potential up to nowadays, similar analysis is widely used for light atoms. Due to exact quantum-mechanical solvability of the Coulomb potential, exact wave functions of electron- states in a hydrogen-like atom can be obtained directly from the corresponding classical orbits [25]. And there- fore, quantum dynamics of the electron in a hydro- gen-like atom is wholly expressed by its classical dy- namics. Classically a two-electron helium-like atom can be represented as a pair of electrons placed at the opposite ends of the straight line with nucleus at the midpoint (see e.g. [26]). This classical model added with quantization condition for electron orbital moment leads to the almost hydrogen-like electron energy spectra, where atomic number Z is substituted for the reduced value Z–1/4 (it means that another electron effectively screens nuclear electric charge). Ground state energies calculated from the obtained relation for some helium-like systems differ from the experimental ones only by 3-6% [27]. Even entirely classical model of helium atom can be success- fully explored numerically to obtain its possible configu- rations [28]: most of the orbits are found to cause auto- ionization via chaotic transients. As for the modern se- miclassical approach based on the conception of periodic classical orbits, it allow visually interpret physical meaning of special quantum numbers inherent to this three-particle system between ground and fragmented states [29]. For many-electron atoms, a reasonable accuracy can be achieved in terms of the self-consistent-field ap- proximation, within which a minimum of the total energy is sought in the class of quasi-classical wave functions [30]. As is well known, many-electron systems such as heavy atoms are characterized by some quantum proper- ties like the electron-shell effects, fluctuations in pa- rameters’ values, discrete electron energy spectrum etc, which are averaged and, therefore, invisible in semi- classical atomic models. However, it was demonstrated that semiclassical treatment of the atomic many-electron system, when it is combined with information-theory- method, reveals resources to describe such kind effects as well [31]. It was demonstrated how based on purely classical no- tions it is possible to reproduce general trends in inelastic scattering atomic form-factors dependences upon quan- tum numbers [32]. Besides, starting from classical rela- tions together with energy conservation law and classi- cal-quantum correspondence principle, it was found ex- pressions of intensity-distribution and line-width of the electron–ion recombination X-ray spectrum, which is in unexpectedly good agreement with these resulted from the accurate quantum-mechanical calculations [33]. Semiclassical quantization rule leading to the exact electron energies in a hydrogen-like atom with Coulomb potential at the same time provides good accuracy of the valence electron energy value in a many-electron atom with model potential in form of sum of the nucleus Cou- lomb potential and a screening term [34]. Substitutions of the electron quantum numbers for their analogues in Thomas–Fermi semiclassical statistical model of atom can be applied for investigation of the excited and ion- ized electron states [35]. Semiclassical electron energy spectrum of Thomas–Fermi atom, which was described in terms of an effective kinetic energy obtained from the corresponding quantization rule formulated in momen- tum space, was found to agree essentially with that in the standard formalism employing an effective potential en- ergy [36]. Semiclassical evaluation of sums over quantum num- bers of electron states in many-electron atoms is known to be an effective tool of obtaining of the integrated atomic characteristics (see e.g. [37]) like the shell and subshell electron densities [38] or averaged electron momentum density [39] in atoms etc. Introducing of the semiclassical self-consistent intra-atomic electric field yields the relative error not more than 22 /1~ n in de- termining of the electronic energies, where n is the prin- cipal quantum number of the highest occupied electron state [40]. Then, accuracy of the semiclassical approxi- mation should quite satisfactory even for light atoms. Effectiveness of the Bohr-type analytical models to the description of the periodic motion of electrons in small- sized molecules also was demonstrated [41]. For a long time, semiclassical asymptotic form was known to pro- vide a fundamental device for studying quantum systems in which non-perturbative effects play an essential role. But, the crucial step was advanced for the bound-state quantization of fermions few-body systems such as mo- lecules. Semiclassical quantization rules were success- fully applied to describe elastic interatomic scattering [42] in spectroscopy of diatomic molecules [43]. Using path integration as a relevant mathematical tool for semi- classical asymptotic form it was obtained semiclassical quantization rule for the periodic mean-field solutions [44]. Therm energies of diatomic K2 molecule calculated by the semiclassical method showed absolute deviations of only ~ 0.05 cm−1 from the quantum-mechanical results [45]. Same approach was found to be a strong method for generating the interatomic potential energy curve for diatomic molecules. It was provided a semiclassical de- scription of the shell-structure in fermions-system: level densities and shell-corrections were obtained from the  Intra-Atomic Electric Field Radial Potentials in Step-Like Presentation Copyright © 2010 SciRes. JEMAA 207 periodic orbit theory [46]. The semiclassical quantifica- tion method has raised increasing interest in relation to approximated method in various physical systems such as not only atoms, but molecules etc. It would serve as a general device for evaluating the bound-state spectra, once the exact or approximate solutions for the mean- field equation are known. Usually, different methods all use only periodic and/or non-closed quasi-periodic clas- sical orbitals as basis for the quantization. Contrary to them, in [47] it was introduced an adapted version of the semiclassical quantization method applied to molecular orbitals into path integrals formalism, and it also gives an alternative procedure for the calculation of the electronic correlation energy of a molecular system. Primitive semiclassical treatment even reveals exis- tence of a classical contribution to the chemical bond in small molecules: ground state electron is found to be exchanged classically between two nuclei [48]. Proceed- ing classical limit for a one-electron orbital model of such many-electron systems with electron periodic mo- tion leads to visualization of its quantum description [49]. Quantum description also can be introduced starting from the formal correspondence between classical harmonics of an electron periodic motion and its quantum jumps, i.e. Fourier-analysis added by the simple quantization condi- tion directly yields steady-state electron energies [50]. Even formation of the electron spin, which is considered as essentially quantum characteristic, can be explained within a classical model [51]. In case of multidimensional systems, the globally uni- form semiclassical approximation for energy eigenstates can be derived explicitly [52]. This is a true semiclassical approximation producing almost accurate wave functions providing with considerable degree of overlap (more than 0.98) between semiclassical and exact quantum eigen- states. Semiclassical method of calculation was used to describe electronic super-shells in metallic clusters [53]. Later, it was supposed a general method of the quasi- classical spectral analysis useful for central potentials with Coulomb singularity or finite value at the center which are characteristic for isolated atoms and spherical clusters, respectively [54]. Atomic clusters and con- densed phases can be calculated in framework of the density-functional theory (DFT) using a quasi-classical expansion of the energy functional [55]. However, as substance is considered as a non-relativ- istic electron system affected by the external field of nu- clei fixed at their sites in structure, its inner potential do not satisfy the standard Wentzel-Kramers-Brillouin (WKB) quasi-classical condition on spatial smoothness due to singularities at nuclear sites and electron shell effects. The success of the above approaches can be ex- plained on the basis of the quasi-classical expressions obtained by Maslov [56] for the energies of bound elec- tronic states. It follows from these expressions that the exact and quasi-classical spectra are similar to each other irrespective of the potential smoothness at 12 2 00 R, where 0 and 0 R are the characteristic values of the potential and its effective range, respectively (hereafter, all relationships will be given in the atomic system of units (a.u.)). 3. Semiclassical Parameterization of the Electric Charge Density and Electric Field Potential Distributions in an Atom The semiclassical parameterization of the atomic electric charge density and electric field potential distributions (see e.g. [57]) can be performed in analytical form if the effective fields acting on any ith electron in a neutral atom (i.e., Zi ,...,3,2,1 with Z as the nucleus charge) are represented by Coulomb-like potentials r Z ri i )( (1) where ||2 iii EnZ (2) is the effective charge of the nucleus screened by other electrons’ cloud dependent on the electron-state principal quantum number i n and its energy 0 i E. Electron charge equals to 1. Therefore, classical turning points radii i r and i r (ii rr ) of the i th electron with orbital quantum number i l can be found as the roots of the equation 2 2 )1( )( r ll rE ii ii (3) As a result, we obtain ||2 )1( 2 i iiii iE llnn r (4) ||2 )1( 2 i iiii iE llnn r (5) Let )( ~r i be the potential of the effective electric field induced by the i th electron. Then, potential )( ~ r of the electric field induced by the whole electron cloud can be written as the sum of the potentials )( ~ r i : Zi iirr 1)( ~ )( ~ (6) Potential of the electric field acting on an arbitrary i th electron of the atom is equal to the sum of the potentials of the nucleus Coulomb field and the field induced by all the electrons, except for the potential of the electron un- der consideration:  Intra-Atomic Electric Field Radial Potentials in Step-Like Presentation Copyright © 2010 SciRes. JEMAA 208 )( ~ )( ~rr r Z r Z i i (7) Now, we sum up such potentials over electrons. As a result, the terms independent of the electron number on the right-hand sides are multiplied by the total number Z of electrons in the atom and the sum of the potentials )( ~r i gives )( ~r. The solution of the obtained equa- tion with respect to )( ~ r has the form r Z ZZ r Zi ii1 1 )( ~1 2 (8) i.e. in this case, effective field of the interaction between nucleus and electron cloud also turns out to be a Cou- lomb-like field. Nucleus charge equals to Z and in the ground state its relative (to the electron cloud) motion corresponds to a zero orbital quantum number. Therefore, the radius of one classical turning point for nucleus is equal to 0 and the radius r ~ of another turning point is a root of the equation )( ~ ~rZE (9) where E ~ is the eigenvalue of the energy associated with the relative motion electron cloud and nucleus. Un- der the assumption that the nucleus has an infinite mass the reduced mass of the system nucleus – electron cloud with Z electrons equals to the cloud total mass Z. There- fore, energy and, consequently, turning point radius for the nucleus motion with respect to the electron cloud are given by the formulas 2 2 1 23 )1(2 )( ~ Z ZZZ E Zi ii (10) )( )1(2 ~ 1 22 Zi ii ZZZ Z r (11) The semiclassical, i.e., initial quasi-classical approxi- mation implies that exponentially decaying partial elec- tron densities are disregarded in the classically forbidden regions and that oscillations of these densities are ig- nored in classically allowed regions. As a result, the ra- dial dependence of the direction-averaged partial charge density of the ith electron state in atom is represented by a piecewise constant function: 33 () 0 3 4() 0 ii ii ii i rrr rrr rr rr (12) A similar averaging for the nucleus motion with re- spect to the electron cloud nucleus is equivalent to aver- aging the nuclear charge over a sphere of radius r ~ : 3 3 () 0 4 0 Z rrr r rr (13) Summation of similar contributions gives the distribu- tion of the total density of the electric charge in the atom in the form of a step radial function k Zi iirrr 1)()( ~ )( kk RrR 1qk,...,3,2,1 (14) where ρk are constants determined from the radii of the classical turning points and Rk coincide with these radii. Here, 1210 0qq RRRRR and Zq 2 is the number of layers with uniform charge densities. Parameter q R plays the role of the quasi-classical atomi- ic radius (the charge density is equal to zero at q Rr ). Mathematically, this representation is equivalent to the volume averaging in layers kk RrR 1. Next, we calculate the fields induced by the charged layers with densities k on the basis of the Gauss theorem and sum these fields. Then, the atomic potential can be written in the form of the continuously differenti- able piecewise analytical function 2 1 133 3 11 1 22 2 1 1 ()1, 2, 3,..., 44 () 33 2 3 2()2 k kk kk ik kiiikk i kk iq kiiikk ik a rbrcRrRk q r aRRR b cRRR (15) However, since the energy of the electronic system is a single-valued functional of the electron density, it is ex- pedient to approximate the above potential by a step function too. Averaging over the volume can adequately perform this: kk kk kkk kk kkk c RR RRb RR RRa r )(5 )(3 )(2 )(3 )( 3 1 3 5 1 5 3 1 3 2 1 2 kk RrR 1 qk,...,3,2,1 (16) 4. Tables The numerical values of parameters k R, k , and k can be found by fitting quasi-classical energetic levels i E to the Hartree–Fock (HF) ab initio ones [58]. Results of calculation are presented in Table 1 below for each 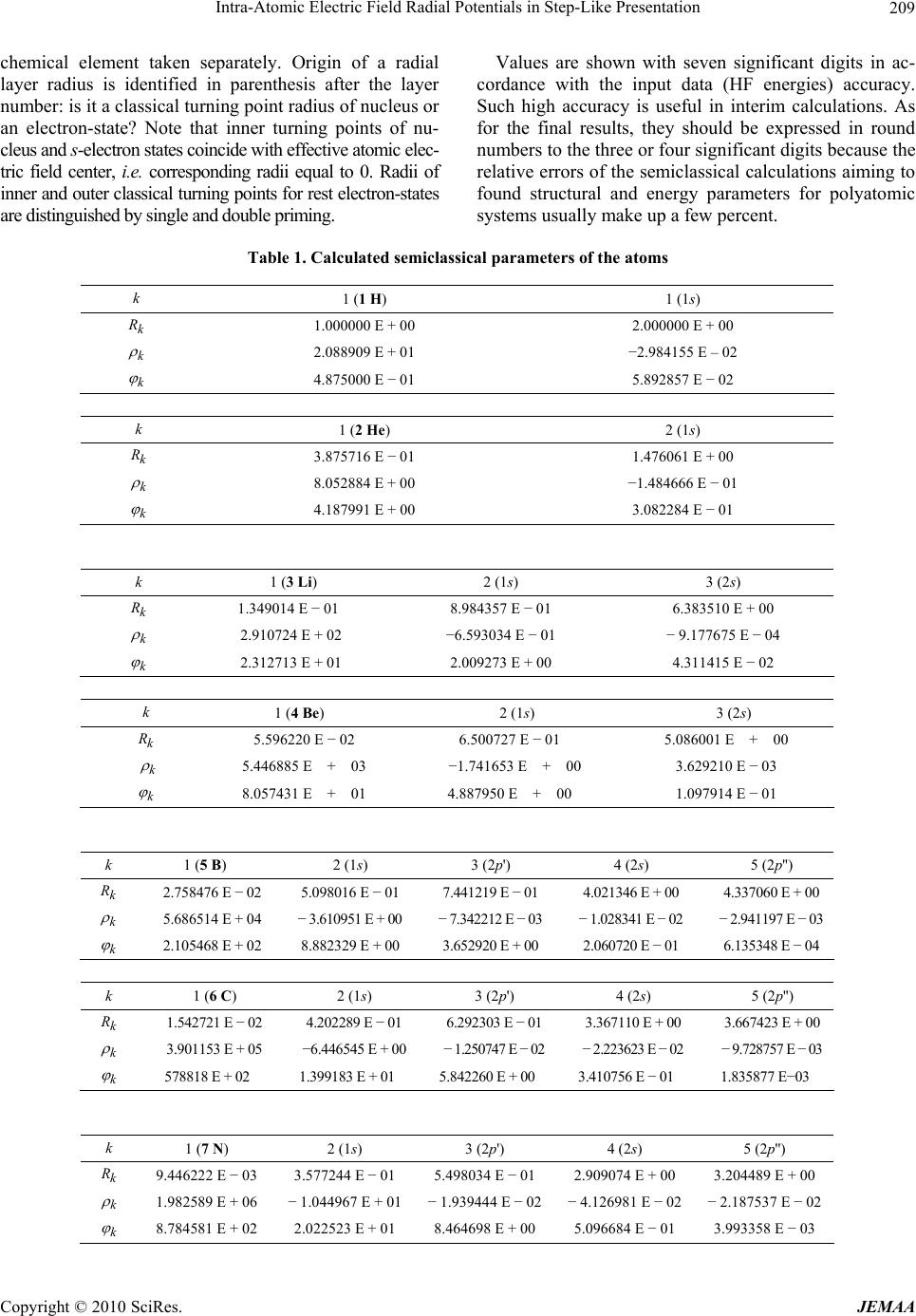 Intra-Atomic Electric Field Radial Potentials in Step-Like Presentation Copyright © 2010 SciRes. JEMAA 209 chemical element taken separately. Origin of a radial layer radius is identified in parenthesis after the layer number: is it a classical turning point radius of nucleus or an electron-state? Note that inner turning points of nu- cleus and s-electron states coincide with effective atomic elec- tric field center, i.e. corresponding radii equal to 0. Radii of inner and outer classical turning points for rest electron-states are distinguished by single and double priming. Values are shown with seven significant digits in ac- cordance with the input data (HF energies) accuracy. Such high accuracy is useful in interim calculations. As for the final results, they should be expressed in round numbers to the three or four significant digits because the relative errors of the semiclassical calculations aiming to found structural and energy parameters for polyatomic systems usually make up a few percent. Table 1. Calculated semiclassical parameters of the atoms k 1 (1 H) 1 (1s) k R 1.000000 E + 00 2.000000 E + 00 k 2.088909 E + 01 −2.984155 E – 02 k 4.875000 E − 01 5.892857 E − 02 k 1 (2 He) 2 (1s) k R 3.875716 E − 01 1.476061 E + 00 k 8.052884 E + 00 −1.484666 E − 01 k 4.187991 E + 00 3.082284 E − 01 k 1 (3 Li) 2 (1s) 3 (2s) k R 1.349014 E − 01 8.984357 E − 01 6.383510 E + 00 k 2.910724 E + 02 −6.593034 E − 01 − 9.177675 E − 04 k 2.312713 E + 01 2.009273 E + 00 4.311415 E − 02 k 1 (4 Be) 2 (1s) 3 (2s) k R 5.596220 E − 02 6.500727 E − 01 5.086001 E + 00 k 5.446885 E + 03 −1.741653 E + 00 3.629210 E − 03 k 8.057431 E + 01 4.887950 E + 00 1.097914 E − 01 k 1 (5 B) 2 (1s) 3 (2p') 4 (2s) 5 (2p'') k R 2.758476 E − 02 5.098016 E − 017.441219 E − 01 4.021346 E + 00 4.337060 E + 00 k 5.686514 E + 04 − 3.610951 E + 00− 7.342212 E − 03− 1.028341 E − 02− 2.941197 E − 03 k 2.105468 E + 02 8.882329 E + 003.652920 E + 00 2.060720 E − 01 6.135348 E − 04 k 1 (6 C) 2 (1s) 3 (2p') 4 (2s) 5 (2p'') k R 1.542721 E − 02 4.202289 E − 01 6.292303 E − 01 3.367110 E + 00 3.667423 E + 00 k 3.901153 E + 05 −6.446545 E + 00− 1.250747 E − 02 − 2.223623 E − 02 − 9.728757 E − 03 k 578818 E + 02 1.399183 E + 01 5.842260 E + 00 3.410756 E − 01 1.835877 E−03 k 1 (7 N) 2 (1s) 3 (2p') 4 (2s) 5 (2p'') k R 9.446222 E − 03 3.577244 E − 01 5.498034 E − 01 2.909074 E + 00 3.204489 E + 00 k 1.982589 E + 06 − 1.044967 E + 01− 1.939444 E − 02− 4.126981 E − 02− 2.187537 E − 02 k 8.784581 E + 02 2.022523 E + 01 8.464698 E + 00 5.096684 E − 01 3.993358 E − 03 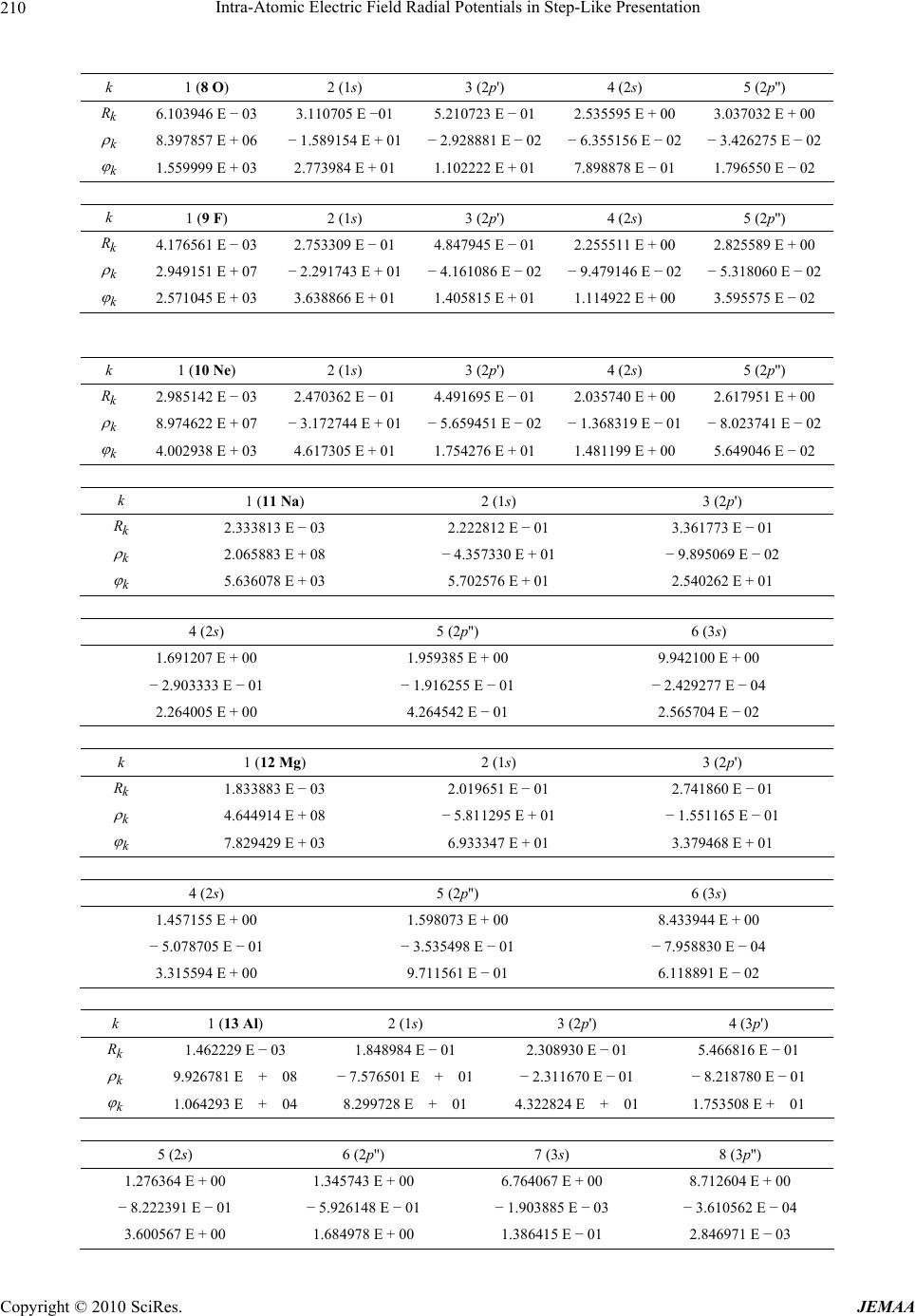 Intra-Atomic Electric Field Radial Potentials in Step-Like Presentation Copyright © 2010 SciRes. JEMAA 210 k 1 (8 O) 2 (1s) 3 (2p') 4 (2s) 5 (2p'') k R 6.103946 E − 03 3.110705 E −01 5.210723 E − 01 2.535595 E + 00 3.037032 E + 00 k 8.397857 E + 06 − 1.589154 E + 01− 2.928881 E − 02− 6.355156 E − 02− 3.426275 E − 02 k 1.559999 E + 03 2.773984 E + 01 1.102222 E + 01 7.898878 E − 01 1.796550 E − 02 k 1 (9 F) 2 (1s) 3 (2p') 4 (2s) 5 (2p'') k R 4.176561 E − 03 2.753309 E − 01 4.847945 E − 01 2.255511 E + 00 2.825589 E + 00 k 2.949151 E + 07 − 2.291743 E + 01− 4.161086 E − 02− 9.479146 E − 02− 5.318060 E − 02 k 2.571045 E + 03 3.638866 E + 01 1.405815 E + 01 1.114922 E + 00 3.595575 E − 02 k 1 (10 Ne) 2 (1s) 3 (2p') 4 (2s) 5 (2p'') k R 2.985142 E − 03 2.470362 E − 01 4.491695 E − 01 2.035740 E + 00 2.617951 E + 00 k 8.974622 E + 07 − 3.172744 E + 01− 5.659451 E − 02− 1.368319 E − 01− 8.023741 E − 02 k 4.002938 E + 03 4.617305 E + 01 1.754276 E + 01 1.481199 E + 00 5.649046 E − 02 k 1 (11 Na) 2 (1s) 3 (2p') k R 2.333813 E − 03 2.222812 E − 01 3.361773 E − 01 k 2.065883 E + 08 − 4.357330 E + 01 − 9.895069 E − 02 k 5.636078 E + 03 5.702576 E + 01 2.540262 E + 01 4 (2s) 5 (2p'') 6 (3s) 1.691207 E + 00 1.959385 E + 00 9.942100 E + 00 − 2.903333 E − 01 − 1.916255 E − 01 − 2.429277 E − 04 2.264005 E + 00 4.264542 E − 01 2.565704 E − 02 k 1 (12 Mg) 2 (1s) 3 (2p') k R 1.833883 E − 03 2.019651 E − 01 2.741860 E − 01 k 4.644914 E + 08 − 5.811295 E + 01 − 1.551165 E − 01 k 7.829429 E + 03 6.933347 E + 01 3.379468 E + 01 4 (2s) 5 (2p'') 6 (3s) 1.457155 E + 00 1.598073 E + 00 8.433944 E + 00 − 5.078705 E − 01 − 3.535498 E − 01 − 7.958830 E − 04 3.315594 E + 00 9.711561 E − 01 6.118891 E − 02 k 1 (13 Al) 2 (1s) 3 (2p') 4 (3p') k R 1.462229 E − 03 1.848984 E − 01 2.308930 E − 01 5.466816 E − 01 k 9.926781 E + 08 − 7.576501 E + 01 − 2.311670 E − 01 − 8.218780 E − 01 k 1.064293 E + 04 8.299728 E + 01 4.322824 E + 01 1.753508 E + 01 5 (2s) 6 (2p'') 7 (3s) 8 (3p'') 1.276364 E + 00 1.345743 E + 00 6.764067 E + 00 8.712604 E + 00 − 8.222391 E − 01 − 5.926148 E − 01 − 1.903885 E − 03 − 3.610562 E − 04 3.600567 E + 00 1.684978 E + 00 1.386415 E − 01 2.846971 E − 03 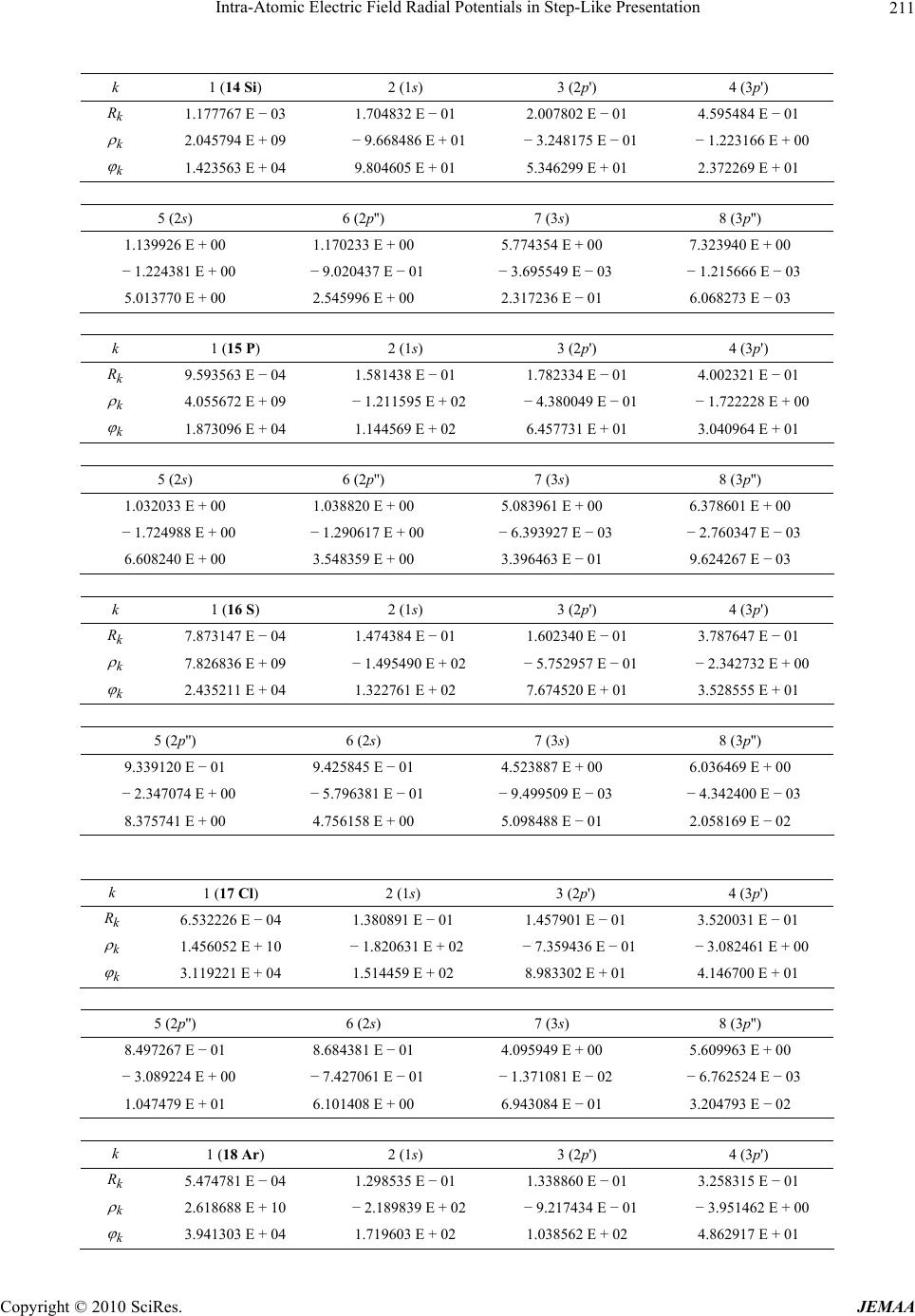 Intra-Atomic Electric Field Radial Potentials in Step-Like Presentation Copyright © 2010 SciRes. JEMAA 211 k 1 (14 Si) 2 (1s) 3 (2p') 4 (3p') k R 1.177767 E − 03 1.704832 E − 01 2.007802 E − 01 4.595484 E − 01 k 2.045794 E + 09 − 9.668486 E + 01 − 3.248175 E − 01 − 1.223166 E + 00 k 1.423563 E + 04 9.804605 E + 01 5.346299 E + 01 2.372269 E + 01 5 (2s) 6 (2p'') 7 (3s) 8 (3p'') 1.139926 E + 00 1.170233 E + 00 5.774354 E + 00 7.323940 E + 00 − 1.224381 E + 00 − 9.020437 E − 01 − 3.695549 E − 03 − 1.215666 E − 03 5.013770 E + 00 2.545996 E + 00 2.317236 E − 01 6.068273 E − 03 k 1 (15 P) 2 (1s) 3 (2p') 4 (3p') k R 9.593563 E − 04 1.581438 E − 01 1.782334 E − 01 4.002321 E − 01 k 4.055672 E + 09 − 1.211595 E + 02 − 4.380049 E − 01 − 1.722228 E + 00 k 1.873096 E + 04 1.144569 E + 02 6.457731 E + 01 3.040964 E + 01 5 (2s) 6 (2p'') 7 (3s) 8 (3p'') 1.032033 E + 00 1.038820 E + 00 5.083961 E + 00 6.378601 E + 00 − 1.724988 E + 00 − 1.290617 E + 00 − 6.393927 E − 03 − 2.760347 E − 03 6.608240 E + 00 3.548359 E + 00 3.396463 E − 01 9.624267 E − 03 k 1 (16 S) 2 (1s) 3 (2p') 4 (3p') k R 7.873147 E − 04 1.474384 E − 01 1.602340 E − 01 3.787647 E − 01 k 7.826836 E + 09 − 1.495490 E + 02 − 5.752957 E − 01 − 2.342732 E + 00 k 2.435211 E + 04 1.322761 E + 02 7.674520 E + 01 3.528555 E + 01 5 (2p'') 6 (2s) 7 (3s) 8 (3p'') 9.339120 E − 01 9.425845 E − 01 4.523887 E + 00 6.036469 E + 00 − 2.347074 E + 00 − 5.796381 E − 01 − 9.499509 E − 03 − 4.342400 E − 03 8.375741 E + 00 4.756158 E + 00 5.098488 E − 01 2.058169 E − 02 k 1 (17 Cl) 2 (1s) 3 (2p') 4 (3p') k R 6.532226 E − 04 1.380891 E − 01 1.457901 E − 01 3.520031 E − 01 k 1.456052 E + 10 − 1.820631 E + 02 − 7.359436 E − 01 − 3.082461 E + 00 k 3.119221 E + 04 1.514459 E + 02 8.983302 E + 01 4.146700 E + 01 5 (2p'') 6 (2s) 7 (3s) 8 (3p'') 8.497267 E − 01 8.684381 E − 01 4.095949 E + 00 5.609963 E + 00 − 3.089224 E + 00 − 7.427061 E − 01 − 1.371081 E − 02 − 6.762524 E − 03 1.047479 E + 01 6.101408 E + 00 6.943084 E − 01 3.204793 E − 02 k 1 (18 Ar) 2 (1s) 3 (2p') 4 (3p') k R 5.474781 E − 04 1.298535 E − 01 1.338860 E − 01 3.258315 E − 01 k 2.618688 E + 10 − 2.189839 E + 02 − 9.217434 E − 01 − 3.951462 E + 00 k 3.941303 E + 04 1.719603 E + 02 1.038562 E + 02 4.862917 E + 01 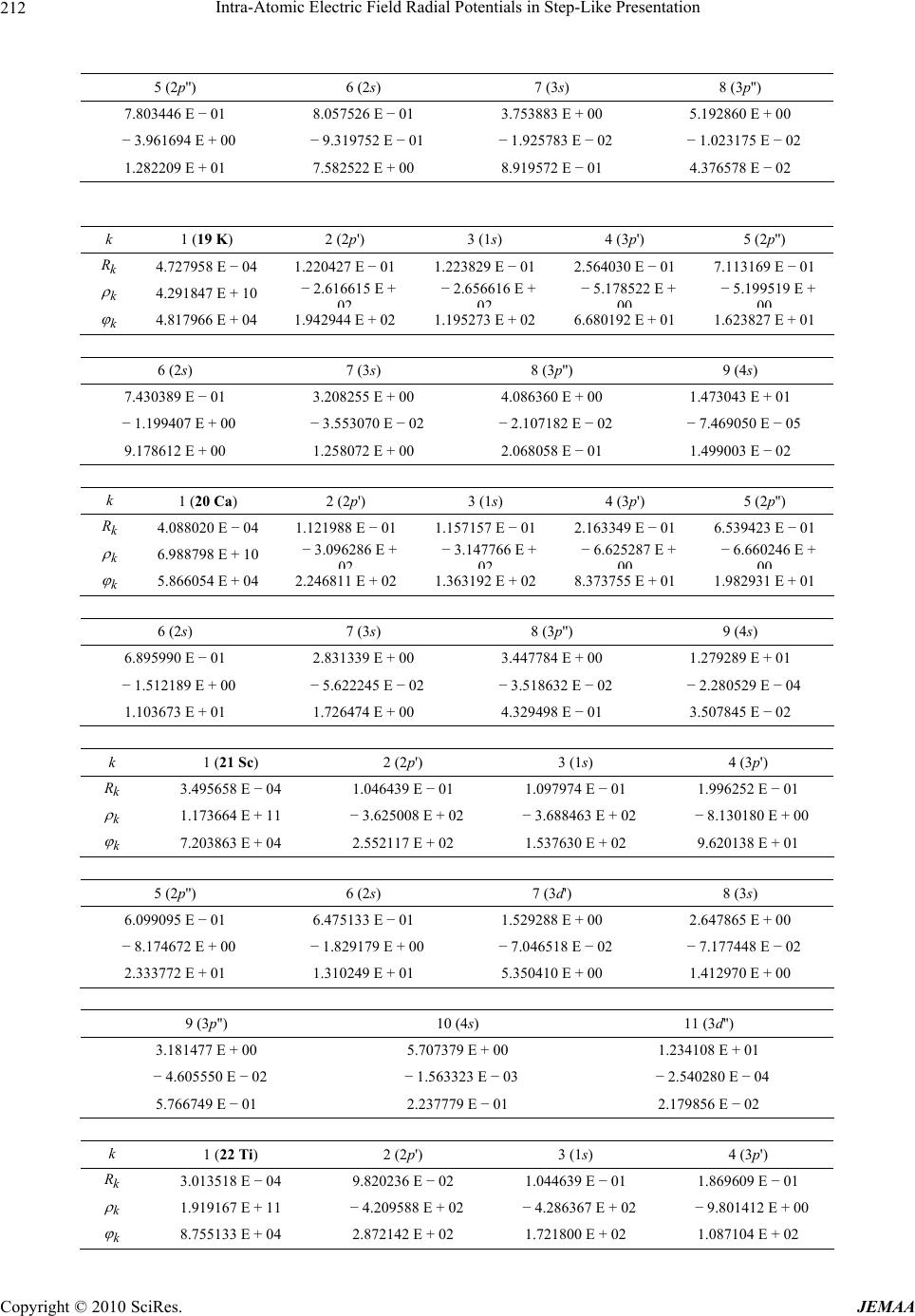 Intra-Atomic Electric Field Radial Potentials in Step-Like Presentation Copyright © 2010 SciRes. JEMAA 212 5 (2p'') 6 (2s) 7 (3s) 8 (3p'') 7.803446 E − 01 8.057526 E − 01 3.753883 E + 00 5.192860 E + 00 − 3.961694 E + 00 − 9.319752 E − 01 − 1.925783 E − 02 − 1.023175 E − 02 1.282209 E + 01 7.582522 E + 00 8.919572 E − 01 4.376578 E − 02 k 1 (19 K) 2 (2p') 3 (1s) 4 (3p') 5 (2p'') k R 4.727958 E − 04 1.220427 E − 01 1.223829 E − 01 2.564030 E − 01 7.113169 E − 01 k 4.291847 E + 10 − 2.616615 E + 02 − 2.656616 E + 02 − 5.178522 E + 00 − 5.199519 E + 00 k 4.817966 E + 04 1.942944 E + 02 1.195273 E + 02 6.680192 E + 01 1.623827 E + 01 6 (2s) 7 (3s) 8 (3p'') 9 (4s) 7.430389 E − 01 3.208255 E + 00 4.086360 E + 00 1.473043 E + 01 − 1.199407 E + 00 − 3.553070 E − 02 − 2.107182 E − 02 − 7.469050 E − 05 9.178612 E + 00 1.258072 E + 00 2.068058 E − 01 1.499003 E − 02 k 1 (20 Ca) 2 (2p') 3 (1s) 4 (3p') 5 (2p'') k R 4.088020 E − 04 1.121988 E − 01 1.157157 E − 01 2.163349 E − 01 6.539423 E − 01 k 6.988798 E + 10 − 3.096286 E + 02 − 3.147766 E + 02 − 6.625287 E + 00 − 6.660246 E + 00 k 5.866054 E + 04 2.246811 E + 02 1.363192 E + 02 8.373755 E + 01 1.982931 E + 01 6 (2s) 7 (3s) 8 (3p'') 9 (4s) 6.895990 E − 01 2.831339 E + 00 3.447784 E + 00 1.279289 E + 01 − 1.512189 E + 00 − 5.622245 E − 02 − 3.518632 E − 02 − 2.280529 E − 04 1.103673 E + 01 1.726474 E + 00 4.329498 E − 01 3.507845 E − 02 k 1 (21 Sc) 2 (2p') 3 (1s) 4 (3p') k R 3.495658 E − 04 1.046439 E − 01 1.097974 E − 01 1.996252 E − 01 k 1.173664 E + 11 − 3.625008 E + 02 − 3.688463 E + 02 − 8.130180 E + 00 k 7.203863 E + 04 2.552117 E + 02 1.537630 E + 02 9.620138 E + 01 5 (2p'') 6 (2s) 7 (3d') 8 (3s) 6.099095 E − 01 6.475133 E − 01 1.529288 E + 00 2.647865 E + 00 − 8.174672 E + 00 − 1.829179 E + 00 − 7.046518 E − 02 − 7.177448 E − 02 2.333772 E + 01 1.310249 E + 01 5.350410 E + 00 1.412970 E + 00 9 (3p'') 10 (4s) 11 (3d'') 3.181477 E + 00 5.707379 E + 00 1.234108 E + 01 − 4.605550 E − 02 − 1.563323 E − 03 − 2.540280 E − 04 5.766749 E − 01 2.237779 E − 01 2.179856 E − 02 k 1 (22 Ti) 2 (2p') 3 (1s) 4 (3p') k R 3.013518 E − 04 9.820236 E − 02 1.044639 E − 01 1.869609 E − 01 k 1.919167 E + 11 − 4.209588 E + 02 − 4.286367 E + 02 − 9.801412 E + 00 k 8.755133 E + 04 2.872142 E + 02 1.721800 E + 02 1.087104 E + 02 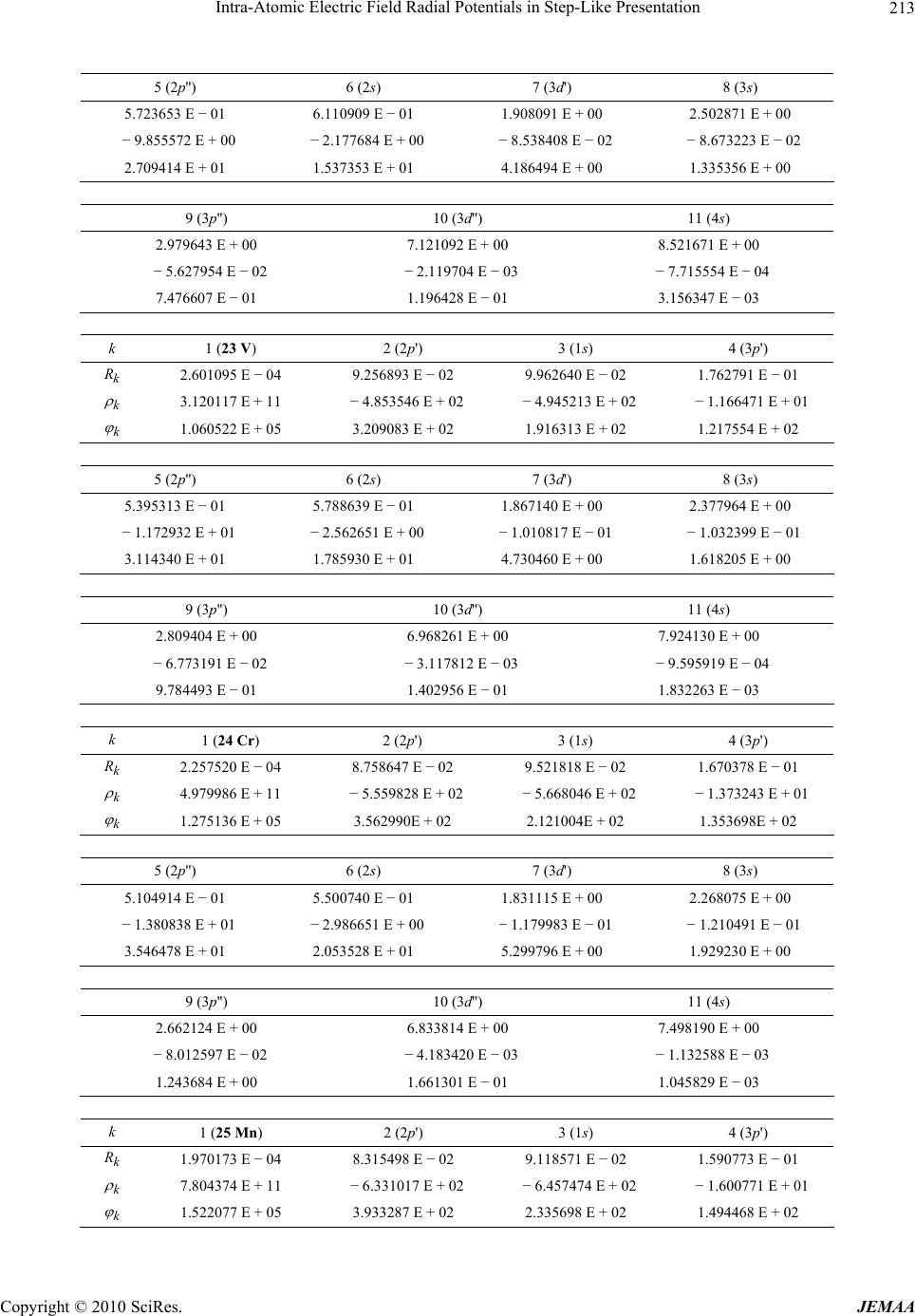 Intra-Atomic Electric Field Radial Potentials in Step-Like Presentation Copyright © 2010 SciRes. JEMAA 213 5 (2p'') 6 (2s) 7 (3d') 8 (3s) 5.723653 E − 01 6.110909 E − 01 1.908091 E + 00 2.502871 E + 00 − 9.855572 E + 00 − 2.177684 E + 00 − 8.538408 E − 02 − 8.673223 E − 02 2.709414 E + 01 1.537353 E + 01 4.186494 E + 00 1.335356 E + 00 9 (3p'') 10 (3d'') 11 (4s) 2.979643 E + 00 7.121092 E + 00 8.521671 E + 00 − 5.627954 E − 02 − 2.119704 E − 03 − 7.715554 E − 04 7.476607 E − 01 1.196428 E − 01 3.156347 E − 03 k 1 (23 V) 2 (2p') 3 (1s) 4 (3p') k R 2.601095 E − 04 9.256893 E − 02 9.962640 E − 02 1.762791 E − 01 k 3.120117 E + 11 − 4.853546 E + 02 − 4.945213 E + 02 − 1.166471 E + 01 k 1.060522 E + 05 3.209083 E + 02 1.916313 E + 02 1.217554 E + 02 5 (2p'') 6 (2s) 7 (3d') 8 (3s) 5.395313 E − 01 5.788639 E − 01 1.867140 E + 00 2.377964 E + 00 − 1.172932 E + 01 − 2.562651 E + 00 − 1.010817 E − 01 − 1.032399 E − 01 3.114340 E + 01 1.785930 E + 01 4.730460 E + 00 1.618205 E + 00 9 (3p'') 10 (3d'') 11 (4s) 2.809404 E + 00 6.968261 E + 00 7.924130 E + 00 − 6.773191 E − 02 − 3.117812 E − 03 − 9.595919 E − 04 9.784493 E − 01 1.402956 E − 01 1.832263 E − 03 k 1 (24 Cr) 2 (2p') 3 (1s) 4 (3p') k R 2.257520 E − 04 8.758647 E − 02 9.521818 E − 02 1.670378 E − 01 k 4.979986 E + 11 − 5.559828 E + 02 − 5.668046 E + 02 − 1.373243 E + 01 k 1.275136 E + 05 3.562990E + 02 2.121004E + 02 1.353698E + 02 5 (2p'') 6 (2s) 7 (3d') 8 (3s) 5.104914 E − 01 5.500740 E − 01 1.831115 E + 00 2.268075 E + 00 − 1.380838 E + 01 − 2.986651 E + 00 − 1.179983 E − 01 − 1.210491 E − 01 3.546478 E + 01 2.053528 E + 01 5.299796 E + 00 1.929230 E + 00 9 (3p'') 10 (3d'') 11 (4s) 2.662124 E + 00 6.833814 E + 00 7.498190 E + 00 − 8.012597 E − 02 − 4.183420 E − 03 − 1.132588 E − 03 1.243684 E + 00 1.661301 E − 01 1.045829 E − 03 k 1 (25 Mn) 2 (2p') 3 (1s) 4 (3p') k R 1.970173 E − 04 8.315498 E − 02 9.118571 E − 02 1.590773 E − 01 k 7.804374 E + 11 − 6.331017 E + 02 − 6.457474 E + 02 − 1.600771 E + 01 k 1.522077 E + 05 3.933287 E + 02 2.335698 E + 02 1.494468 E + 02 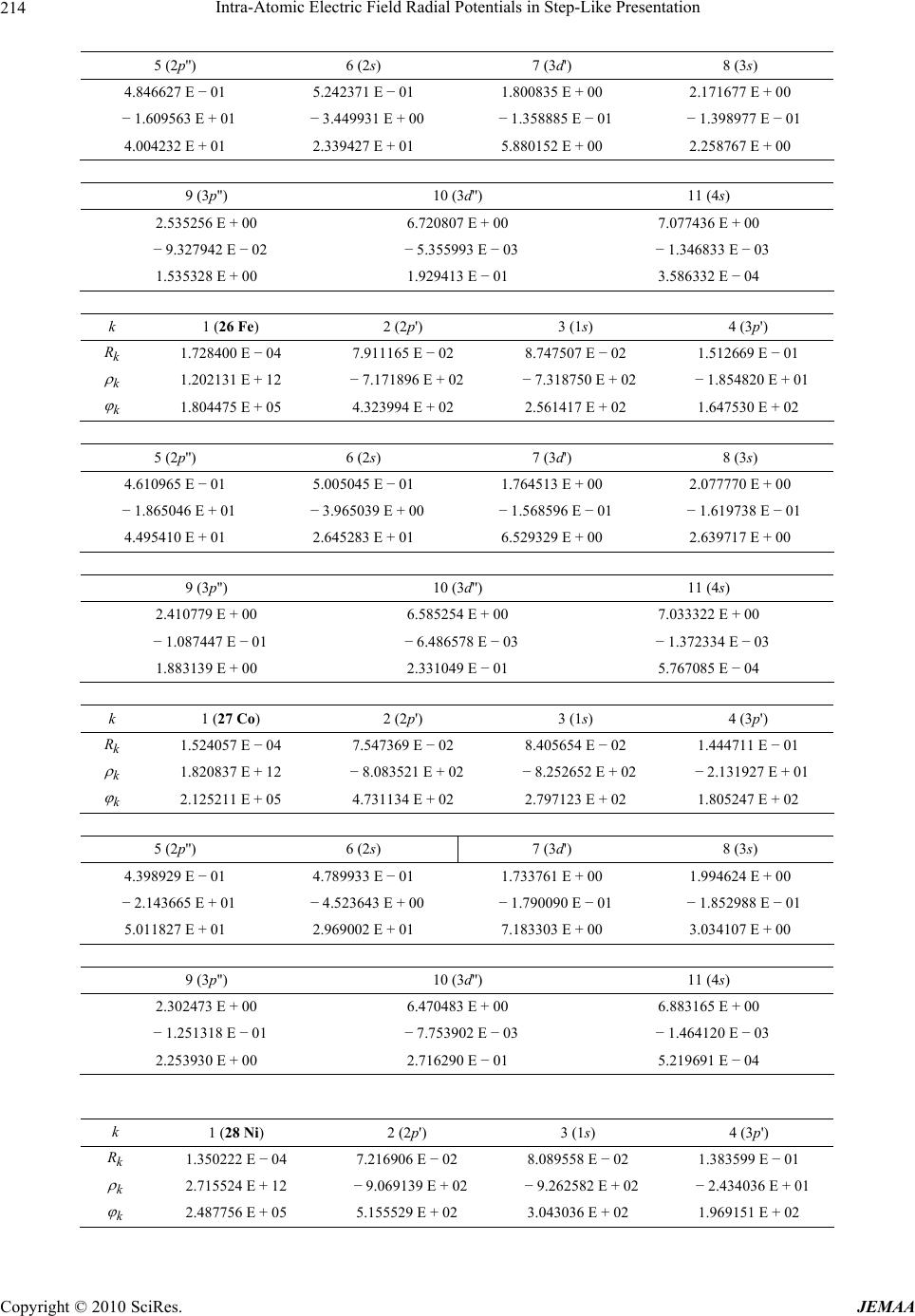 Intra-Atomic Electric Field Radial Potentials in Step-Like Presentation Copyright © 2010 SciRes. JEMAA 214 5 (2p'') 6 (2s) 7 (3d') 8 (3s) 4.846627 E − 01 5.242371 E − 01 1.800835 E + 00 2.171677 E + 00 − 1.609563 E + 01 − 3.449931 E + 00 − 1.358885 E − 01 − 1.398977 E − 01 4.004232 E + 01 2.339427 E + 01 5.880152 E + 00 2.258767 E + 00 9 (3p'') 10 (3d'') 11 (4s) 2.535256 E + 00 6.720807 E + 00 7.077436 E + 00 − 9.327942 E − 02 − 5.355993 E − 03 − 1.346833 E − 03 1.535328 E + 00 1.929413 E − 01 3.586332 E − 04 k 1 (26 Fe) 2 (2p') 3 (1s) 4 (3p') k R 1.728400 E − 04 7.911165 E − 02 8.747507 E − 02 1.512669 E − 01 k 1.202131 E + 12 − 7.171896 E + 02 − 7.318750 E + 02 − 1.854820 E + 01 k 1.804475 E + 05 4.323994 E + 02 2.561417 E + 02 1.647530 E + 02 5 (2p'') 6 (2s) 7 (3d') 8 (3s) 4.610965 E − 01 5.005045 E − 01 1.764513 E + 00 2.077770 E + 00 − 1.865046 E + 01 − 3.965039 E + 00 − 1.568596 E − 01 − 1.619738 E − 01 4.495410 E + 01 2.645283 E + 01 6.529329 E + 00 2.639717 E + 00 9 (3p'') 10 (3d'') 11 (4s) 2.410779 E + 00 6.585254 E + 00 7.033322 E + 00 − 1.087447 E − 01 − 6.486578 E − 03 − 1.372334 E − 03 1.883139 E + 00 2.331049 E − 01 5.767085 E − 04 k 1 (27 Co) 2 (2p') 3 (1s) 4 (3p') k R 1.524057 E − 04 7.547369 E − 02 8.405654 E − 02 1.444711 E − 01 k 1.820837 E + 12 − 8.083521 E + 02 − 8.252652 E + 02 − 2.131927 E + 01 k 2.125211 E + 05 4.731134 E + 02 2.797123 E + 02 1.805247 E + 02 5 (2p'') 6 (2s) 7 (3d') 8 (3s) 4.398929 E − 01 4.789933 E − 01 1.733761 E + 00 1.994624 E + 00 − 2.143665 E + 01 − 4.523643 E + 00 − 1.790090 E − 01 − 1.852988 E − 01 5.011827 E + 01 2.969002 E + 01 7.183303 E + 00 3.034107 E + 00 9 (3p'') 10 (3d'') 11 (4s) 2.302473 E + 00 6.470483 E + 00 6.883165 E + 00 − 1.251318 E − 01 − 7.753902 E − 03 − 1.464120 E − 03 2.253930 E + 00 2.716290 E − 01 5.219691 E − 04 k 1 (28 Ni) 2 (2p') 3 (1s) 4 (3p') k R 1.350222 E − 04 7.216906 E − 02 8.089558 E − 02 1.383599 E − 01 k 2.715524 E + 12 − 9.069139 E + 02 − 9.262582 E + 02 − 2.434036 E + 01 k 2.487756 E + 05 5.155529 E + 02 3.043036 E + 02 1.969151 E + 02 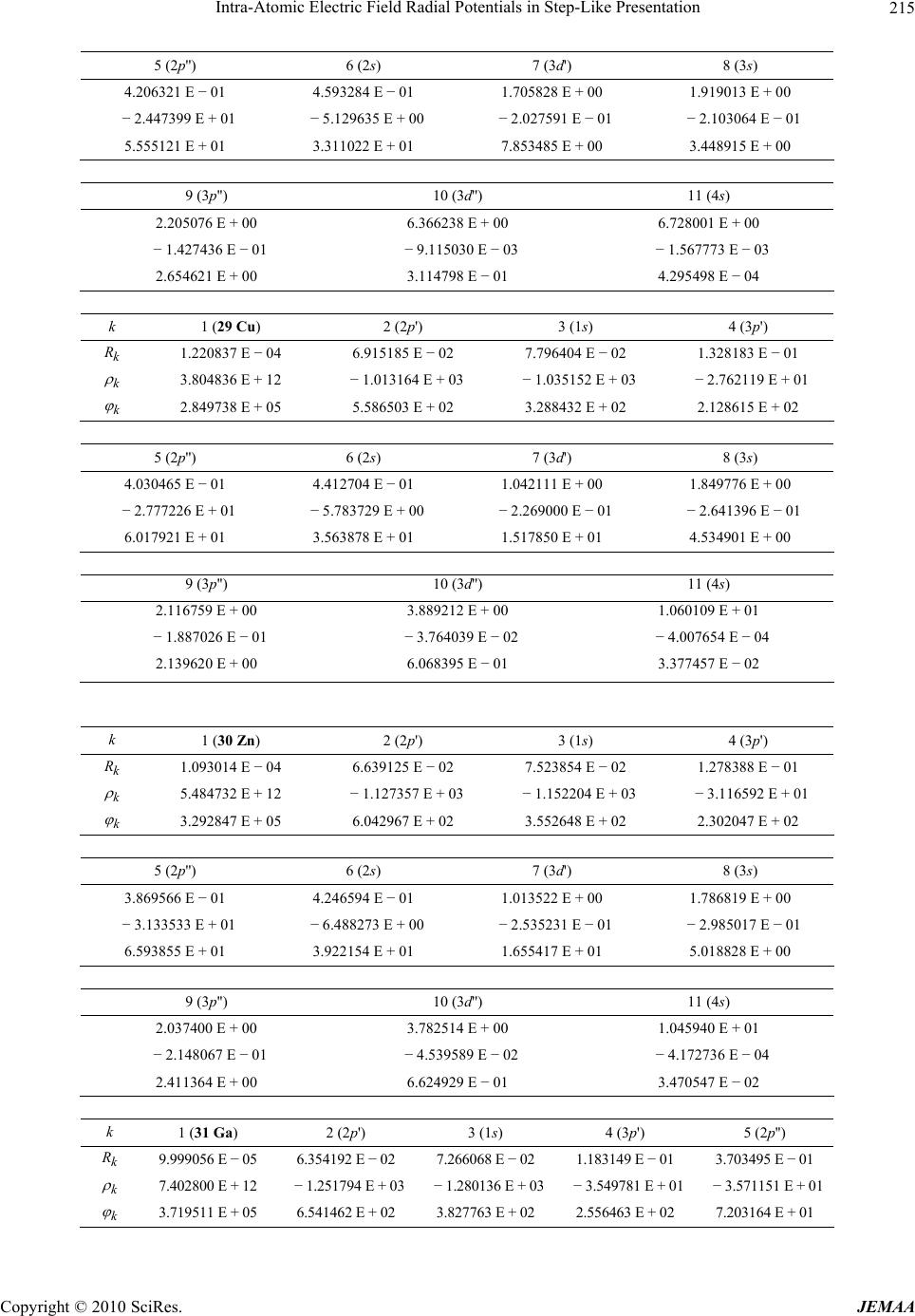 Intra-Atomic Electric Field Radial Potentials in Step-Like Presentation Copyright © 2010 SciRes. JEMAA 215 5 (2p'') 6 (2s) 7 (3d') 8 (3s) 4.206321 E − 01 4.593284 E − 01 1.705828 E + 00 1.919013 E + 00 − 2.447399 E + 01 − 5.129635 E + 00 − 2.027591 E − 01 − 2.103064 E − 01 5.555121 E + 01 3.311022 E + 01 7.853485 E + 00 3.448915 E + 00 9 (3p'') 10 (3d'') 11 (4s) 2.205076 E + 00 6.366238 E + 00 6.728001 E + 00 − 1.427436 E − 01 − 9.115030 E − 03 − 1.567773 E − 03 2.654621 E + 00 3.114798 E − 01 4.295498 E − 04 k 1 (29 Cu) 2 (2p') 3 (1s) 4 (3p') k R 1.220837 E − 04 6.915185 E − 02 7.796404 E − 02 1.328183 E − 01 k 3.804836 E + 12 − 1.013164 E + 03 − 1.035152 E + 03 − 2.762119 E + 01 k 2.849738 E + 05 5.586503 E + 02 3.288432 E + 02 2.128615 E + 02 5 (2p'') 6 (2s) 7 (3d') 8 (3s) 4.030465 E − 01 4.412704 E − 01 1.042111 E + 00 1.849776 E + 00 − 2.777226 E + 01 − 5.783729 E + 00 − 2.269000 E − 01 − 2.641396 E − 01 6.017921 E + 01 3.563878 E + 01 1.517850 E + 01 4.534901 E + 00 9 (3p'') 10 (3d'') 11 (4s) 2.116759 E + 00 3.889212 E + 00 1.060109 E + 01 − 1.887026 E − 01 − 3.764039 E − 02 − 4.007654 E − 04 2.139620 E + 00 6.068395 E − 01 3.377457 E − 02 k 1 (30 Zn) 2 (2p') 3 (1s) 4 (3p') k R 1.093014 E − 04 6.639125 E − 02 7.523854 E − 02 1.278388 E − 01 k 5.484732 E + 12 − 1.127357 E + 03 − 1.152204 E + 03 − 3.116592 E + 01 k 3.292847 E + 05 6.042967 E + 02 3.552648 E + 02 2.302047 E + 02 5 (2p'') 6 (2s) 7 (3d') 8 (3s) 3.869566 E − 01 4.246594 E − 01 1.013522 E + 00 1.786819 E + 00 − 3.133533 E + 01 − 6.488273 E + 00 − 2.535231 E − 01 − 2.985017 E − 01 6.593855 E + 01 3.922154 E + 01 1.655417 E + 01 5.018828 E + 00 9 (3p'') 10 (3d'') 11 (4s) 2.037400 E + 00 3.782514 E + 00 1.045940 E + 01 − 2.148067 E − 01 − 4.539589 E − 02 − 4.172736 E − 04 2.411364 E + 00 6.624929 E − 01 3.470547 E − 02 k 1 (31 Ga) 2 (2p') 3 (1s) 4 (3p') 5 (2p'') k R 9.999056 E − 05 6.354192 E − 02 7.266068 E − 02 1.183149 E − 01 3.703495 E − 01 k 7.402800 E + 12 − 1.251794 E + 03 − 1.280136 E + 03 − 3.549781 E + 01 − 3.571151 E + 01 k 3.719511 E + 05 6.541462 E + 02 3.827763 E + 02 2.556463 E + 02 7.203164 E + 01  Intra-Atomic Electric Field Radial Potentials in Step-Like Presentation Copyright © 2010 SciRes. JEMAA 216 6 (4p') 7 (2s) 8 (3d') 9 (3s) 4.000629 E − 01 4.075339 E − 01 8.207280 E − 01 1.677752 E + 00 − 7.369772 E + 00 − 7.369910 E + 00 − 3.156732 E − 01 − 4.003778 E − 01 4.294966 E + 01 4.044939 E + 01 2.158163 E + 01 5.860955 E + 00 10 (3p'') 11 (3d'') 12 (4s) 13 (4p'') 1.885615 E + 00 3.062999 E + 00 8.681390 E + 00 1.198854 E + 01 − 2.992761 E − 01 − 8.557296 E − 02 − 8.683064 E − 04 − 1.385572 E − 04 2.525737 E + 00 9.701396 E − 01 8.953004 E − 02 3.130941 E − 03 k 1 (32 Ge) 2 (2p') 3 (1s) 4 (3p') 5 (4p') k R 9.152975 E − 05 6.091629 E − 02 7.025164 E − 02 1.102557 E − 01 3.407788 E − 01 k 9.962644 E + 12 − 1.385189 E + 03 − 1.417356 E + 03 − 4.023520 E + 01 − 4.049928 E + 01 k 4.194475 E + 05 7.062215 E + 02 4.114887 E + 02 2.818673 E + 02 8.232897 E + 01 6 (2p'') 7 (2s) 8 (3d') 9 (3s) 3.550461 E − 01 3.916665 E − 01 7.011994 E − 01 1.582128 E + 00 − 4.049972 E + 01 − 8.332968 E + 00 − 3.861721 E − 01 − 5.219974 E − 01 5.031343 E + 01 4.588502 E + 01 2.635623 E + 01 6.739995 E + 00 10 (3p'') 11 (3d'') 12 (4s) 13 (4p'') 1.757173 E + 00 2.616912 E + 00 7.604480 E + 00 1.021199 E + 01 − 4.014337 E − 01 − 1.373594 E − 01 − 1.534117 E − 03 − 4.483593 E − 04 2.784645 E + 00 1.339787 E + 00 1.525060 E − 01 6.312235 E − 03 k 1 (33 As) 2 (2p') 3 (1s) 4 (3p') 5 (4p') k R 8.390709 E − 05 5.848880 E − 026.799527 E − 021.032950 E − 013.005274 E − 01 k 1.333612 E + 13 − 1.527877 E + 03− 1.564217 E + 03− 4.540040 E + 01− 4.572154 E + 01 k 4.718582 E + 05 7.604931 E + 024.413606 E + 023.089537 E + 029.868202 E + 01 6 (2p'') 7 (2s) 8 (3d') 9 (3s) 3.408977 E − 01 3.769232 E − 01 6.168400 E − 01 1.497231 E + 00 − 4.572252 E + 01 − 9.382132 E + 00 − 4.658747 E − 01 − 6.653952 E − 01 5.747501 E + 01 4.960630 E + 01 3.109604 E + 01 7.683508 E + 00 10 (3p'') 11 (3d'') 12 (4s) 13 (4p'') 1.646238 E + 00 2.302078 E + 00 6.830392 E + 00 9.005793 E + 00 − 5.231378 E − 01 − 2.019994 E − 01 − 2.478899 E − 03 − 9.805796 E − 04 3.142610 E + 00 1.769067 E + 00 2.236234 E − 01 9.621769 E − 03 k 1 (34 Se) 2 (2p') 3 (1s) 4 (3p') 5 (4p') k R 7.701127 E − 05 5.622750 E − 02 6.587597 E − 02 9.705244 E − 02 2.878107 E − 01 k 1.777164 E + 13 − 1.680309 E + 03− 1.721213 E + 03− 5.104387 E + 01− 5.143105 E + 01 k 5.296960 E + 05 8.171156 E + 02 4.724217 E + 02 3.373356 E + 02 1.075189 E + 02 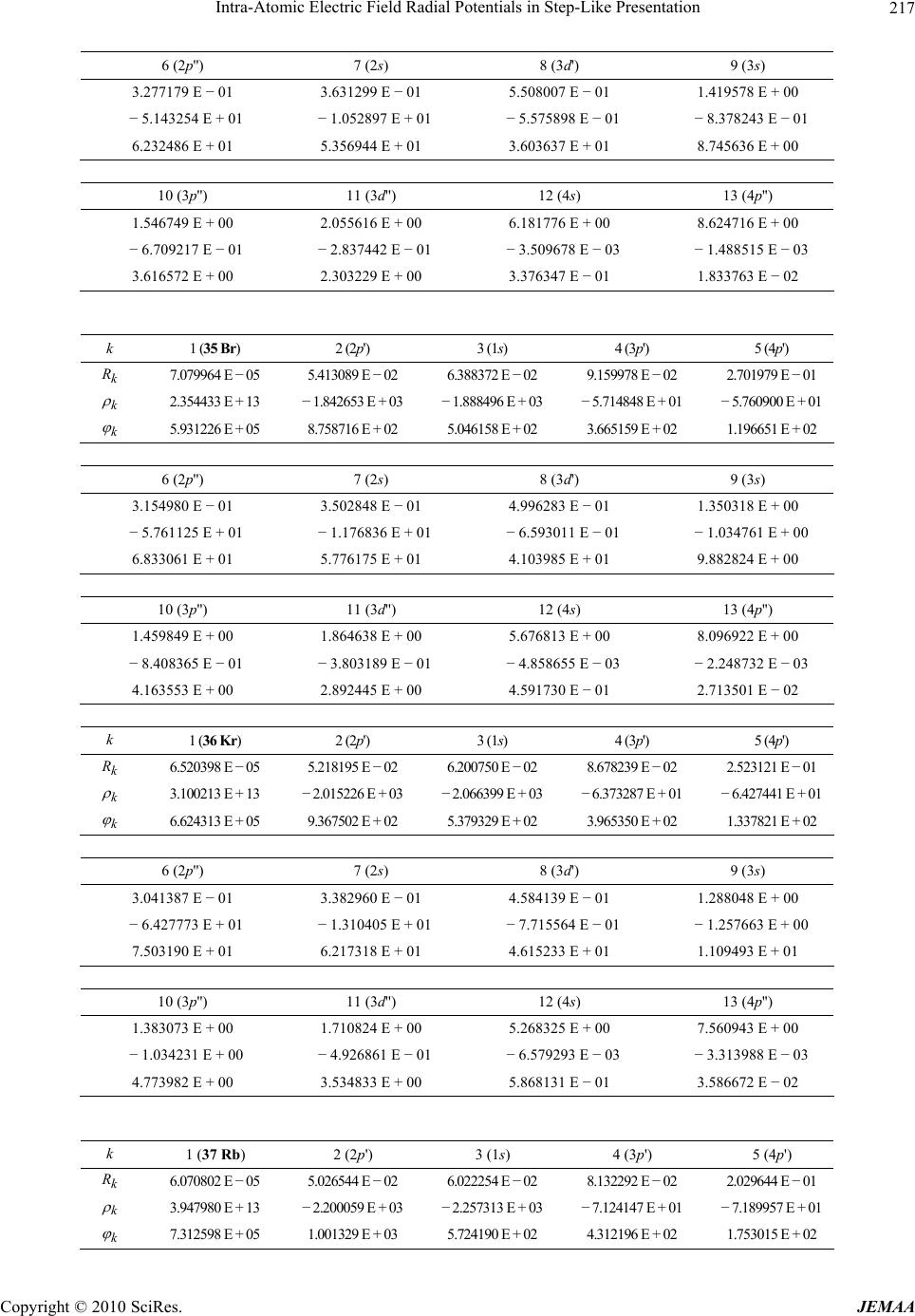 Intra-Atomic Electric Field Radial Potentials in Step-Like Presentation Copyright © 2010 SciRes. JEMAA 217 6 (2p'') 7 (2s) 8 (3d') 9 (3s) 3.277179 E − 01 3.631299 E − 01 5.508007 E − 01 1.419578 E + 00 − 5.143254 E + 01 − 1.052897 E + 01 − 5.575898 E − 01 − 8.378243 E − 01 6.232486 E + 01 5.356944 E + 01 3.603637 E + 01 8.745636 E + 00 10 (3p'') 11 (3d'') 12 (4s) 13 (4p'') 1.546749 E + 00 2.055616 E + 00 6.181776 E + 00 8.624716 E + 00 − 6.709217 E − 01 − 2.837442 E − 01 − 3.509678 E − 03 − 1.488515 E − 03 3.616572 E + 00 2.303229 E + 00 3.376347 E − 01 1.833763 E − 02 k 1 (35 Br) 2 (2p') 3 (1s) 4 (3p') 5 (4p') k R 7.079964 E − 05 5.413089 E − 02 6.388372 E − 02 9.159978 E − 02 2.701979 E − 01 k 2.354433 E + 13 − 1.842653 E + 03 − 1.888496 E + 03 − 5.714848 E + 01 − 5.760900 E + 01 k 5.931226 E + 05 8.758716 E + 02 5.046158 E + 02 3.665159 E + 02 1.196651 E + 02 6 (2p'') 7 (2s) 8 (3d') 9 (3s) 3.154980 E − 01 3.502848 E − 01 4.996283 E − 01 1.350318 E + 00 − 5.761125 E + 01 − 1.176836 E + 01 − 6.593011 E − 01 − 1.034761 E + 00 6.833061 E + 01 5.776175 E + 01 4.103985 E + 01 9.882824 E + 00 10 (3p'') 11 (3d'') 12 (4s) 13 (4p'') 1.459849 E + 00 1.864638 E + 00 5.676813 E + 00 8.096922 E + 00 − 8.408365 E − 01 − 3.803189 E − 01 − 4.858655 E − 03 − 2.248732 E − 03 4.163553 E + 00 2.892445 E + 00 4.591730 E − 01 2.713501 E − 02 k 1 (36 Kr) 2 (2p') 3 (1s) 4 (3p') 5 (4p') k R 6.520398 E − 05 5.218195 E − 02 6.200750 E − 02 8.678239 E − 02 2.523121 E − 01 k 3.100213 E + 13 − 2.015226 E + 03 − 2.066399 E + 03 − 6.373287 E + 01 − 6.427441 E + 01 k 6.624313 E + 05 9.367502 E + 02 5.379329 E + 02 3.965350 E + 02 1.337821 E + 02 6 (2p'') 7 (2s) 8 (3d') 9 (3s) 3.041387 E − 01 3.382960 E − 01 4.584139 E − 01 1.288048 E + 00 − 6.427773 E + 01 − 1.310405 E + 01 − 7.715564 E − 01 − 1.257663 E + 00 7.503190 E + 01 6.217318 E + 01 4.615233 E + 01 1.109493 E + 01 10 (3p'') 11 (3d'') 12 (4s) 13 (4p'') 1.383073 E + 00 1.710824 E + 00 5.268325 E + 00 7.560943 E + 00 − 1.034231 E + 00 − 4.926861 E − 01 − 6.579293 E − 03 − 3.313988 E − 03 4.773982 E + 00 3.534833 E + 00 5.868131 E − 01 3.586672 E − 02 k 1 (37 Rb) 2 (2p') 3 (1s) 4 (3p') 5 (4p') k R 6.070802 E − 05 5.026544 E − 02 6.022254 E − 02 8.132292 E − 02 2.029644 E − 01 k 3.947980 E + 13 − 2.200059 E + 03 − 2.257313 E + 03 − 7.124147 E + 01 − 7.189957 E + 01 k 7.312598 E + 05 1.001329 E + 03 5.724190 E + 02 4.312196 E + 02 1.753015 E + 02 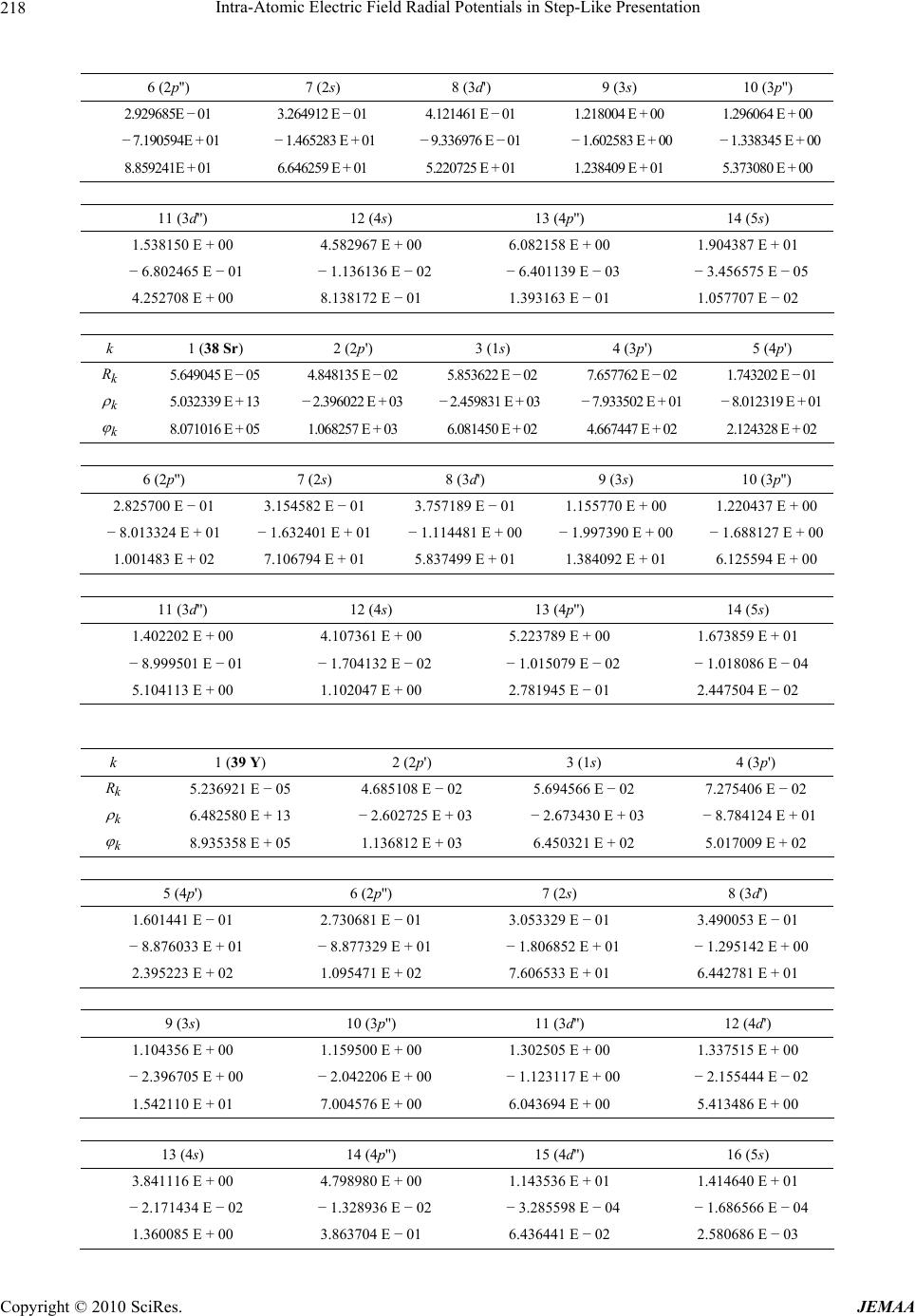 Intra-Atomic Electric Field Radial Potentials in Step-Like Presentation Copyright © 2010 SciRes. JEMAA 218 6 (2p'') 7 (2s) 8 (3d') 9 (3s) 10 (3p'') 2.929685E − 01 3.264912 E − 01 4.121461 E − 01 1.218004 E + 00 1.296064 E + 00 − 7.190594E + 01 − 1.465283 E + 01 − 9.336976 E − 01 − 1.602583 E + 00 − 1.338345 E + 00 8.859241E + 01 6.646259 E + 01 5.220725 E + 01 1.238409 E + 01 5.373080 E + 00 11 (3d'') 12 (4s) 13 (4p'') 14 (5s) 1.538150 E + 00 4.582967 E + 00 6.082158 E + 00 1.904387 E + 01 − 6.802465 E − 01 − 1.136136 E − 02 − 6.401139 E − 03 − 3.456575 E − 05 4.252708 E + 00 8.138172 E − 01 1.393163 E − 01 1.057707 E − 02 k 1 (38 Sr) 2 (2p') 3 (1s) 4 (3p') 5 (4p') k R 5.649045 E − 05 4.848135 E − 02 5.853622 E − 02 7.657762 E − 02 1.743202 E − 01 k 5.032339 E + 13 − 2.396022 E + 03 − 2.459831 E + 03 − 7.933502 E + 01 − 8.012319 E + 01 k 8.071016 E + 05 1.068257 E + 03 6.081450 E + 02 4.667447 E + 02 2.124328 E + 02 6 (2p'') 7 (2s) 8 (3d') 9 (3s) 10 (3p'') 2.825700 E − 01 3.154582 E − 01 3.757189 E − 01 1.155770 E + 00 1.220437 E + 00 − 8.013324 E + 01 − 1.632401 E + 01− 1.114481 E + 00− 1.997390 E + 00− 1.688127 E + 00 1.001483 E + 02 7.106794 E + 01 5.837499 E + 01 1.384092 E + 01 6.125594 E + 00 11 (3d'') 12 (4s) 13 (4p'') 14 (5s) 1.402202 E + 00 4.107361 E + 00 5.223789 E + 00 1.673859 E + 01 − 8.999501 E − 01 − 1.704132 E − 02 − 1.015079 E − 02 − 1.018086 E − 04 5.104113 E + 00 1.102047 E + 00 2.781945 E − 01 2.447504 E − 02 k 1 (39 Y) 2 (2p') 3 (1s) 4 (3p') k R 5.236921 E − 05 4.685108 E − 02 5.694566 E − 02 7.275406 E − 02 k 6.482580 E + 13 − 2.602725 E + 03 − 2.673430 E + 03 − 8.784124 E + 01 k 8.935358 E + 05 1.136812 E + 03 6.450321 E + 02 5.017009 E + 02 5 (4p') 6 (2p'') 7 (2s) 8 (3d') 1.601441 E − 01 2.730681 E − 01 3.053329 E − 01 3.490053 E − 01 − 8.876033 E + 01 − 8.877329 E + 01 − 1.806852 E + 01 − 1.295142 E + 00 2.395223 E + 02 1.095471 E + 02 7.606533 E + 01 6.442781 E + 01 9 (3s) 10 (3p'') 11 (3d'') 12 (4d') 1.104356 E + 00 1.159500 E + 00 1.302505 E + 00 1.337515 E + 00 − 2.396705 E + 00 − 2.042206 E + 00 − 1.123117 E + 00 − 2.155444 E − 02 1.542110 E + 01 7.004576 E + 00 6.043694 E + 00 5.413486 E + 00 13 (4s) 14 (4p'') 15 (4d'') 16 (5s) 3.841116 E + 00 4.798980 E + 00 1.143536 E + 01 1.414640 E + 01 − 2.171434 E − 02 − 1.328936 E − 02 − 3.285598 E − 04 − 1.686566 E − 04 1.360085 E + 00 3.863704 E − 01 6.436441 E − 02 2.580686 E − 03 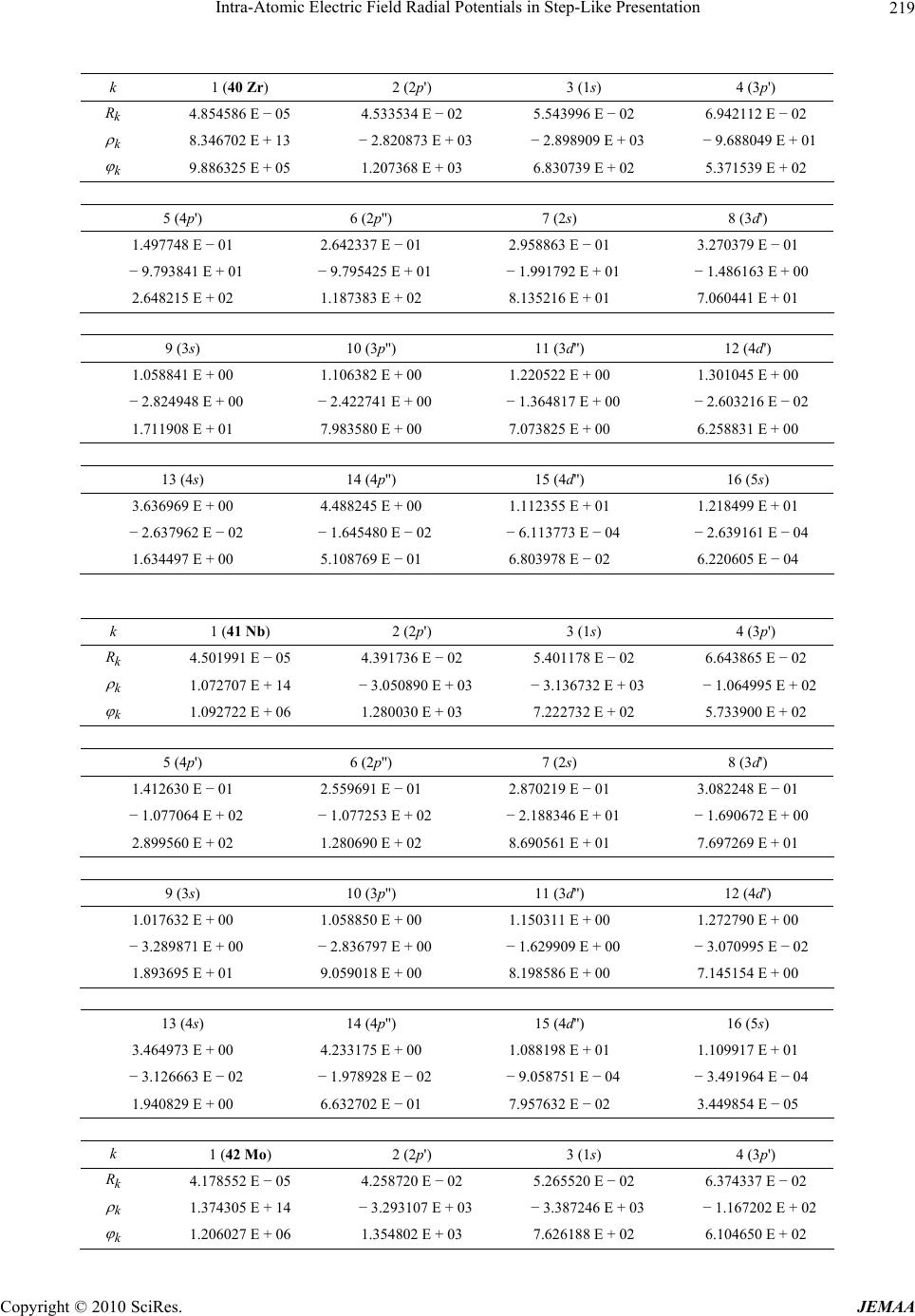 Intra-Atomic Electric Field Radial Potentials in Step-Like Presentation Copyright © 2010 SciRes. JEMAA 219 k 1 (40 Zr) 2 (2p') 3 (1s) 4 (3p') k R 4.854586 E − 05 4.533534 E − 02 5.543996 E − 02 6.942112 E − 02 k 8.346702 E + 13 − 2.820873 E + 03 − 2.898909 E + 03 − 9.688049 E + 01 k 9.886325 E + 05 1.207368 E + 03 6.830739 E + 02 5.371539 E + 02 5 (4p') 6 (2p'') 7 (2s) 8 (3d') 1.497748 E − 01 2.642337 E − 01 2.958863 E − 01 3.270379 E − 01 − 9.793841 E + 01 − 9.795425 E + 01 − 1.991792 E + 01 − 1.486163 E + 00 2.648215 E + 02 1.187383 E + 02 8.135216 E + 01 7.060441 E + 01 9 (3s) 10 (3p'') 11 (3d'') 12 (4d') 1.058841 E + 00 1.106382 E + 00 1.220522 E + 00 1.301045 E + 00 − 2.824948 E + 00 − 2.422741 E + 00 − 1.364817 E + 00 − 2.603216 E − 02 1.711908 E + 01 7.983580 E + 00 7.073825 E + 00 6.258831 E + 00 13 (4s) 14 (4p'') 15 (4d'') 16 (5s) 3.636969 E + 00 4.488245 E + 00 1.112355 E + 01 1.218499 E + 01 − 2.637962 E − 02 − 1.645480 E − 02 − 6.113773 E − 04 − 2.639161 E − 04 1.634497 E + 00 5.108769 E − 01 6.803978 E − 02 6.220605 E − 04 k 1 (41 Nb) 2 (2p') 3 (1s) 4 (3p') k R 4.501991 E − 05 4.391736 E − 02 5.401178 E − 02 6.643865 E − 02 k 1.072707 E + 14 − 3.050890 E + 03 − 3.136732 E + 03 − 1.064995 E + 02 k 1.092722 E + 06 1.280030 E + 03 7.222732 E + 02 5.733900 E + 02 5 (4p') 6 (2p'') 7 (2s) 8 (3d') 1.412630 E − 01 2.559691 E − 01 2.870219 E − 01 3.082248 E − 01 − 1.077064 E + 02 − 1.077253 E + 02 − 2.188346 E + 01 − 1.690672 E + 00 2.899560 E + 02 1.280690 E + 02 8.690561 E + 01 7.697269 E + 01 9 (3s) 10 (3p'') 11 (3d'') 12 (4d') 1.017632 E + 00 1.058850 E + 00 1.150311 E + 00 1.272790 E + 00 − 3.289871 E + 00 − 2.836797 E + 00 − 1.629909 E + 00 − 3.070995 E − 02 1.893695 E + 01 9.059018 E + 00 8.198586 E + 00 7.145154 E + 00 13 (4s) 14 (4p'') 15 (4d'') 16 (5s) 3.464973 E + 00 4.233175 E + 00 1.088198 E + 01 1.109917 E + 01 − 3.126663 E − 02 − 1.978928 E − 02 − 9.058751 E − 04 − 3.491964 E − 04 1.940829 E + 00 6.632702 E − 01 7.957632 E − 02 3.449854 E − 05 k 1 (42 Mo) 2 (2p') 3 (1s) 4 (3p') k R 4.178552 E − 05 4.258720 E − 02 5.265520 E − 02 6.374337 E − 02 k 1.374305 E + 14 − 3.293107 E + 03 − 3.387246 E + 03 − 1.167202 E + 02 k 1.206027 E + 06 1.354802 E + 03 7.626188 E + 02 6.104650 E + 02 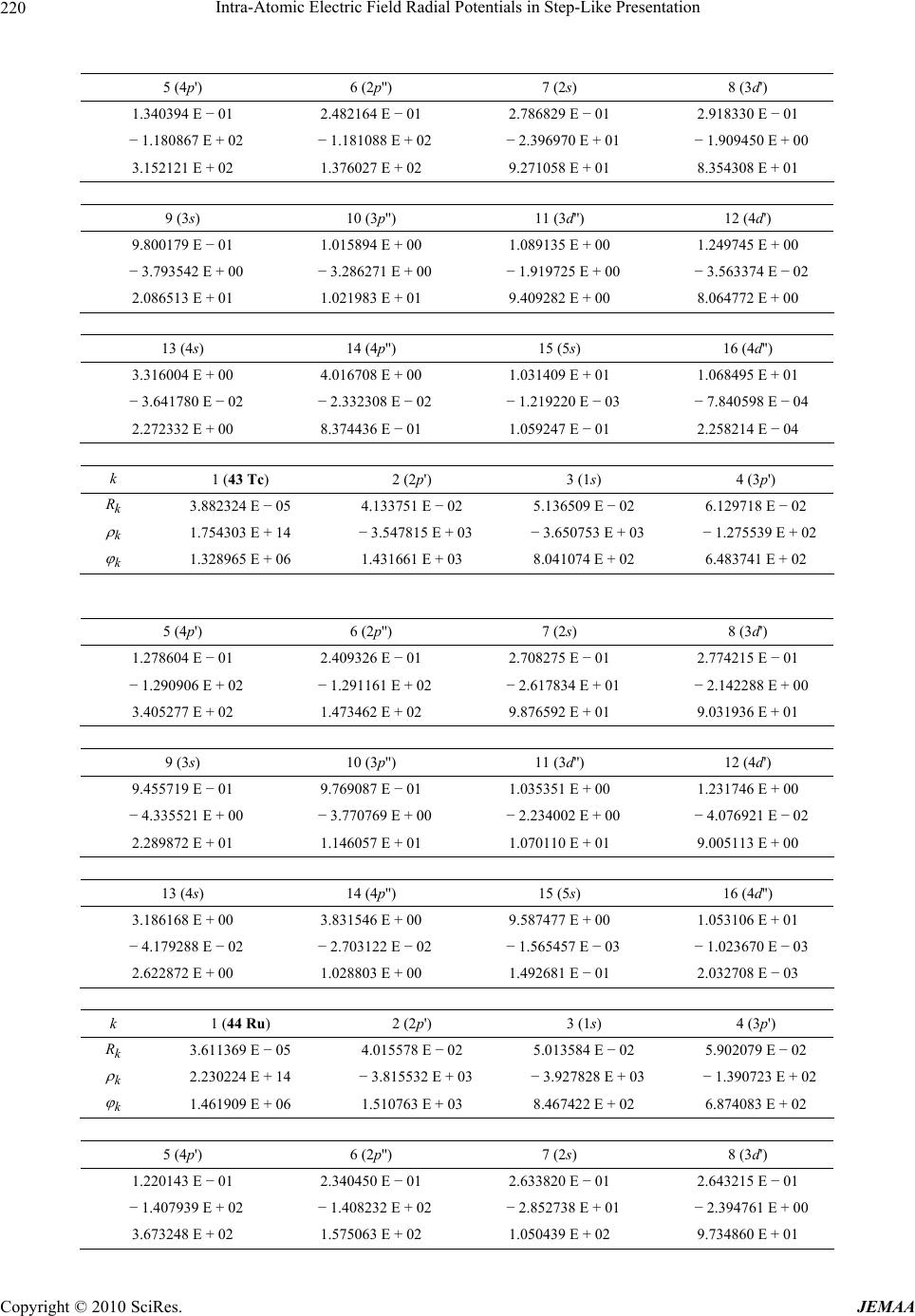 Intra-Atomic Electric Field Radial Potentials in Step-Like Presentation Copyright © 2010 SciRes. JEMAA 220 5 (4p') 6 (2p'') 7 (2s) 8 (3d') 1.340394 E − 01 2.482164 E − 01 2.786829 E − 01 2.918330 E − 01 − 1.180867 E + 02 − 1.181088 E + 02 − 2.396970 E + 01 − 1.909450 E + 00 3.152121 E + 02 1.376027 E + 02 9.271058 E + 01 8.354308 E + 01 9 (3s) 10 (3p'') 11 (3d'') 12 (4d') 9.800179 E − 01 1.015894 E + 00 1.089135 E + 00 1.249745 E + 00 − 3.793542 E + 00 − 3.286271 E + 00 − 1.919725 E + 00 − 3.563374 E − 02 2.086513 E + 01 1.021983 E + 01 9.409282 E + 00 8.064772 E + 00 13 (4s) 14 (4p'') 15 (5s) 16 (4d'') 3.316004 E + 00 4.016708 E + 00 1.031409 E + 01 1.068495 E + 01 − 3.641780 E − 02 − 2.332308 E − 02 − 1.219220 E − 03 − 7.840598 E − 04 2.272332 E + 00 8.374436 E − 01 1.059247 E − 01 2.258214 E − 04 k 1 (43 Tc) 2 (2p') 3 (1s) 4 (3p') k R 3.882324 E − 05 4.133751 E − 02 5.136509 E − 02 6.129718 E − 02 k 1.754303 E + 14 − 3.547815 E + 03 − 3.650753 E + 03 − 1.275539 E + 02 k 1.328965 E + 06 1.431661 E + 03 8.041074 E + 02 6.483741 E + 02 5 (4p') 6 (2p'') 7 (2s) 8 (3d') 1.278604 E − 01 2.409326 E − 01 2.708275 E − 01 2.774215 E − 01 − 1.290906 E + 02 − 1.291161 E + 02 − 2.617834 E + 01 − 2.142288 E + 00 3.405277 E + 02 1.473462 E + 02 9.876592 E + 01 9.031936 E + 01 9 (3s) 10 (3p'') 11 (3d'') 12 (4d') 9.455719 E − 01 9.769087 E − 01 1.035351 E + 00 1.231746 E + 00 − 4.335521 E + 00 − 3.770769 E + 00 − 2.234002 E + 00 − 4.076921 E − 02 2.289872 E + 01 1.146057 E + 01 1.070110 E + 01 9.005113 E + 00 13 (4s) 14 (4p'') 15 (5s) 16 (4d'') 3.186168 E + 00 3.831546 E + 00 9.587477 E + 00 1.053106 E + 01 − 4.179288 E − 02 − 2.703122 E − 02 − 1.565457 E − 03 − 1.023670 E − 03 2.622872 E + 00 1.028803 E + 00 1.492681 E − 01 2.032708 E − 03 k 1 (44 Ru) 2 (2p') 3 (1s) 4 (3p') k R 3.611369 E − 05 4.015578 E − 02 5.013584 E − 02 5.902079 E − 02 k 2.230224 E + 14 − 3.815532 E + 03 − 3.927828 E + 03 − 1.390723 E + 02 k 1.461909 E + 06 1.510763 E + 03 8.467422 E + 02 6.874083 E + 02 5 (4p') 6 (2p'') 7 (2s) 8 (3d') 1.220143 E − 01 2.340450 E − 01 2.633820 E − 01 2.643215 E − 01 − 1.407939 E + 02 − 1.408232 E + 02 − 2.852738 E + 01 − 2.394761 E + 00 3.673248 E + 02 1.575063 E + 02 1.050439 E + 02 9.734860 E + 01 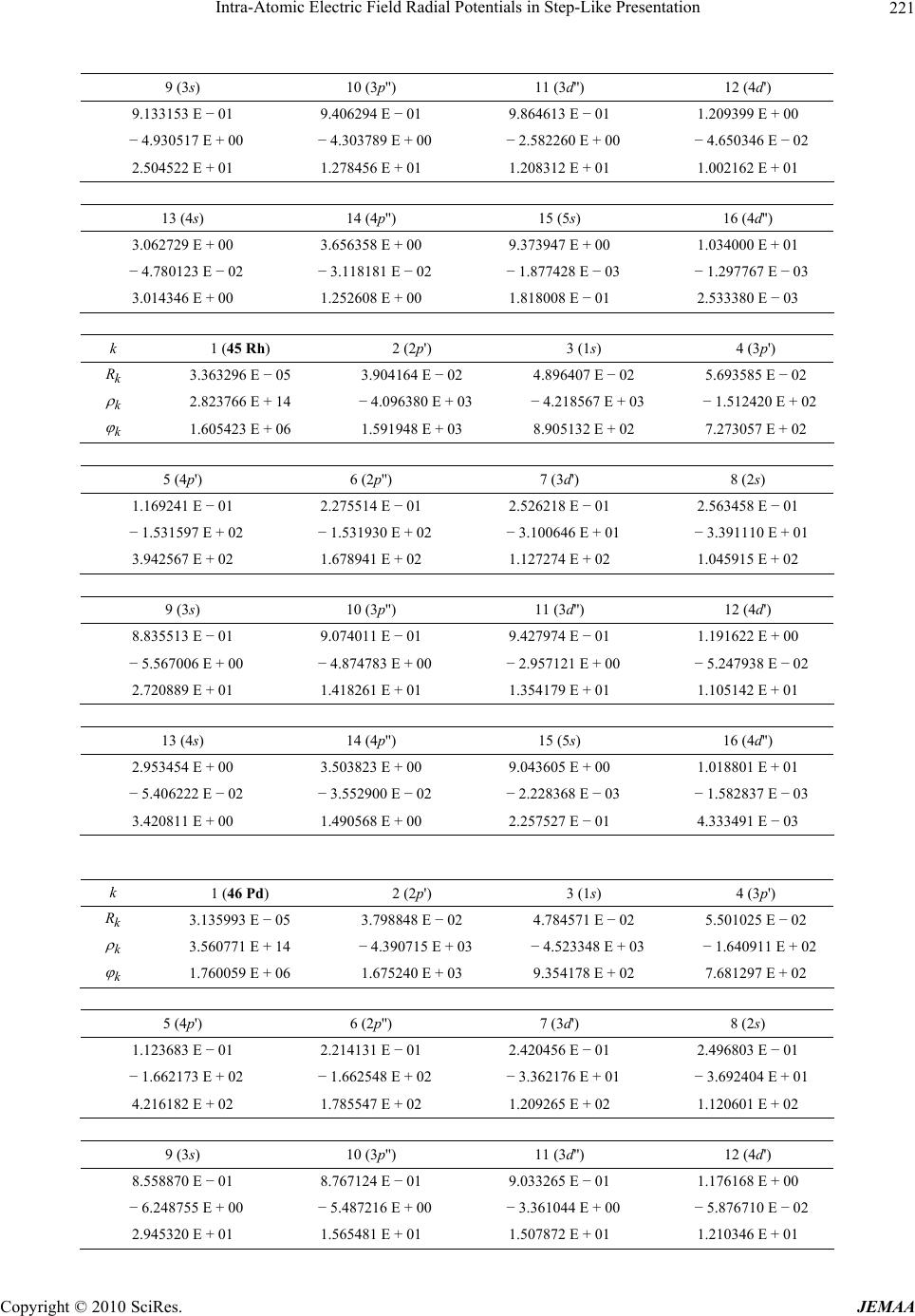 Intra-Atomic Electric Field Radial Potentials in Step-Like Presentation Copyright © 2010 SciRes. JEMAA 221 9 (3s) 10 (3p'') 11 (3d'') 12 (4d') 9.133153 E − 01 9.406294 E − 01 9.864613 E − 01 1.209399 E + 00 − 4.930517 E + 00 − 4.303789 E + 00 − 2.582260 E + 00 − 4.650346 E − 02 2.504522 E + 01 1.278456 E + 01 1.208312 E + 01 1.002162 E + 01 13 (4s) 14 (4p'') 15 (5s) 16 (4d'') 3.062729 E + 00 3.656358 E + 00 9.373947 E + 00 1.034000 E + 01 − 4.780123 E − 02 − 3.118181 E − 02 − 1.877428 E − 03 − 1.297767 E − 03 3.014346 E + 00 1.252608 E + 00 1.818008 E − 01 2.533380 E − 03 k 1 (45 Rh) 2 (2p') 3 (1s) 4 (3p') k R 3.363296 E − 05 3.904164 E − 02 4.896407 E − 02 5.693585 E − 02 k 2.823766 E + 14 − 4.096380 E + 03 − 4.218567 E + 03 − 1.512420 E + 02 k 1.605423 E + 06 1.591948 E + 03 8.905132 E + 02 7.273057 E + 02 5 (4p') 6 (2p'') 7 (3d') 8 (2s) 1.169241 E − 01 2.275514 E − 01 2.526218 E − 01 2.563458 E − 01 − 1.531597 E + 02 − 1.531930 E + 02 − 3.100646 E + 01 − 3.391110 E + 01 3.942567 E + 02 1.678941 E + 02 1.127274 E + 02 1.045915 E + 02 9 (3s) 10 (3p'') 11 (3d'') 12 (4d') 8.835513 E − 01 9.074011 E − 01 9.427974 E − 01 1.191622 E + 00 − 5.567006 E + 00 − 4.874783 E + 00 − 2.957121 E + 00 − 5.247938 E − 02 2.720889 E + 01 1.418261 E + 01 1.354179 E + 01 1.105142 E + 01 13 (4s) 14 (4p'') 15 (5s) 16 (4d'') 2.953454 E + 00 3.503823 E + 00 9.043605 E + 00 1.018801 E + 01 − 5.406222 E − 02 − 3.552900 E − 02 − 2.228368 E − 03 − 1.582837 E − 03 3.420811 E + 00 1.490568 E + 00 2.257527 E − 01 4.333491 E − 03 k 1 (46 Pd) 2 (2p') 3 (1s) 4 (3p') k R 3.135993 E − 05 3.798848 E − 02 4.784571 E − 02 5.501025 E − 02 k 3.560771 E + 14 − 4.390715 E + 03 − 4.523348 E + 03 − 1.640911 E + 02 k 1.760059 E + 06 1.675240 E + 03 9.354178 E + 02 7.681297 E + 02 5 (4p') 6 (2p'') 7 (3d') 8 (2s) 1.123683 E − 01 2.214131 E − 01 2.420456 E − 01 2.496803 E − 01 − 1.662173 E + 02 − 1.662548 E + 02 − 3.362176 E + 01 − 3.692404 E + 01 4.216182 E + 02 1.785547 E + 02 1.209265 E + 02 1.120601 E + 02 9 (3s) 10 (3p'') 11 (3d'') 12 (4d') 8.558870 E − 01 8.767124 E − 01 9.033265 E − 01 1.176168 E + 00 − 6.248755 E + 00 − 5.487216 E + 00 − 3.361044 E + 00 − 5.876710 E − 02 2.945320 E + 01 1.565481 E + 01 1.507872 E + 01 1.210346 E + 01 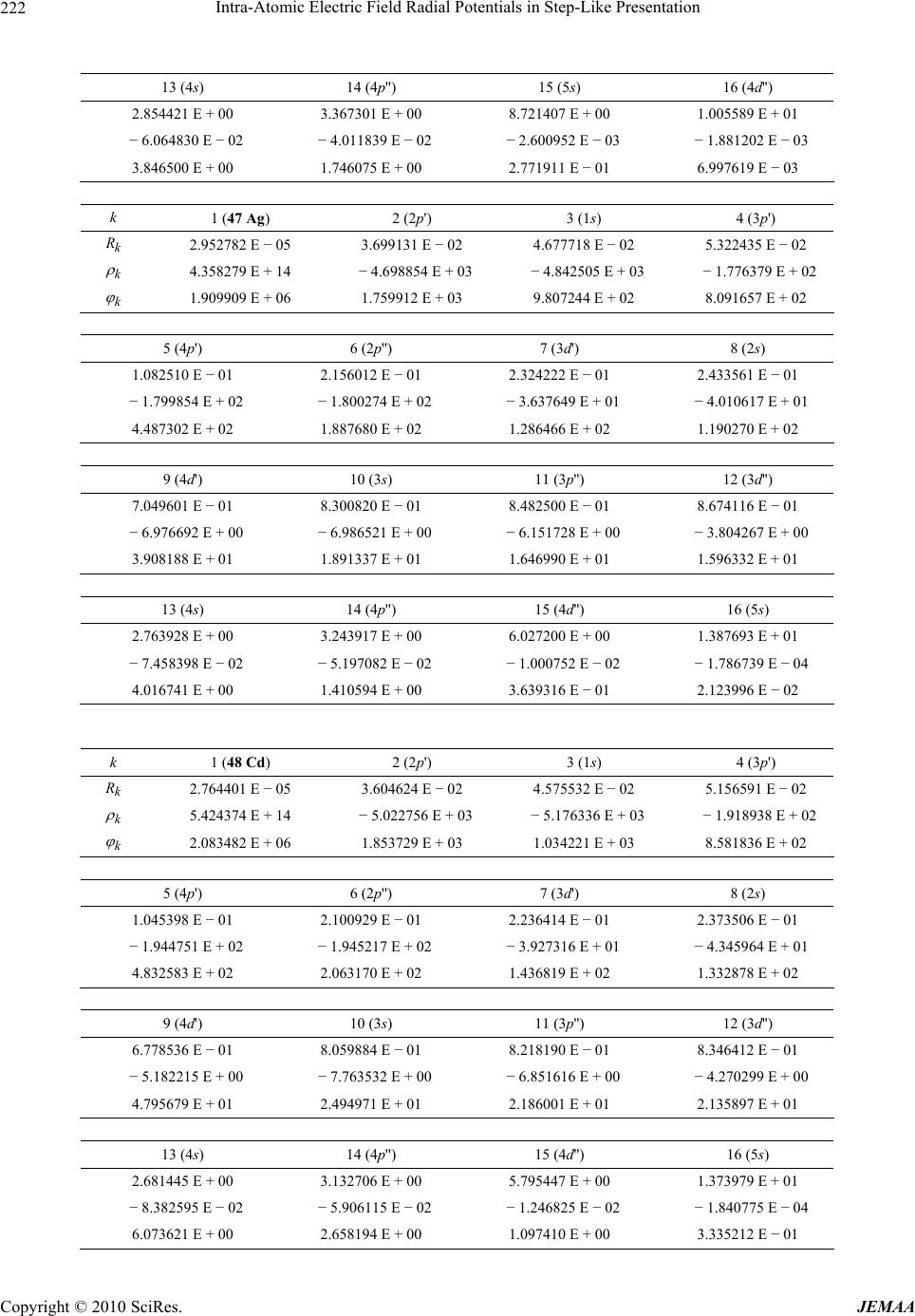 Intra-Atomic Electric Field Radial Potentials in Step-Like Presentation Copyright © 2010 SciRes. JEMAA 222 13 (4s) 14 (4p'') 15 (5s) 16 (4d'') 2.854421 E + 00 3.367301 E + 00 8.721407 E + 00 1.005589 E + 01 − 6.064830 E − 02 − 4.011839 E − 02 − 2.600952 E − 03 − 1.881202 E − 03 3.846500 E + 00 1.746075 E + 00 2.771911 E − 01 6.997619 E − 03 k 1 (47 Ag) 2 (2p') 3 (1s) 4 (3p') k R 2.952782 E − 05 3.699131 E − 02 4.677718 E − 02 5.322435 E − 02 k 4.358279 E + 14 − 4.698854 E + 03 − 4.842505 E + 03 − 1.776379 E + 02 k 1.909909 E + 06 1.759912 E + 03 9.807244 E + 02 8.091657 E + 02 5 (4p') 6 (2p'') 7 (3d') 8 (2s) 1.082510 E − 01 2.156012 E − 01 2.324222 E − 01 2.433561 E − 01 − 1.799854 E + 02 − 1.800274 E + 02 − 3.637649 E + 01 − 4.010617 E + 01 4.487302 E + 02 1.887680 E + 02 1.286466 E + 02 1.190270 E + 02 9 (4d') 10 (3s) 11 (3p'') 12 (3d'') 7.049601 E − 01 8.300820 E − 01 8.482500 E − 01 8.674116 E − 01 − 6.976692 E + 00 − 6.986521 E + 00 − 6.151728 E + 00 − 3.804267 E + 00 3.908188 E + 01 1.891337 E + 01 1.646990 E + 01 1.596332 E + 01 13 (4s) 14 (4p'') 15 (4d'') 16 (5s) 2.763928 E + 00 3.243917 E + 00 6.027200 E + 00 1.387693 E + 01 − 7.458398 E − 02 − 5.197082 E − 02 − 1.000752 E − 02 − 1.786739 E − 04 4.016741 E + 00 1.410594 E + 00 3.639316 E − 01 2.123996 E − 02 k 1 (48 Cd) 2 (2p') 3 (1s) 4 (3p') k R 2.764401 E − 05 3.604624 E − 02 4.575532 E − 02 5.156591 E − 02 k 5.424374 E + 14 − 5.022756 E + 03 − 5.176336 E + 03 − 1.918938 E + 02 k 2.083482 E + 06 1.853729 E + 03 1.034221 E + 03 8.581836 E + 02 5 (4p') 6 (2p'') 7 (3d') 8 (2s) 1.045398 E − 01 2.100929 E − 01 2.236414 E − 01 2.373506 E − 01 − 1.944751 E + 02 − 1.945217 E + 02 − 3.927316 E + 01 − 4.345964 E + 01 4.832583 E + 02 2.063170 E + 02 1.436819 E + 02 1.332878 E + 02 9 (4d') 10 (3s) 11 (3p'') 12 (3d'') 6.778536 E − 01 8.059884 E − 01 8.218190 E − 01 8.346412 E − 01 − 5.182215 E + 00 − 7.763532 E + 00 − 6.851616 E + 00 − 4.270299 E + 00 4.795679 E + 01 2.494971 E + 01 2.186001 E + 01 2.135897 E + 01 13 (4s) 14 (4p'') 15 (4d'') 16 (5s) 2.681445 E + 00 3.132706 E + 00 5.795447 E + 00 1.373979 E + 01 − 8.382595 E − 02 − 5.906115 E − 02 − 1.246825 E − 02 − 1.840775 E − 04 6.073621 E + 00 2.658194 E + 00 1.097410 E + 00 3.335212 E − 01 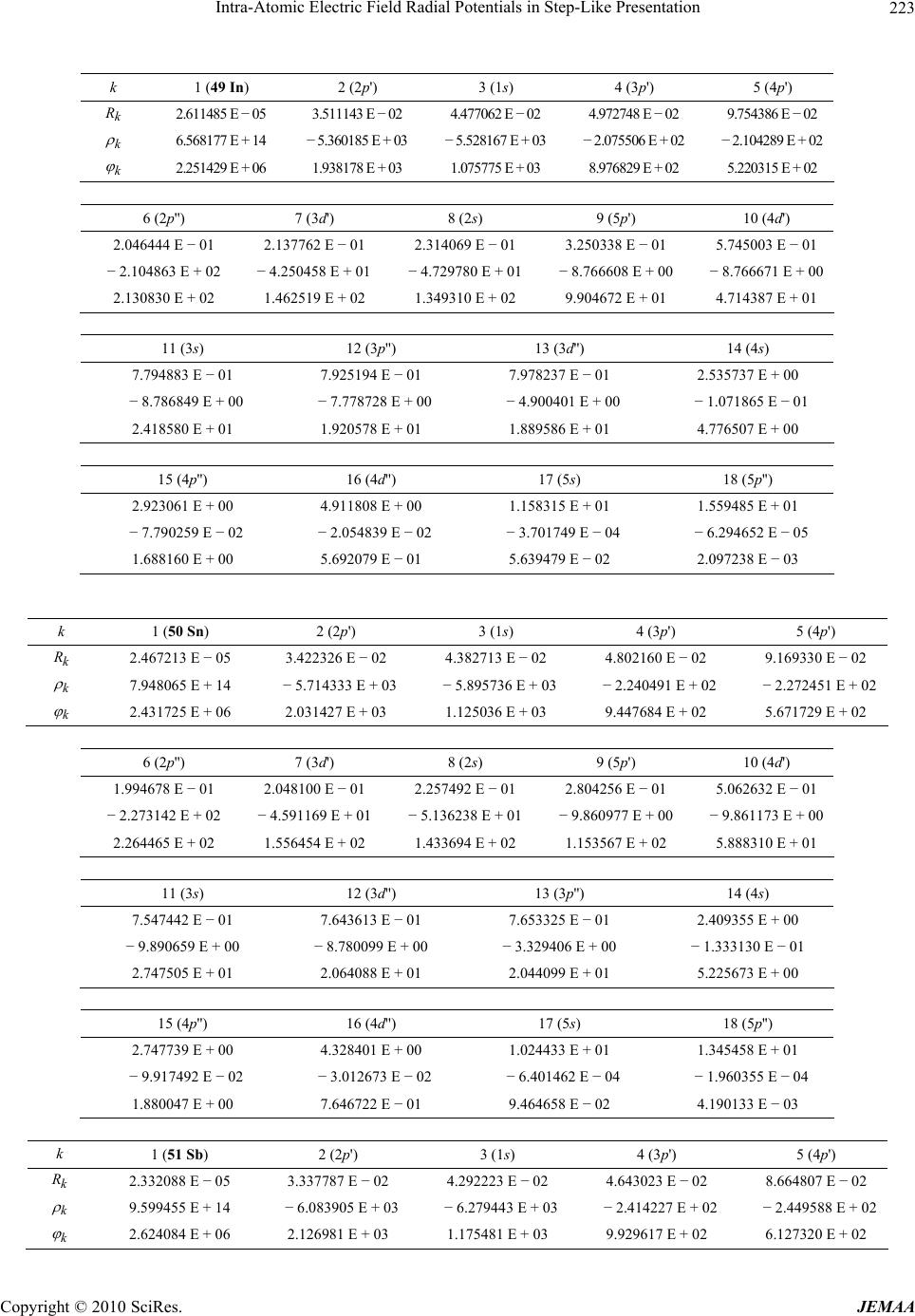 Intra-Atomic Electric Field Radial Potentials in Step-Like Presentation Copyright © 2010 SciRes. JEMAA 223 k 1 (49 In) 2 (2p') 3 (1s) 4 (3p') 5 (4p') k R 2.611485 E − 05 3.511143 E − 02 4.477062 E − 02 4.972748 E − 02 9.754386 E − 02 k 6.568177 E + 14 − 5.360185 E + 03 − 5.528167 E + 03 − 2.075506 E + 02 − 2.104289 E + 02 k 2.251429 E + 06 1.938178 E + 03 1.075775 E + 03 8.976829 E + 02 5.220315 E + 02 6 (2p'') 7 (3d') 8 (2s) 9 (5p') 10 (4d') 2.046444 E − 01 2.137762 E − 01 2.314069 E − 01 3.250338 E − 01 5.745003 E − 01 − 2.104863 E + 02 − 4.250458 E + 01− 4.729780 E + 01− 8.766608 E + 00− 8.766671 E + 00 2.130830 E + 02 1.462519 E + 02 1.349310 E + 02 9.904672 E + 01 4.714387 E + 01 11 (3s) 12 (3p'') 13 (3d'') 14 (4s) 7.794883 E − 01 7.925194 E − 01 7.978237 E − 01 2.535737 E + 00 − 8.786849 E + 00 − 7.778728 E + 00 − 4.900401 E + 00 − 1.071865 E − 01 2.418580 E + 01 1.920578 E + 01 1.889586 E + 01 4.776507 E + 00 15 (4p'') 16 (4d'') 17 (5s) 18 (5p'') 2.923061 E + 00 4.911808 E + 00 1.158315 E + 01 1.559485 E + 01 − 7.790259 E − 02 − 2.054839 E − 02 − 3.701749 E − 04 − 6.294652 E − 05 1.688160 E + 00 5.692079 E − 01 5.639479 E − 02 2.097238 E − 03 k 1 (50 Sn) 2 (2p') 3 (1s) 4 (3p') 5 (4p') k R 2.467213 E − 05 3.422326 E − 02 4.382713 E − 02 4.802160 E − 02 9.169330 E − 02 k 7.948065 E + 14 − 5.714333 E + 03 − 5.895736 E + 03 − 2.240491 E + 02 − 2.272451 E + 02 k 2.431725 E + 06 2.031427 E + 03 1.125036 E + 03 9.447684 E + 02 5.671729 E + 02 6 (2p'') 7 (3d') 8 (2s) 9 (5p') 10 (4d') 1.994678 E − 01 2.048100 E − 01 2.257492 E − 01 2.804256 E − 01 5.062632 E − 01 − 2.273142 E + 02 − 4.591169 E + 01− 5.136238 E + 01− 9.860977 E + 00− 9.861173 E + 00 2.264465 E + 02 1.556454 E + 02 1.433694 E + 02 1.153567 E + 02 5.888310 E + 01 11 (3s) 12 (3d'') 13 (3p'') 14 (4s) 7.547442 E − 01 7.643613 E − 01 7.653325 E − 01 2.409355 E + 00 − 9.890659 E + 00 − 8.780099 E + 00 − 3.329406 E + 00 − 1.333130 E − 01 2.747505 E + 01 2.064088 E + 01 2.044099 E + 01 5.225673 E + 00 15 (4p'') 16 (4d'') 17 (5s) 18 (5p'') 2.747739 E + 00 4.328401 E + 00 1.024433 E + 01 1.345458 E + 01 − 9.917492 E − 02 − 3.012673 E − 02 − 6.401462 E − 04 − 1.960355 E − 04 1.880047 E + 00 7.646722 E − 01 9.464658 E − 02 4.190133 E − 03 k 1 (51 Sb) 2 (2p') 3 (1s) 4 (3p') 5 (4p') k R 2.332088 E − 05 3.337787 E − 02 4.292223 E − 02 4.643023 E − 02 8.664807 E − 02 k 9.599455 E + 14 − 6.083905 E + 03 − 6.279443 E + 03 − 2.414227 E + 02 − 2.449588 E + 02 k 2.624084 E + 06 2.126981 E + 03 1.175481 E + 03 9.929617 E + 02 6.127320 E + 02 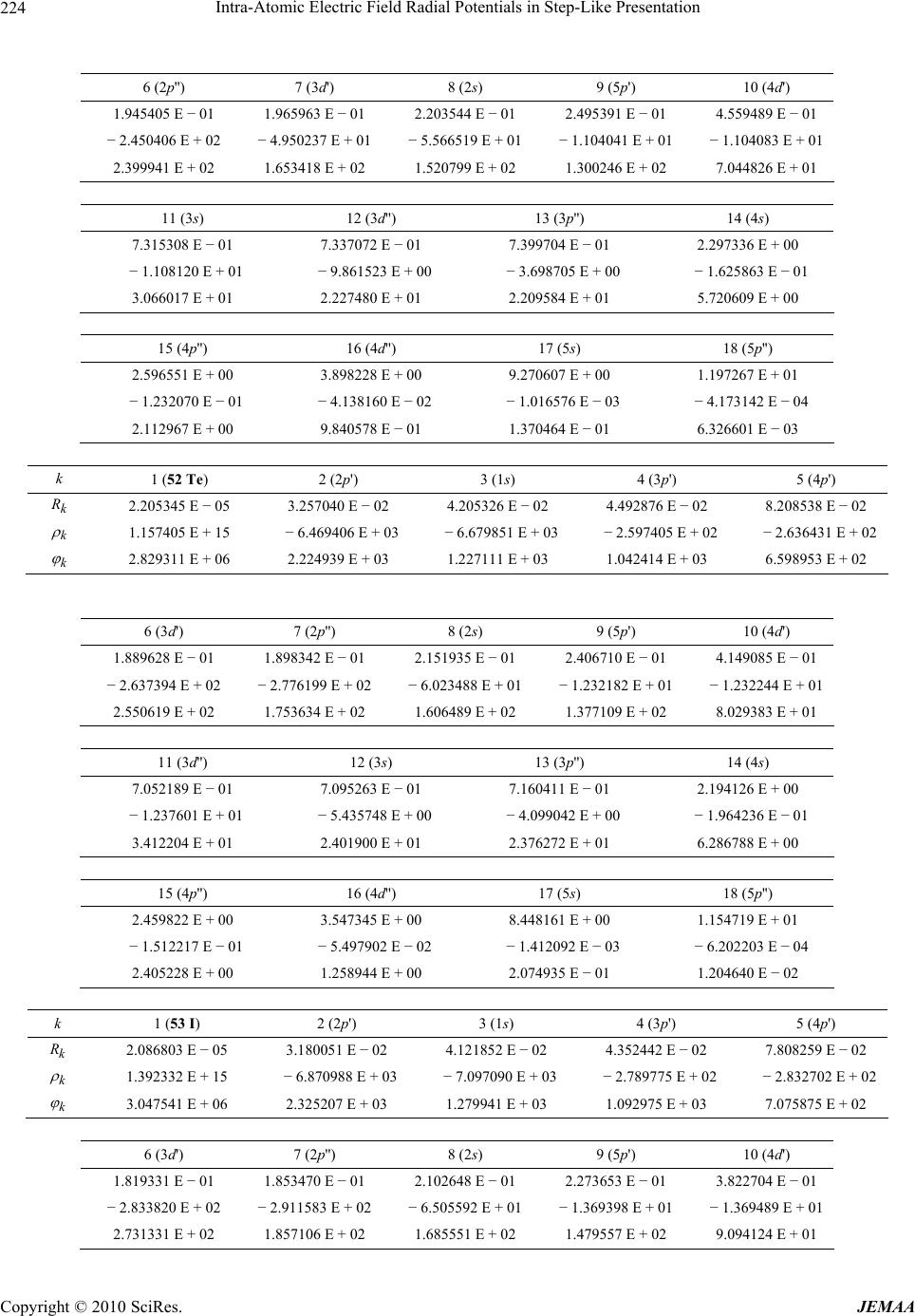 Intra-Atomic Electric Field Radial Potentials in Step-Like Presentation Copyright © 2010 SciRes. JEMAA 224 6 (2p'') 7 (3d') 8 (2s) 9 (5p') 10 (4d') 1.945405 E − 01 1.965963 E − 01 2.203544 E − 01 2.495391 E − 01 4.559489 E − 01 − 2.450406 E + 02 − 4.950237 E + 01− 5.566519 E + 01− 1.104041 E + 01− 1.104083 E + 01 2.399941 E + 02 1.653418 E + 02 1.520799 E + 02 1.300246 E + 02 7.044826 E + 01 11 (3s) 12 (3d'') 13 (3p'') 14 (4s) 7.315308 E − 01 7.337072 E − 01 7.399704 E − 01 2.297336 E + 00 − 1.108120 E + 01 − 9.861523 E + 00 − 3.698705 E + 00 − 1.625863 E − 01 3.066017 E + 01 2.227480 E + 01 2.209584 E + 01 5.720609 E + 00 15 (4p'') 16 (4d'') 17 (5s) 18 (5p'') 2.596551 E + 00 3.898228 E + 00 9.270607 E + 00 1.197267 E + 01 − 1.232070 E − 01 − 4.138160 E − 02 − 1.016576 E − 03 − 4.173142 E − 04 2.112967 E + 00 9.840578 E − 01 1.370464 E − 01 6.326601 E − 03 k 1 (52 Te) 2 (2p') 3 (1s) 4 (3p') 5 (4p') k R 2.205345 E − 05 3.257040 E − 02 4.205326 E − 02 4.492876 E − 02 8.208538 E − 02 k 1.157405 E + 15 − 6.469406 E + 03 − 6.679851 E + 03 − 2.597405 E + 02 − 2.636431 E + 02 k 2.829311 E + 06 2.224939 E + 03 1.227111 E + 03 1.042414 E + 03 6.598953 E + 02 6 (3d') 7 (2p'') 8 (2s) 9 (5p') 10 (4d') 1.889628 E − 01 1.898342 E − 01 2.151935 E − 01 2.406710 E − 01 4.149085 E − 01 − 2.637394 E + 02 − 2.776199 E + 02− 6.023488 E + 01− 1.232182 E + 01− 1.232244 E + 01 2.550619 E + 02 1.753634 E + 02 1.606489 E + 02 1.377109 E + 02 8.029383 E + 01 11 (3d'') 12 (3s) 13 (3p'') 14 (4s) 7.052189 E − 01 7.095263 E − 01 7.160411 E − 01 2.194126 E + 00 − 1.237601 E + 01 − 5.435748 E + 00 − 4.099042 E + 00 − 1.964236 E − 01 3.412204 E + 01 2.401900 E + 01 2.376272 E + 01 6.286788 E + 00 15 (4p'') 16 (4d'') 17 (5s) 18 (5p'') 2.459822 E + 00 3.547345 E + 00 8.448161 E + 00 1.154719 E + 01 − 1.512217 E − 01 − 5.497902 E − 02 − 1.412092 E − 03 − 6.202203 E − 04 2.405228 E + 00 1.258944 E + 00 2.074935 E − 01 1.204640 E − 02 k 1 (53 I) 2 (2p') 3 (1s) 4 (3p') 5 (4p') k R 2.086803 E − 05 3.180051 E − 02 4.121852 E − 02 4.352442 E − 02 7.808259 E − 02 k 1.392332 E + 15 − 6.870988 E + 03 − 7.097090 E + 03 − 2.789775 E + 02 − 2.832702 E + 02 k 3.047541 E + 06 2.325207 E + 03 1.279941 E + 03 1.092975 E + 03 7.075875 E + 02 6 (3d') 7 (2p'') 8 (2s) 9 (5p') 10 (4d') 1.819331 E − 01 1.853470 E − 01 2.102648 E − 01 2.273653 E − 01 3.822704 E − 01 − 2.833820 E + 02 − 2.911583 E + 02− 6.505592 E + 01− 1.369398 E + 01− 1.369489 E + 01 2.731331 E + 02 1.857106 E + 02 1.685551 E + 02 1.479557 E + 02 9.094124 E + 01 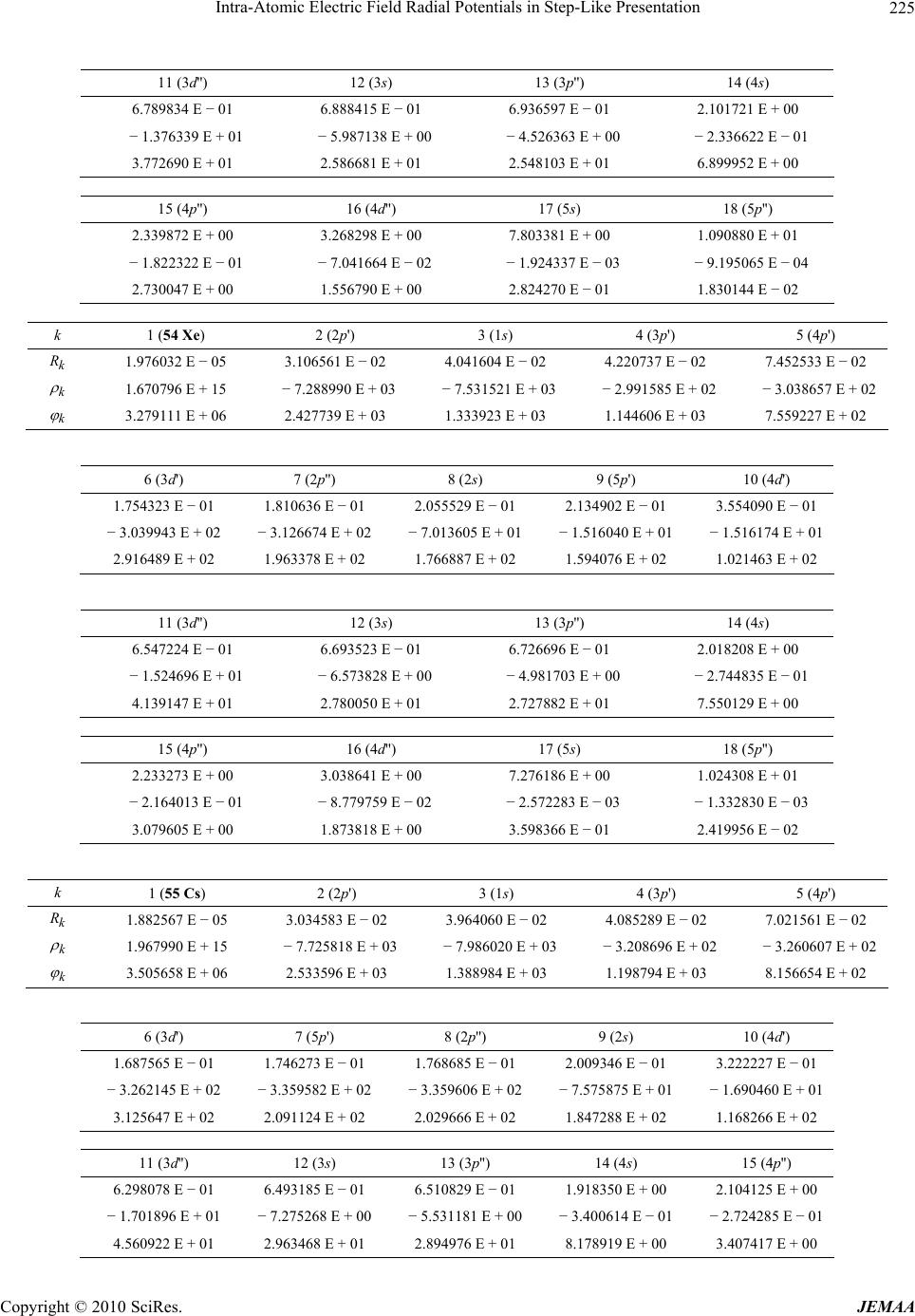 Intra-Atomic Electric Field Radial Potentials in Step-Like Presentation Copyright © 2010 SciRes. JEMAA 225 11 (3d'') 12 (3s) 13 (3p'') 14 (4s) 6.789834 E − 01 6.888415 E − 01 6.936597 E − 01 2.101721 E + 00 − 1.376339 E + 01 − 5.987138 E + 00 − 4.526363 E + 00 − 2.336622 E − 01 3.772690 E + 01 2.586681 E + 01 2.548103 E + 01 6.899952 E + 00 15 (4p'') 16 (4d'') 17 (5s) 18 (5p'') 2.339872 E + 00 3.268298 E + 00 7.803381 E + 00 1.090880 E + 01 − 1.822322 E − 01 − 7.041664 E − 02 − 1.924337 E − 03 − 9.195065 E − 04 2.730047 E + 00 1.556790 E + 00 2.824270 E − 01 1.830144 E − 02 k 1 (54 Xe) 2 (2p') 3 (1s) 4 (3p') 5 (4p') k R 1.976032 E − 05 3.106561 E − 02 4.041604 E − 02 4.220737 E − 02 7.452533 E − 02 k 1.670796 E + 15 − 7.288990 E + 03 − 7.531521 E + 03 − 2.991585 E + 02 − 3.038657 E + 02 k 3.279111 E + 06 2.427739 E + 03 1.333923 E + 03 1.144606 E + 03 7.559227 E + 02 6 (3d') 7 (2p'') 8 (2s) 9 (5p') 10 (4d') 1.754323 E − 01 1.810636 E − 01 2.055529 E − 01 2.134902 E − 01 3.554090 E − 01 − 3.039943 E + 02 − 3.126674 E + 02− 7.013605 E + 01− 1.516040 E + 01− 1.516174 E + 01 2.916489 E + 02 1.963378 E + 02 1.766887 E + 02 1.594076 E + 02 1.021463 E + 02 11 (3d'') 12 (3s) 13 (3p'') 14 (4s) 6.547224 E − 01 6.693523 E − 01 6.726696 E − 01 2.018208 E + 00 − 1.524696 E + 01 − 6.573828 E + 00 − 4.981703 E + 00 − 2.744835 E − 01 4.139147 E + 01 2.780050 E + 01 2.727882 E + 01 7.550129 E + 00 15 (4p'') 16 (4d'') 17 (5s) 18 (5p'') 2.233273 E + 00 3.038641 E + 00 7.276186 E + 00 1.024308 E + 01 − 2.164013 E − 01 − 8.779759 E − 02 − 2.572283 E − 03 − 1.332830 E − 03 3.079605 E + 00 1.873818 E + 00 3.598366 E − 01 2.419956 E − 02 k 1 (55 Cs) 2 (2p') 3 (1s) 4 (3p') 5 (4p') k R 1.882567 E − 05 3.034583 E − 02 3.964060 E − 02 4.085289 E − 02 7.021561 E − 02 k 1.967990 E + 15 − 7.725818 E + 03 − 7.986020 E + 03 − 3.208696 E + 02 − 3.260607 E + 02 k 3.505658 E + 06 2.533596 E + 03 1.388984 E + 03 1.198794 E + 03 8.156654 E + 02 6 (3d') 7 (5p') 8 (2p'') 9 (2s) 10 (4d') 1.687565 E − 01 1.746273 E − 01 1.768685 E − 01 2.009346 E − 01 3.222227 E − 01 − 3.262145 E + 02 − 3.359582 E + 02− 3.359606 E + 02− 7.575875 E + 01− 1.690460 E + 01 3.125647 E + 02 2.091124 E + 02 2.029666 E + 02 1.847288 E + 02 1.168266 E + 02 11 (3d'') 12 (3s) 13 (3p'') 14 (4s) 15 (4p'') 6.298078 E − 01 6.493185 E − 01 6.510829 E − 01 1.918350 E + 00 2.104125 E + 00 − 1.701896 E + 01 − 7.275268 E + 00− 5.531181 E + 00− 3.400614 E − 01− 2.724285 E − 01 4.560922 E + 01 2.963468 E + 01 2.894976 E + 01 8.178919 E + 00 3.407417 E + 00 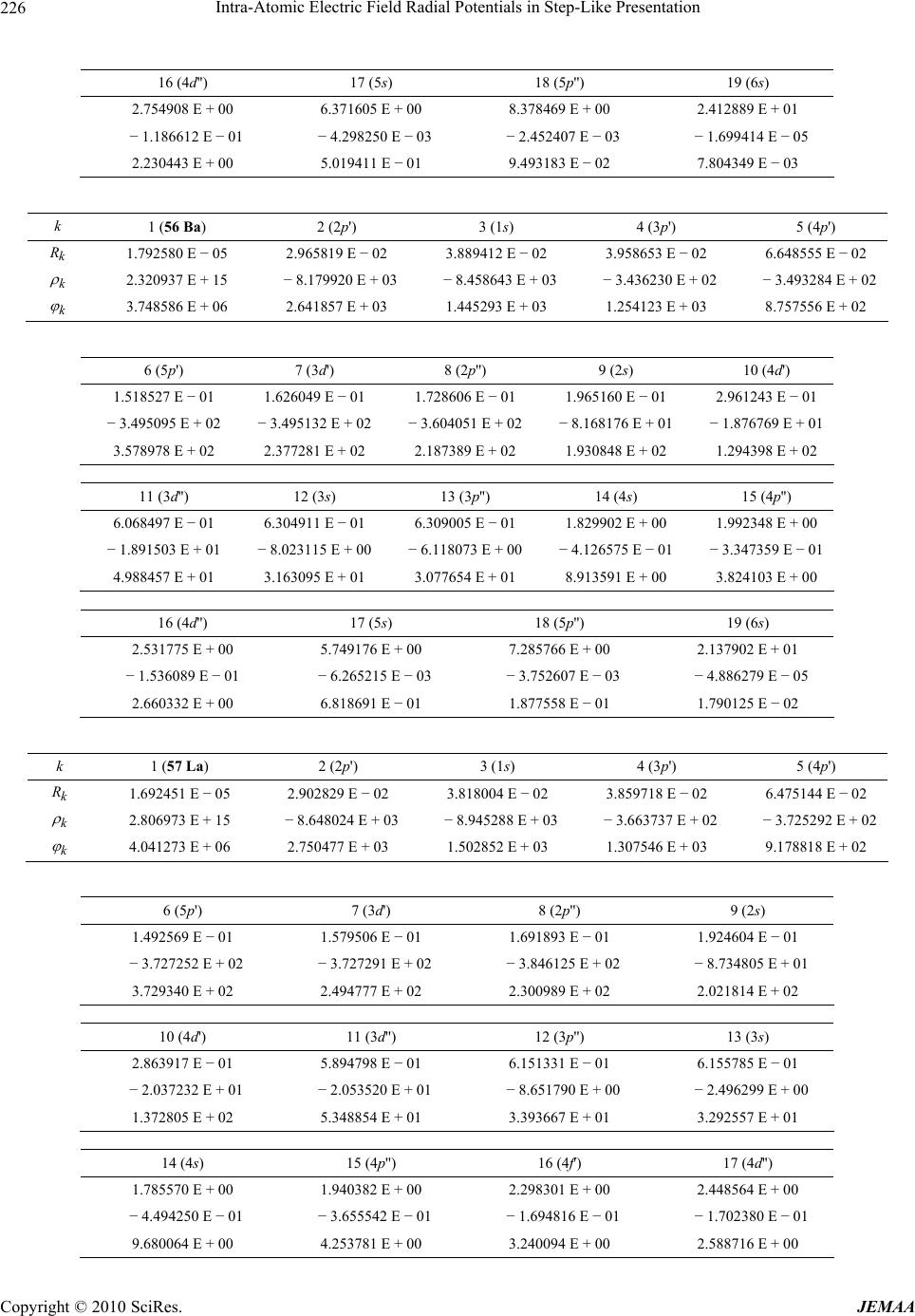 Intra-Atomic Electric Field Radial Potentials in Step-Like Presentation Copyright © 2010 SciRes. JEMAA 226 16 (4d'') 17 (5s) 18 (5p'') 19 (6s) 2.754908 E + 00 6.371605 E + 00 8.378469 E + 00 2.412889 E + 01 − 1.186612 E − 01 − 4.298250 E − 03 − 2.452407 E − 03 − 1.699414 E − 05 2.230443 E + 00 5.019411 E − 01 9.493183 E − 02 7.804349 E − 03 k 1 (56 Ba) 2 (2p') 3 (1s) 4 (3p') 5 (4p') k R 1.792580 E − 05 2.965819 E − 02 3.889412 E − 02 3.958653 E − 02 6.648555 E − 02 k 2.320937 E + 15 − 8.179920 E + 03 − 8.458643 E + 03 − 3.436230 E + 02 − 3.493284 E + 02 k 3.748586 E + 06 2.641857 E + 03 1.445293 E + 03 1.254123 E + 03 8.757556 E + 02 6 (5p') 7 (3d') 8 (2p'') 9 (2s) 10 (4d') 1.518527 E − 01 1.626049 E − 01 1.728606 E − 01 1.965160 E − 01 2.961243 E − 01 − 3.495095 E + 02 − 3.495132 E + 02− 3.604051 E + 02− 8.168176 E + 01− 1.876769 E + 01 3.578978 E + 02 2.377281 E + 02 2.187389 E + 02 1.930848 E + 02 1.294398 E + 02 11 (3d'') 12 (3s) 13 (3p'') 14 (4s) 15 (4p'') 6.068497 E − 01 6.304911 E − 01 6.309005 E − 01 1.829902 E + 00 1.992348 E + 00 − 1.891503 E + 01 − 8.023115 E + 00− 6.118073 E + 00− 4.126575 E − 01− 3.347359 E − 01 4.988457 E + 01 3.163095 E + 01 3.077654 E + 01 8.913591 E + 00 3.824103 E + 00 16 (4d'') 17 (5s) 18 (5p'') 19 (6s) 2.531775 E + 00 5.749176 E + 00 7.285766 E + 00 2.137902 E + 01 − 1.536089 E − 01 − 6.265215 E − 03 − 3.752607 E − 03 − 4.886279 E − 05 2.660332 E + 00 6.818691 E − 01 1.877558 E − 01 1.790125 E − 02 k 1 (57 La) 2 (2p') 3 (1s) 4 (3p') 5 (4p') k R 1.692451 E − 05 2.902829 E − 02 3.818004 E − 02 3.859718 E − 02 6.475144 E − 02 k 2.806973 E + 15 − 8.648024 E + 03 − 8.945288 E + 03 − 3.663737 E + 02 − 3.725292 E + 02 k 4.041273 E + 06 2.750477 E + 03 1.502852 E + 03 1.307546 E + 03 9.178818 E + 02 6 (5p') 7 (3d') 8 (2p'') 9 (2s) 1.492569 E − 01 1.579506 E − 01 1.691893 E − 01 1.924604 E − 01 − 3.727252 E + 02 − 3.727291 E + 02 − 3.846125 E + 02 − 8.734805 E + 01 3.729340 E + 02 2.494777 E + 02 2.300989 E + 02 2.021814 E + 02 10 (4d') 11 (3d'') 12 (3p'') 13 (3s) 2.863917 E − 01 5.894798 E − 01 6.151331 E − 01 6.155785 E − 01 − 2.037232 E + 01 − 2.053520 E + 01 − 8.651790 E + 00 − 2.496299 E + 00 1.372805 E + 02 5.348854 E + 01 3.393667 E + 01 3.292557 E + 01 14 (4s) 15 (4p'') 16 (4f') 17 (4d'') 1.785570 E + 00 1.940382 E + 00 2.298301 E + 00 2.448564 E + 00 − 4.494250 E − 01 − 3.655542 E − 01 − 1.694816 E − 01 − 1.702380 E − 01 9.680064 E + 00 4.253781 E + 00 3.240094 E + 00 2.588716 E + 00 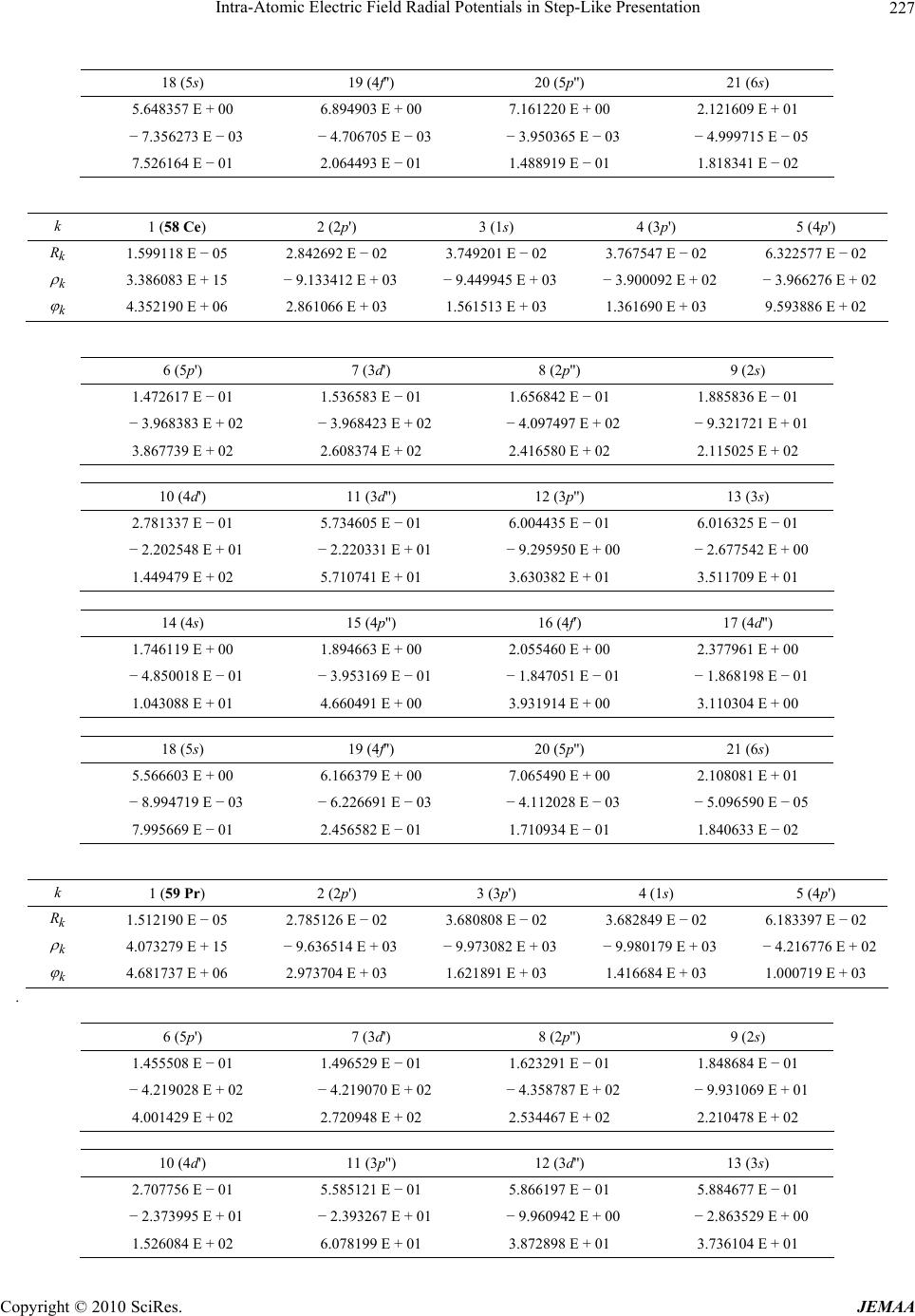 Intra-Atomic Electric Field Radial Potentials in Step-Like Presentation Copyright © 2010 SciRes. JEMAA 227 18 (5s) 19 (4f'') 20 (5p'') 21 (6s) 5.648357 E + 00 6.894903 E + 00 7.161220 E + 00 2.121609 E + 01 − 7.356273 E − 03 − 4.706705 E − 03 − 3.950365 E − 03 − 4.999715 E − 05 7.526164 E − 01 2.064493 E − 01 1.488919 E − 01 1.818341 E − 02 k 1 (58 Ce) 2 (2p') 3 (1s) 4 (3p') 5 (4p') k R 1.599118 E − 05 2.842692 E − 02 3.749201 E − 02 3.767547 E − 02 6.322577 E − 02 k 3.386083 E + 15 − 9.133412 E + 03 − 9.449945 E + 03 − 3.900092 E + 02 − 3.966276 E + 02 k 4.352190 E + 06 2.861066 E + 03 1.561513 E + 03 1.361690 E + 03 9.593886 E + 02 6 (5p') 7 (3d') 8 (2p'') 9 (2s) 1.472617 E − 01 1.536583 E − 01 1.656842 E − 01 1.885836 E − 01 − 3.968383 E + 02 − 3.968423 E + 02 − 4.097497 E + 02 − 9.321721 E + 01 3.867739 E + 02 2.608374 E + 02 2.416580 E + 02 2.115025 E + 02 10 (4d') 11 (3d'') 12 (3p'') 13 (3s) 2.781337 E − 01 5.734605 E − 01 6.004435 E − 01 6.016325 E − 01 − 2.202548 E + 01 − 2.220331 E + 01 − 9.295950 E + 00 − 2.677542 E + 00 1.449479 E + 02 5.710741 E + 01 3.630382 E + 01 3.511709 E + 01 14 (4s) 15 (4p'') 16 (4f') 17 (4d'') 1.746119 E + 00 1.894663 E + 00 2.055460 E + 00 2.377961 E + 00 − 4.850018 E − 01 − 3.953169 E − 01 − 1.847051 E − 01 − 1.868198 E − 01 1.043088 E + 01 4.660491 E + 00 3.931914 E + 00 3.110304 E + 00 18 (5s) 19 (4f'') 20 (5p'') 21 (6s) 5.566603 E + 00 6.166379 E + 00 7.065490 E + 00 2.108081 E + 01 − 8.994719 E − 03 − 6.226691 E − 03 − 4.112028 E − 03 − 5.096590 E − 05 7.995669 E − 01 2.456582 E − 01 1.710934 E − 01 1.840633 E − 02 k 1 (59 Pr) 2 (2p') 3 (3p') 4 (1s) 5 (4p') k R 1.512190 E − 05 2.785126 E − 02 3.680808 E − 02 3.682849 E − 02 6.183397 E − 02 k 4.073279 E + 15 − 9.636514 E + 03 − 9.973082 E + 03 − 9.980179 E + 03 − 4.216776 E + 02 k 4.681737 E + 06 2.973704 E + 03 1.621891 E + 03 1.416684 E + 03 1.000719 E + 03 . 6 (5p') 7 (3d') 8 (2p'') 9 (2s) 1.455508 E − 01 1.496529 E − 01 1.623291 E − 01 1.848684 E − 01 − 4.219028 E + 02 − 4.219070 E + 02 − 4.358787 E + 02 − 9.931069 E + 01 4.001429 E + 02 2.720948 E + 02 2.534467 E + 02 2.210478 E + 02 10 (4d') 11 (3p'') 12 (3d'') 13 (3s) 2.707756 E − 01 5.585121 E − 01 5.866197 E − 01 5.884677 E − 01 − 2.373995 E + 01 − 2.393267 E + 01 − 9.960942 E + 00 − 2.863529 E + 00 1.526084 E + 02 6.078199 E + 01 3.872898 E + 01 3.736104 E + 01 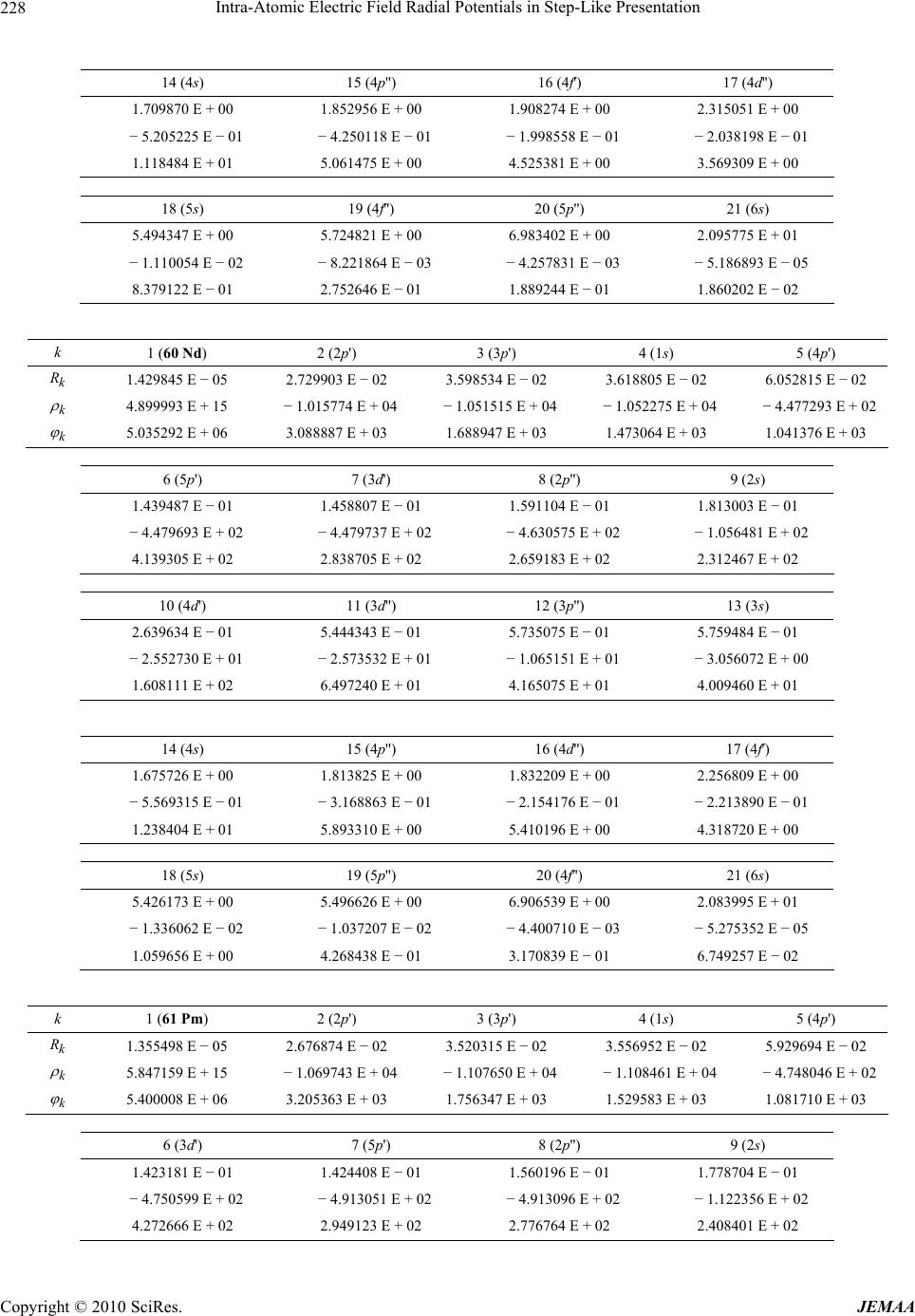 Intra-Atomic Electric Field Radial Potentials in Step-Like Presentation Copyright © 2010 SciRes. JEMAA 228 14 (4s) 15 (4p'') 16 (4f') 17 (4d'') 1.709870 E + 00 1.852956 E + 00 1.908274 E + 00 2.315051 E + 00 − 5.205225 E − 01 − 4.250118 E − 01 − 1.998558 E − 01 − 2.038198 E − 01 1.118484 E + 01 5.061475 E + 00 4.525381 E + 00 3.569309 E + 00 18 (5s) 19 (4f'') 20 (5p'') 21 (6s) 5.494347 E + 00 5.724821 E + 00 6.983402 E + 00 2.095775 E + 01 − 1.110054 E − 02 − 8.221864 E − 03 − 4.257831 E − 03 − 5.186893 E − 05 8.379122 E − 01 2.752646 E − 01 1.889244 E − 01 1.860202 E − 02 k 1 (60 Nd) 2 (2p') 3 (3p') 4 (1s) 5 (4p') k R 1.429845 E − 05 2.729903 E − 02 3.598534 E − 02 3.618805 E − 02 6.052815 E − 02 k 4.899993 E + 15 − 1.015774 E + 04 − 1.051515 E + 04 − 1.052275 E + 04 − 4.477293 E + 02 k 5.035292 E + 06 3.088887 E + 03 1.688947 E + 03 1.473064 E + 03 1.041376 E + 03 6 (5p') 7 (3d') 8 (2p'') 9 (2s) 1.439487 E − 01 1.458807 E − 01 1.591104 E − 01 1.813003 E − 01 − 4.479693 E + 02 − 4.479737 E + 02 − 4.630575 E + 02 − 1.056481 E + 02 4.139305 E + 02 2.838705 E + 02 2.659183 E + 02 2.312467 E + 02 10 (4d') 11 (3d'') 12 (3p'') 13 (3s) 2.639634 E − 01 5.444343 E − 01 5.735075 E − 01 5.759484 E − 01 − 2.552730 E + 01 − 2.573532 E + 01 − 1.065151 E + 01 − 3.056072 E + 00 1.608111 E + 02 6.497240 E + 01 4.165075 E + 01 4.009460 E + 01 14 (4s) 15 (4p'') 16 (4d'') 17 (4f') 1.675726 E + 00 1.813825 E + 00 1.832209 E + 00 2.256809 E + 00 − 5.569315 E − 01 − 3.168863 E − 01 − 2.154176 E − 01 − 2.213890 E − 01 1.238404 E + 01 5.893310 E + 00 5.410196 E + 00 4.318720 E + 00 18 (5s) 19 (5p'') 20 (4f'') 21 (6s) 5.426173 E + 00 5.496626 E + 00 6.906539 E + 00 2.083995 E + 01 − 1.336062 E − 02 − 1.037207 E − 02 − 4.400710 E − 03 − 5.275352 E − 05 1.059656 E + 00 4.268438 E − 01 3.170839 E − 01 6.749257 E − 02 k 1 (61 Pm) 2 (2p') 3 (3p') 4 (1s) 5 (4p') k R 1.355498 E − 05 2.676874 E − 02 3.520315 E − 02 3.556952 E − 02 5.929694 E − 02 k 5.847159 E + 15 − 1.069743 E + 04 − 1.107650 E + 04 − 1.108461 E + 04 − 4.748046 E + 02 k 5.400008 E + 06 3.205363 E + 03 1.756347 E + 03 1.529583 E + 03 1.081710 E + 03 6 (3d') 7 (5p') 8 (2p'') 9 (2s) 1.423181 E − 01 1.424408 E − 01 1.560196 E − 01 1.778704 E − 01 − 4.750599 E + 02 − 4.913051 E + 02 − 4.913096 E + 02 − 1.122356 E + 02 4.272666 E + 02 2.949123 E + 02 2.776764 E + 02 2.408401 E + 02 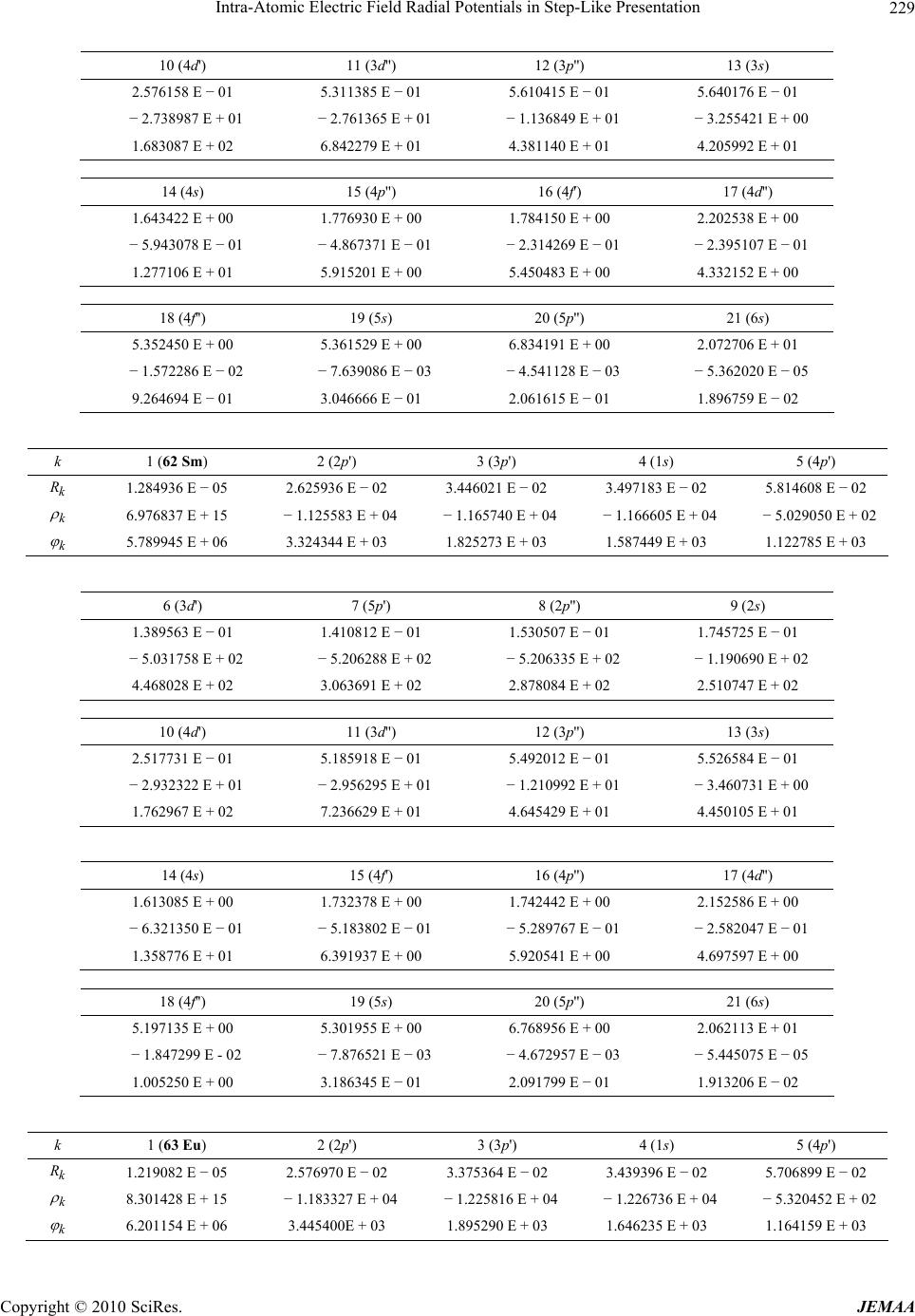 Intra-Atomic Electric Field Radial Potentials in Step-Like Presentation Copyright © 2010 SciRes. JEMAA 229 10 (4d') 11 (3d'') 12 (3p'') 13 (3s) 2.576158 E − 01 5.311385 E − 01 5.610415 E − 01 5.640176 E − 01 − 2.738987 E + 01 − 2.761365 E + 01 − 1.136849 E + 01 − 3.255421 E + 00 1.683087 E + 02 6.842279 E + 01 4.381140 E + 01 4.205992 E + 01 14 (4s) 15 (4p'') 16 (4f') 17 (4d'') 1.643422 E + 00 1.776930 E + 00 1.784150 E + 00 2.202538 E + 00 − 5.943078 E − 01 − 4.867371 E − 01 − 2.314269 E − 01 − 2.395107 E − 01 1.277106 E + 01 5.915201 E + 00 5.450483 E + 00 4.332152 E + 00 18 (4f'') 19 (5s) 20 (5p'') 21 (6s) 5.352450 E + 00 5.361529 E + 00 6.834191 E + 00 2.072706 E + 01 − 1.572286 E − 02 − 7.639086 E − 03 − 4.541128 E − 03 − 5.362020 E − 05 9.264694 E − 01 3.046666 E − 01 2.061615 E − 01 1.896759 E − 02 k 1 (62 Sm) 2 (2p') 3 (3p') 4 (1s) 5 (4p') k R 1.284936 E − 05 2.625936 E − 02 3.446021 E − 02 3.497183 E − 02 5.814608 E − 02 k 6.976837 E + 15 − 1.125583 E + 04 − 1.165740 E + 04 − 1.166605 E + 04 − 5.029050 E + 02 k 5.789945 E + 06 3.324344 E + 03 1.825273 E + 03 1.587449 E + 03 1.122785 E + 03 6 (3d') 7 (5p') 8 (2p'') 9 (2s) 1.389563 E − 01 1.410812 E − 01 1.530507 E − 01 1.745725 E − 01 − 5.031758 E + 02 − 5.206288 E + 02 − 5.206335 E + 02 − 1.190690 E + 02 4.468028 E + 02 3.063691 E + 02 2.878084 E + 02 2.510747 E + 02 10 (4d') 11 (3d'') 12 (3p'') 13 (3s) 2.517731 E − 01 5.185918 E − 01 5.492012 E − 01 5.526584 E − 01 − 2.932322 E + 01 − 2.956295 E + 01 − 1.210992 E + 01 − 3.460731 E + 00 1.762967 E + 02 7.236629 E + 01 4.645429 E + 01 4.450105 E + 01 14 (4s) 15 (4f') 16 (4p'') 17 (4d'') 1.613085 E + 00 1.732378 E + 00 1.742442 E + 00 2.152586 E + 00 − 6.321350 E − 01 − 5.183802 E − 01 − 5.289767 E − 01 − 2.582047 E − 01 1.358776 E + 01 6.391937 E + 00 5.920541 E + 00 4.697597 E + 00 18 (4f'') 19 (5s) 20 (5p'') 21 (6s) 5.197135 E + 00 5.301955 E + 00 6.768956 E + 00 2.062113 E + 01 − 1.847299 E - 02 − 7.876521 E − 03 − 4.672957 E − 03 − 5.445075 E − 05 1.005250 E + 00 3.186345 E − 01 2.091799 E − 01 1.913206 E − 02 k 1 (63 Eu) 2 (2p') 3 (3p') 4 (1s) 5 (4p') k R 1.219082 E − 05 2.576970 E − 02 3.375364 E − 02 3.439396 E − 02 5.706899 E − 02 k 8.301428 E + 15 − 1.183327 E + 04 − 1.225816 E + 04 − 1.226736 E + 04 − 5.320452 E + 02 k 6.201154 E + 06 3.445400E + 03 1.895290 E + 03 1.646235 E + 03 1.164159 E + 03 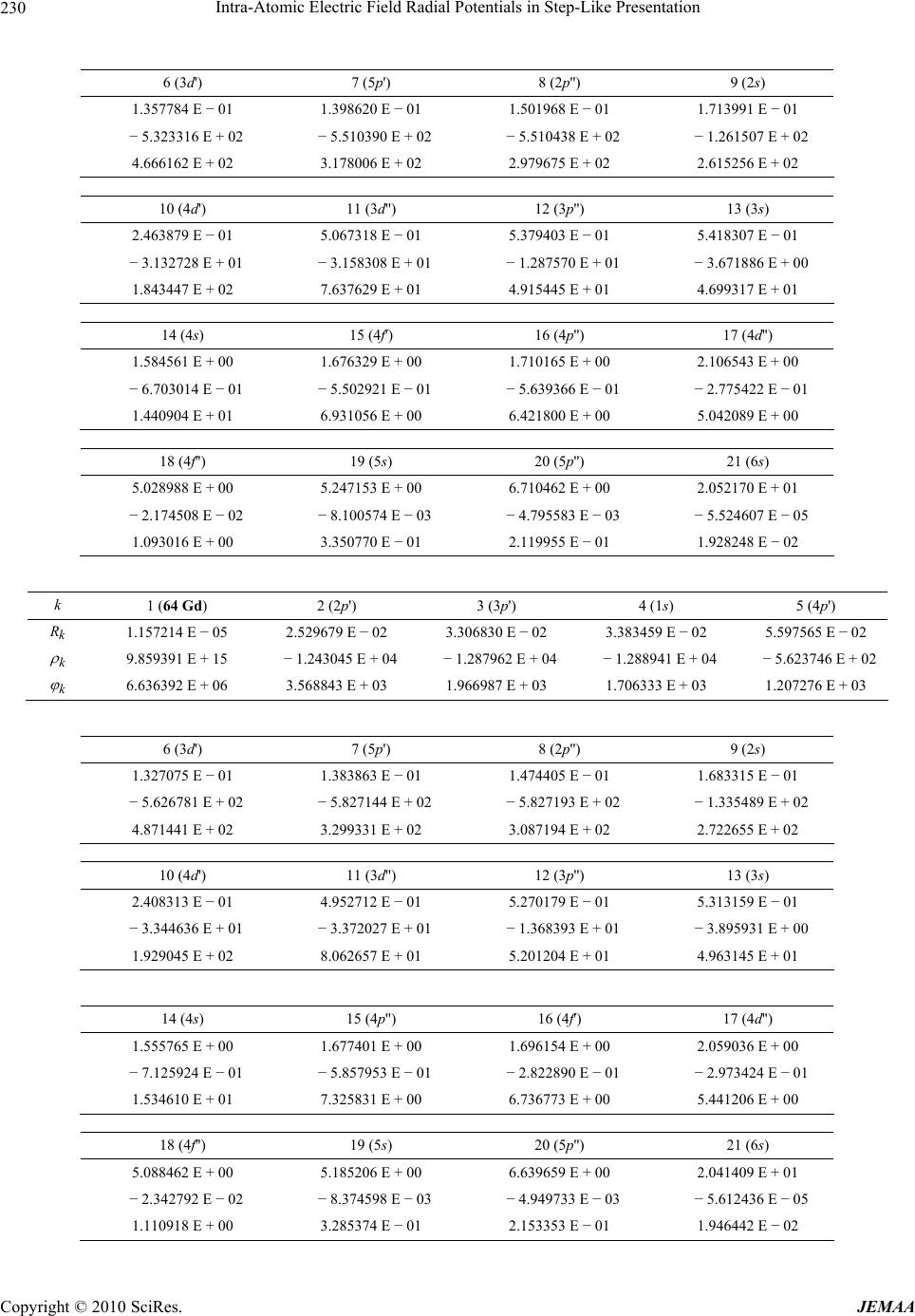 Intra-Atomic Electric Field Radial Potentials in Step-Like Presentation Copyright © 2010 SciRes. JEMAA 230 6 (3d') 7 (5p') 8 (2p'') 9 (2s) 1.357784 E − 01 1.398620 E − 01 1.501968 E − 01 1.713991 E − 01 − 5.323316 E + 02 − 5.510390 E + 02 − 5.510438 E + 02 − 1.261507 E + 02 4.666162 E + 02 3.178006 E + 02 2.979675 E + 02 2.615256 E + 02 10 (4d') 11 (3d'') 12 (3p'') 13 (3s) 2.463879 E − 01 5.067318 E − 01 5.379403 E − 01 5.418307 E − 01 − 3.132728 E + 01 − 3.158308 E + 01 − 1.287570 E + 01 − 3.671886 E + 00 1.843447 E + 02 7.637629 E + 01 4.915445 E + 01 4.699317 E + 01 14 (4s) 15 (4f') 16 (4p'') 17 (4d'') 1.584561 E + 00 1.676329 E + 00 1.710165 E + 00 2.106543 E + 00 − 6.703014 E − 01 − 5.502921 E − 01 − 5.639366 E − 01 − 2.775422 E − 01 1.440904 E + 01 6.931056 E + 00 6.421800 E + 00 5.042089 E + 00 18 (4f'') 19 (5s) 20 (5p'') 21 (6s) 5.028988 E + 00 5.247153 E + 00 6.710462 E + 00 2.052170 E + 01 − 2.174508 E − 02 − 8.100574 E − 03 − 4.795583 E − 03 − 5.524607 E − 05 1.093016 E + 00 3.350770 E − 01 2.119955 E − 01 1.928248 E − 02 k 1 (64 Gd) 2 (2p') 3 (3p') 4 (1s) 5 (4p') k R 1.157214 E − 05 2.529679 E − 02 3.306830 E − 02 3.383459 E − 02 5.597565 E − 02 k 9.859391 E + 15 − 1.243045 E + 04 − 1.287962 E + 04 − 1.288941 E + 04 − 5.623746 E + 02 k 6.636392 E + 06 3.568843 E + 03 1.966987 E + 03 1.706333 E + 03 1.207276 E + 03 6 (3d') 7 (5p') 8 (2p'') 9 (2s) 1.327075 E − 01 1.383863 E − 01 1.474405 E − 01 1.683315 E − 01 − 5.626781 E + 02 − 5.827144 E + 02 − 5.827193 E + 02 − 1.335489 E + 02 4.871441 E + 02 3.299331 E + 02 3.087194 E + 02 2.722655 E + 02 10 (4d') 11 (3d'') 12 (3p'') 13 (3s) 2.408313 E − 01 4.952712 E − 01 5.270179 E − 01 5.313159 E − 01 − 3.344636 E + 01 − 3.372027 E + 01 − 1.368393 E + 01 − 3.895931 E + 00 1.929045 E + 02 8.062657 E + 01 5.201204 E + 01 4.963145 E + 01 14 (4s) 15 (4p'') 16 (4f') 17 (4d'') 1.555765 E + 00 1.677401 E + 00 1.696154 E + 00 2.059036 E + 00 − 7.125924 E − 01 − 5.857953 E − 01 − 2.822890 E − 01 − 2.973424 E − 01 1.534610 E + 01 7.325831 E + 00 6.736773 E + 00 5.441206 E + 00 18 (4f'') 19 (5s) 20 (5p'') 21 (6s) 5.088462 E + 00 5.185206 E + 00 6.639659 E + 00 2.041409 E + 01 − 2.342792 E − 02 − 8.374598 E − 03 − 4.949733 E − 03 − 5.612436 E − 05 1.110918 E + 00 3.285374 E − 01 2.153353 E − 01 1.946442 E − 02 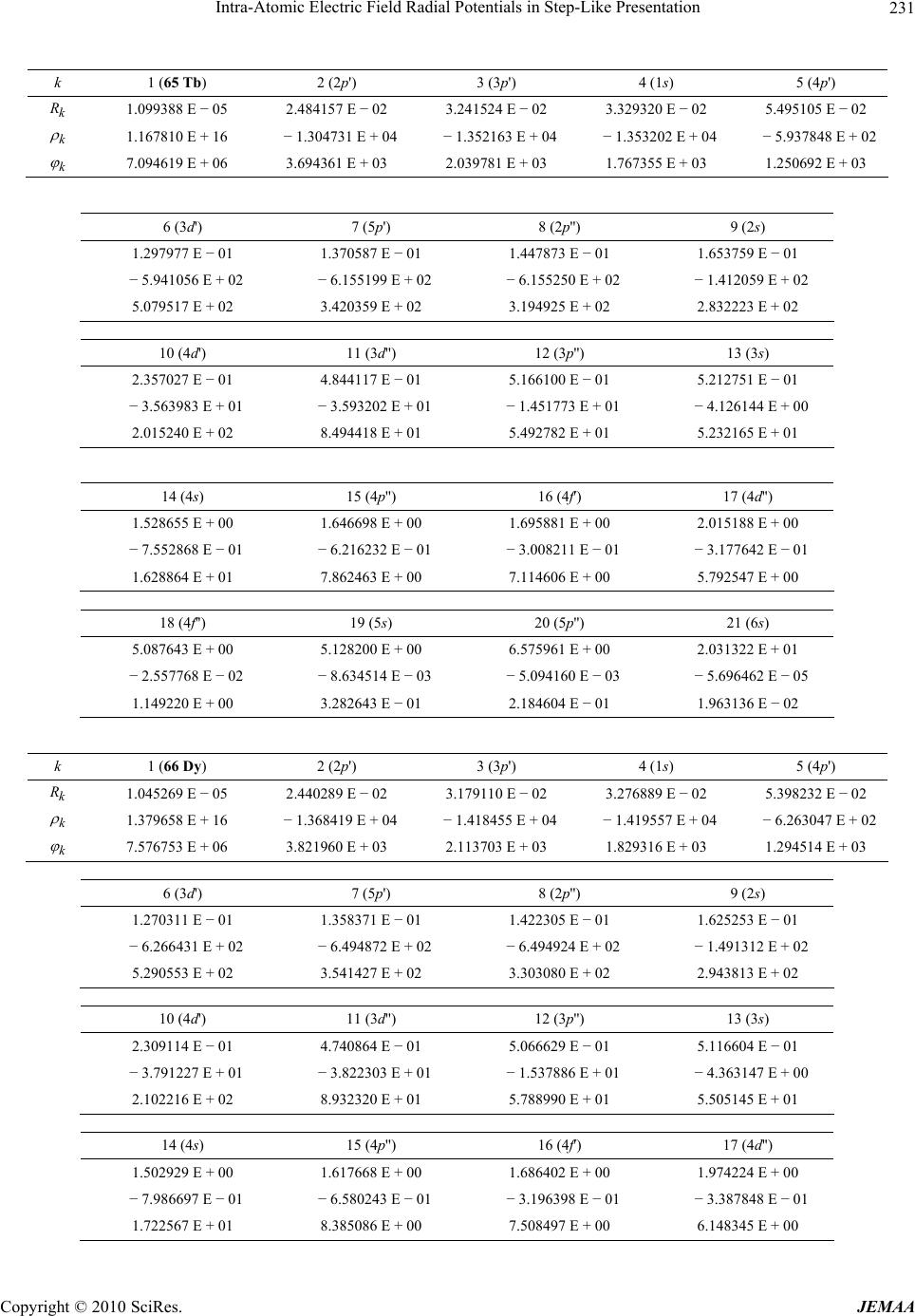 Intra-Atomic Electric Field Radial Potentials in Step-Like Presentation Copyright © 2010 SciRes. JEMAA 231 k 1 (65 Tb) 2 (2p') 3 (3p') 4 (1s) 5 (4p') k R 1.099388 E − 05 2.484157 E − 02 3.241524 E − 02 3.329320 E − 02 5.495105 E − 02 k 1.167810 E + 16 − 1.304731 E + 04 − 1.352163 E + 04 − 1.353202 E + 04 − 5.937848 E + 02 k 7.094619 E + 06 3.694361 E + 03 2.039781 E + 03 1.767355 E + 03 1.250692 E + 03 6 (3d') 7 (5p') 8 (2p'') 9 (2s) 1.297977 E − 01 1.370587 E − 01 1.447873 E − 01 1.653759 E − 01 − 5.941056 E + 02 − 6.155199 E + 02 − 6.155250 E + 02 − 1.412059 E + 02 5.079517 E + 02 3.420359 E + 02 3.194925 E + 02 2.832223 E + 02 10 (4d') 11 (3d'') 12 (3p'') 13 (3s) 2.357027 E − 01 4.844117 E − 01 5.166100 E − 01 5.212751 E − 01 − 3.563983 E + 01 − 3.593202 E + 01 − 1.451773 E + 01 − 4.126144 E + 00 2.015240 E + 02 8.494418 E + 01 5.492782 E + 01 5.232165 E + 01 14 (4s) 15 (4p'') 16 (4f') 17 (4d'') 1.528655 E + 00 1.646698 E + 00 1.695881 E + 00 2.015188 E + 00 − 7.552868 E − 01 − 6.216232 E − 01 − 3.008211 E − 01 − 3.177642 E − 01 1.628864 E + 01 7.862463 E + 00 7.114606 E + 00 5.792547 E + 00 18 (4f'') 19 (5s) 20 (5p'') 21 (6s) 5.087643 E + 00 5.128200 E + 00 6.575961 E + 00 2.031322 E + 01 − 2.557768 E − 02 − 8.634514 E − 03 − 5.094160 E − 03 − 5.696462 E − 05 1.149220 E + 00 3.282643 E − 01 2.184604 E − 01 1.963136 E − 02 k 1 (66 Dy) 2 (2p') 3 (3p') 4 (1s) 5 (4p') k R 1.045269 E − 05 2.440289 E − 02 3.179110 E − 02 3.276889 E − 02 5.398232 E − 02 k 1.379658 E + 16 − 1.368419 E + 04 − 1.418455 E + 04 − 1.419557 E + 04 − 6.263047 E + 02 k 7.576753 E + 06 3.821960 E + 03 2.113703 E + 03 1.829316 E + 03 1.294514 E + 03 6 (3d') 7 (5p') 8 (2p'') 9 (2s) 1.270311 E − 01 1.358371 E − 01 1.422305 E − 01 1.625253 E − 01 − 6.266431 E + 02 − 6.494872 E + 02 − 6.494924 E + 02 − 1.491312 E + 02 5.290553 E + 02 3.541427 E + 02 3.303080 E + 02 2.943813 E + 02 10 (4d') 11 (3d'') 12 (3p'') 13 (3s) 2.309114 E − 01 4.740864 E − 01 5.066629 E − 01 5.116604 E − 01 − 3.791227 E + 01 − 3.822303 E + 01 − 1.537886 E + 01 − 4.363147 E + 00 2.102216 E + 02 8.932320 E + 01 5.788990 E + 01 5.505145 E + 01 14 (4s) 15 (4p'') 16 (4f') 17 (4d'') 1.502929 E + 00 1.617668 E + 00 1.686402 E + 00 1.974224 E + 00 − 7.986697 E − 01 − 6.580243 E − 01 − 3.196398 E − 01 − 3.387848 E − 01 1.722567 E + 01 8.385086 E + 00 7.508497 E + 00 6.148345 E + 00 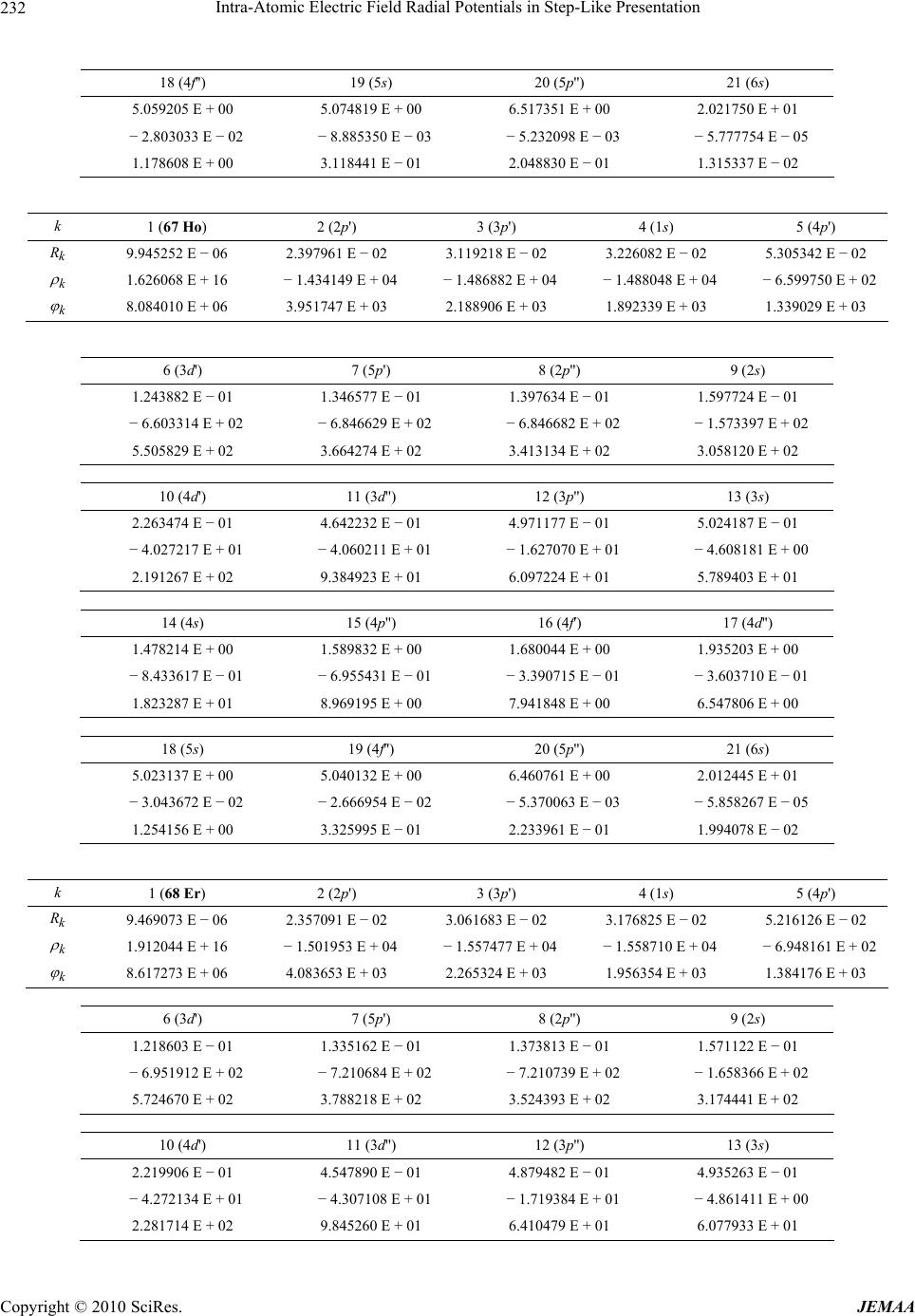 Intra-Atomic Electric Field Radial Potentials in Step-Like Presentation Copyright © 2010 SciRes. JEMAA 232 18 (4f'') 19 (5s) 20 (5p'') 21 (6s) 5.059205 E + 00 5.074819 E + 00 6.517351 E + 00 2.021750 E + 01 − 2.803033 E − 02 − 8.885350 E − 03 − 5.232098 E − 03 − 5.777754 E − 05 1.178608 E + 00 3.118441 E − 01 2.048830 E − 01 1.315337 E − 02 k 1 (67 Ho) 2 (2p') 3 (3p') 4 (1s) 5 (4p') k R 9.945252 E − 06 2.397961 E − 02 3.119218 E − 02 3.226082 E − 02 5.305342 E − 02 k 1.626068 E + 16 − 1.434149 E + 04 − 1.486882 E + 04 − 1.488048 E + 04 − 6.599750 E + 02 k 8.084010 E + 06 3.951747 E + 03 2.188906 E + 03 1.892339 E + 03 1.339029 E + 03 6 (3d') 7 (5p') 8 (2p'') 9 (2s) 1.243882 E − 01 1.346577 E − 01 1.397634 E − 01 1.597724 E − 01 − 6.603314 E + 02 − 6.846629 E + 02 − 6.846682 E + 02 − 1.573397 E + 02 5.505829 E + 02 3.664274 E + 02 3.413134 E + 02 3.058120 E + 02 10 (4d') 11 (3d'') 12 (3p'') 13 (3s) 2.263474 E − 01 4.642232 E − 01 4.971177 E − 01 5.024187 E − 01 − 4.027217 E + 01 − 4.060211 E + 01 − 1.627070 E + 01 − 4.608181 E + 00 2.191267 E + 02 9.384923 E + 01 6.097224 E + 01 5.789403 E + 01 14 (4s) 15 (4p'') 16 (4f') 17 (4d'') 1.478214 E + 00 1.589832 E + 00 1.680044 E + 00 1.935203 E + 00 − 8.433617 E − 01 − 6.955431 E − 01 − 3.390715 E − 01 − 3.603710 E − 01 1.823287 E + 01 8.969195 E + 00 7.941848 E + 00 6.547806 E + 00 18 (5s) 19 (4f'') 20 (5p'') 21 (6s) 5.023137 E + 00 5.040132 E + 00 6.460761 E + 00 2.012445 E + 01 − 3.043672 E − 02 − 2.666954 E − 02 − 5.370063 E − 03 − 5.858267 E − 05 1.254156 E + 00 3.325995 E − 01 2.233961 E − 01 1.994078 E − 02 k 1 (68 Er) 2 (2p') 3 (3p') 4 (1s) 5 (4p') k R 9.469073 E − 06 2.357091 E − 02 3.061683 E − 02 3.176825 E − 02 5.216126 E − 02 k 1.912044 E + 16 − 1.501953 E + 04 − 1.557477 E + 04 − 1.558710 E + 04 − 6.948161 E + 02 k 8.617273 E + 06 4.083653 E + 03 2.265324 E + 03 1.956354 E + 03 1.384176 E + 03 6 (3d') 7 (5p') 8 (2p'') 9 (2s) 1.218603 E − 01 1.335162 E − 01 1.373813 E − 01 1.571122 E − 01 − 6.951912 E + 02 − 7.210684 E + 02 − 7.210739 E + 02 − 1.658366 E + 02 5.724670 E + 02 3.788218 E + 02 3.524393 E + 02 3.174441 E + 02 10 (4d') 11 (3d'') 12 (3p'') 13 (3s) 2.219906 E − 01 4.547890 E − 01 4.879482 E − 01 4.935263 E − 01 − 4.272134 E + 01 − 4.307108 E + 01 − 1.719384 E + 01 − 4.861411 E + 00 2.281714 E + 02 9.845260 E + 01 6.410479 E + 01 6.077933 E + 01 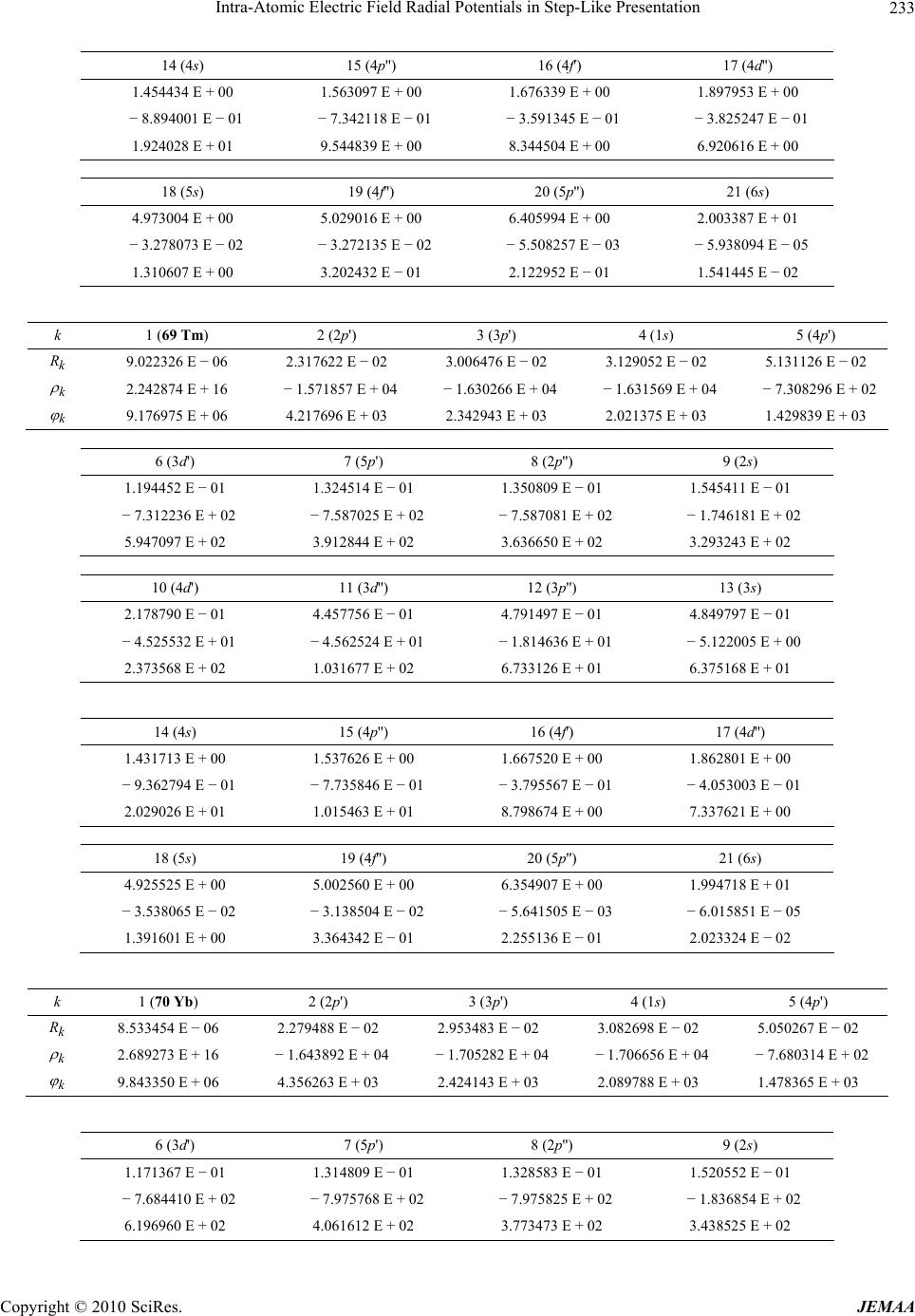 Intra-Atomic Electric Field Radial Potentials in Step-Like Presentation Copyright © 2010 SciRes. JEMAA 233 14 (4s) 15 (4p'') 16 (4f') 17 (4d'') 1.454434 E + 00 1.563097 E + 00 1.676339 E + 00 1.897953 E + 00 − 8.894001 E − 01 − 7.342118 E − 01 − 3.591345 E − 01 − 3.825247 E − 01 1.924028 E + 01 9.544839 E + 00 8.344504 E + 00 6.920616 E + 00 18 (5s) 19 (4f'') 20 (5p'') 21 (6s) 4.973004 E + 00 5.029016 E + 00 6.405994 E + 00 2.003387 E + 01 − 3.278073 E − 02 − 3.272135 E − 02 − 5.508257 E − 03 − 5.938094 E − 05 1.310607 E + 00 3.202432 E − 01 2.122952 E − 01 1.541445 E − 02 k 1 (69 Tm) 2 (2p') 3 (3p') 4 (1s) 5 (4p') k R 9.022326 E − 06 2.317622 E − 02 3.006476 E − 02 3.129052 E − 02 5.131126 E − 02 k 2.242874 E + 16 − 1.571857 E + 04 − 1.630266 E + 04 − 1.631569 E + 04 − 7.308296 E + 02 k 9.176975 E + 06 4.217696 E + 03 2.342943 E + 03 2.021375 E + 03 1.429839 E + 03 6 (3d') 7 (5p') 8 (2p'') 9 (2s) 1.194452 E − 01 1.324514 E − 01 1.350809 E − 01 1.545411 E − 01 − 7.312236 E + 02 − 7.587025 E + 02 − 7.587081 E + 02 − 1.746181 E + 02 5.947097 E + 02 3.912844 E + 02 3.636650 E + 02 3.293243 E + 02 10 (4d') 11 (3d'') 12 (3p'') 13 (3s) 2.178790 E − 01 4.457756 E − 01 4.791497 E − 01 4.849797 E − 01 − 4.525532 E + 01 − 4.562524 E + 01 − 1.814636 E + 01 − 5.122005 E + 00 2.373568 E + 02 1.031677 E + 02 6.733126 E + 01 6.375168 E + 01 14 (4s) 15 (4p'') 16 (4f') 17 (4d'') 1.431713 E + 00 1.537626 E + 00 1.667520 E + 00 1.862801 E + 00 − 9.362794 E − 01 − 7.735846 E − 01 − 3.795567 E − 01 − 4.053003 E − 01 2.029026 E + 01 1.015463 E + 01 8.798674 E + 00 7.337621 E + 00 18 (5s) 19 (4f'') 20 (5p'') 21 (6s) 4.925525 E + 00 5.002560 E + 00 6.354907 E + 00 1.994718 E + 01 − 3.538065 E − 02 − 3.138504 E − 02 − 5.641505 E − 03 − 6.015851 E − 05 1.391601 E + 00 3.364342 E − 01 2.255136 E − 01 2.023324 E − 02 k 1 (70 Yb) 2 (2p') 3 (3p') 4 (1s) 5 (4p') k R 8.533454 E − 06 2.279488 E − 02 2.953483 E − 02 3.082698 E − 02 5.050267 E − 02 k 2.689273 E + 16 − 1.643892 E + 04 − 1.705282 E + 04 − 1.706656 E + 04 − 7.680314 E + 02 k 9.843350 E + 06 4.356263 E + 03 2.424143 E + 03 2.089788 E + 03 1.478365 E + 03 6 (3d') 7 (5p') 8 (2p'') 9 (2s) 1.171367 E − 01 1.314809 E − 01 1.328583 E − 01 1.520552 E − 01 − 7.684410 E + 02 − 7.975768 E + 02 − 7.975825 E + 02 − 1.836854 E + 02 6.196960 E + 02 4.061612 E + 02 3.773473 E + 02 3.438525 E + 02 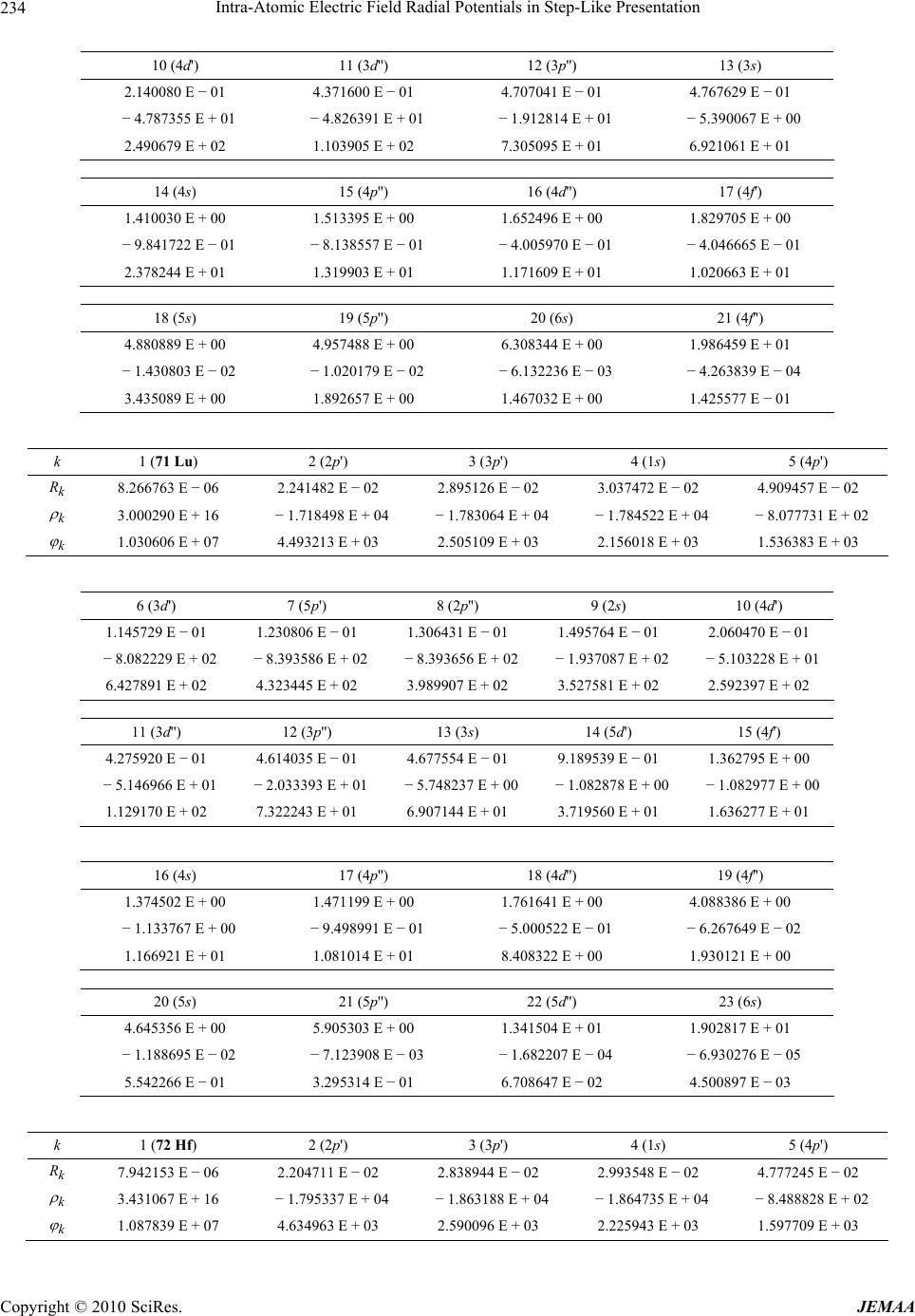 Intra-Atomic Electric Field Radial Potentials in Step-Like Presentation Copyright © 2010 SciRes. JEMAA 234 10 (4d') 11 (3d'') 12 (3p'') 13 (3s) 2.140080 E − 01 4.371600 E − 01 4.707041 E − 01 4.767629 E − 01 − 4.787355 E + 01 − 4.826391 E + 01 − 1.912814 E + 01 − 5.390067 E + 00 2.490679 E + 02 1.103905 E + 02 7.305095 E + 01 6.921061 E + 01 14 (4s) 15 (4p'') 16 (4d'') 17 (4f') 1.410030 E + 00 1.513395 E + 00 1.652496 E + 00 1.829705 E + 00 − 9.841722 E − 01 − 8.138557 E − 01 − 4.005970 E − 01 − 4.046665 E − 01 2.378244 E + 01 1.319903 E + 01 1.171609 E + 01 1.020663 E + 01 18 (5s) 19 (5p'') 20 (6s) 21 (4f'') 4.880889 E + 00 4.957488 E + 00 6.308344 E + 00 1.986459 E + 01 − 1.430803 E − 02 − 1.020179 E − 02 − 6.132236 E − 03 − 4.263839 E − 04 3.435089 E + 00 1.892657 E + 00 1.467032 E + 00 1.425577 E − 01 k 1 (71 Lu) 2 (2p') 3 (3p') 4 (1s) 5 (4p') k R 8.266763 E − 06 2.241482 E − 02 2.895126 E − 02 3.037472 E − 02 4.909457 E − 02 k 3.000290 E + 16 − 1.718498 E + 04 − 1.783064 E + 04 − 1.784522 E + 04 − 8.077731 E + 02 k 1.030606 E + 07 4.493213 E + 03 2.505109 E + 03 2.156018 E + 03 1.536383 E + 03 6 (3d') 7 (5p') 8 (2p'') 9 (2s) 10 (4d') 1.145729 E − 01 1.230806 E − 01 1.306431 E − 01 1.495764 E − 01 2.060470 E − 01 − 8.082229 E + 02 − 8.393586 E + 02 − 8.393656 E + 02 − 1.937087 E + 02 − 5.103228 E + 01 6.427891 E + 02 4.323445 E + 02 3.989907 E + 02 3.527581 E + 02 2.592397 E + 02 11 (3d'') 12 (3p'') 13 (3s) 14 (5d') 15 (4f') 4.275920 E − 01 4.614035 E − 01 4.677554 E − 01 9.189539 E − 01 1.362795 E + 00 − 5.146966 E + 01 − 2.033393 E + 01 − 5.748237 E + 00 − 1.082878 E + 00 − 1.082977 E + 00 1.129170 E + 02 7.322243 E + 01 6.907144 E + 01 3.719560 E + 01 1.636277 E + 01 16 (4s) 17 (4p'') 18 (4d'') 19 (4f'') 1.374502 E + 00 1.471199 E + 00 1.761641 E + 00 4.088386 E + 00 − 1.133767 E + 00 − 9.498991 E − 01 − 5.000522 E − 01 − 6.267649 E − 02 1.166921 E + 01 1.081014 E + 01 8.408322 E + 00 1.930121 E + 00 20 (5s) 21 (5p'') 22 (5d'') 23 (6s) 4.645356 E + 00 5.905303 E + 00 1.341504 E + 01 1.902817 E + 01 − 1.188695 E − 02 − 7.123908 E − 03 − 1.682207 E − 04 − 6.930276 E − 05 5.542266 E − 01 3.295314 E − 01 6.708647 E − 02 4.500897 E − 03 k 1 (72 Hf) 2 (2p') 3 (3p') 4 (1s) 5 (4p') k R 7.942153 E − 06 2.204711 E − 02 2.838944 E − 02 2.993548 E − 02 4.777245 E − 02 k 3.431067 E + 16 − 1.795337 E + 04 − 1.863188 E + 04 − 1.864735 E + 04 − 8.488828 E + 02 k 1.087839 E + 07 4.634963 E + 03 2.590096 E + 03 2.225943 E + 03 1.597709 E + 03 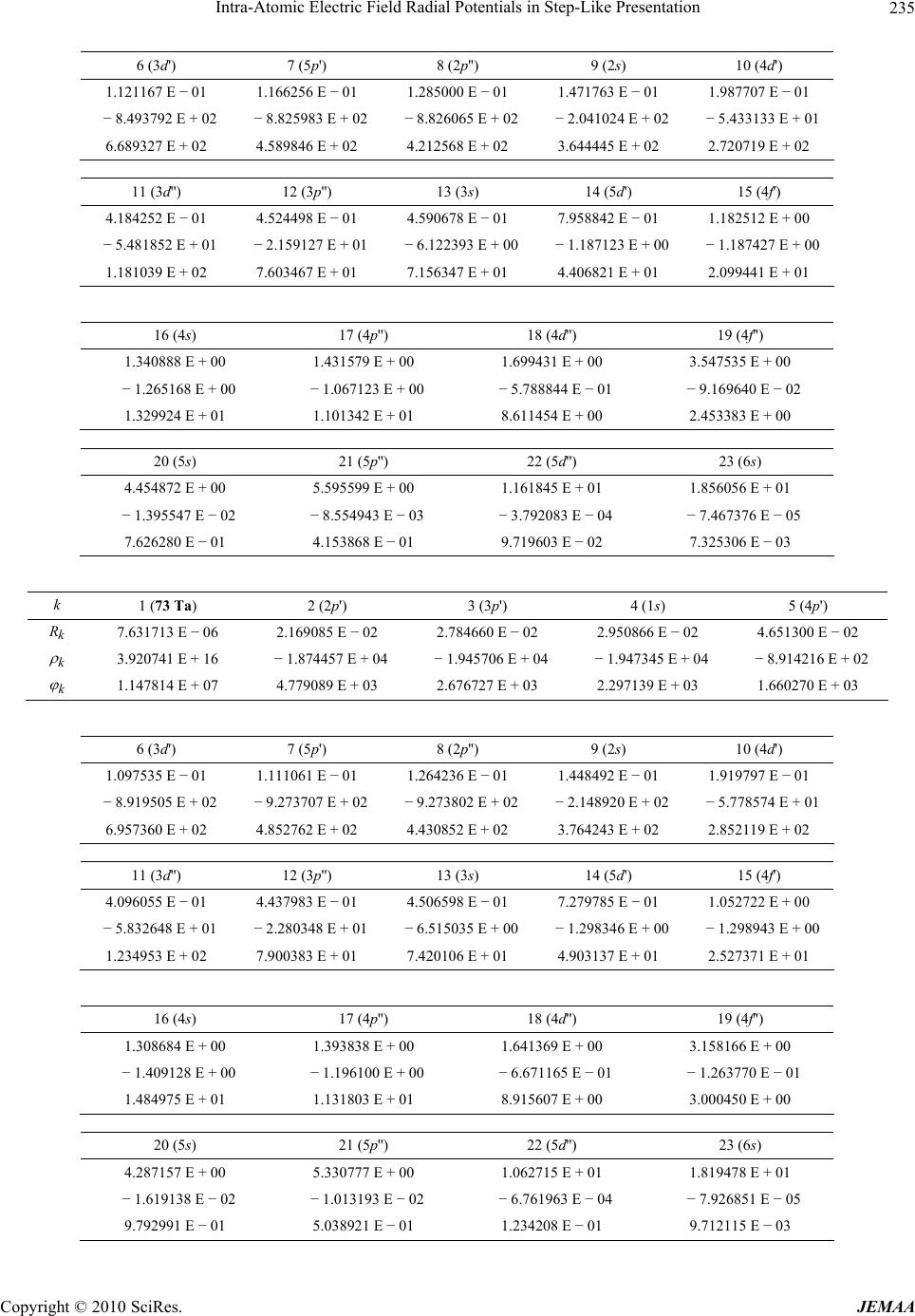 Intra-Atomic Electric Field Radial Potentials in Step-Like Presentation Copyright © 2010 SciRes. JEMAA 235 6 (3d') 7 (5p') 8 (2p'') 9 (2s) 10 (4d') 1.121167 E − 01 1.166256 E − 01 1.285000 E − 01 1.471763 E − 01 1.987707 E − 01 − 8.493792 E + 02 − 8.825983 E + 02 − 8.826065 E + 02 − 2.041024 E + 02 − 5.433133 E + 01 6.689327 E + 02 4.589846 E + 02 4.212568 E + 02 3.644445 E + 02 2.720719 E + 02 11 (3d'') 12 (3p'') 13 (3s) 14 (5d') 15 (4f') 4.184252 E − 01 4.524498 E − 01 4.590678 E − 01 7.958842 E − 01 1.182512 E + 00 − 5.481852 E + 01 − 2.159127 E + 01 − 6.122393 E + 00 − 1.187123 E + 00 − 1.187427 E + 00 1.181039 E + 02 7.603467 E + 01 7.156347 E + 01 4.406821 E + 01 2.099441 E + 01 16 (4s) 17 (4p'') 18 (4d'') 19 (4f'') 1.340888 E + 00 1.431579 E + 00 1.699431 E + 00 3.547535 E + 00 − 1.265168 E + 00 − 1.067123 E + 00 − 5.788844 E − 01 − 9.169640 E − 02 1.329924 E + 01 1.101342 E + 01 8.611454 E + 00 2.453383 E + 00 20 (5s) 21 (5p'') 22 (5d'') 23 (6s) 4.454872 E + 00 5.595599 E + 00 1.161845 E + 01 1.856056 E + 01 − 1.395547 E − 02 − 8.554943 E − 03 − 3.792083 E − 04 − 7.467376 E − 05 7.626280 E − 01 4.153868 E − 01 9.719603 E − 02 7.325306 E − 03 k 1 (73 Ta) 2 (2p') 3 (3p') 4 (1s) 5 (4p') k R 7.631713 E − 06 2.169085 E − 02 2.784660 E − 02 2.950866 E − 02 4.651300 E − 02 k 3.920741 E + 16 − 1.874457 E + 04 − 1.945706 E + 04 − 1.947345 E + 04 − 8.914216 E + 02 k 1.147814 E + 07 4.779089 E + 03 2.676727 E + 03 2.297139 E + 03 1.660270 E + 03 6 (3d') 7 (5p') 8 (2p'') 9 (2s) 10 (4d') 1.097535 E − 01 1.111061 E − 01 1.264236 E − 01 1.448492 E − 01 1.919797 E − 01 − 8.919505 E + 02 − 9.273707 E + 02 − 9.273802 E + 02 − 2.148920 E + 02 − 5.778574 E + 01 6.957360 E + 02 4.852762 E + 02 4.430852 E + 02 3.764243 E + 02 2.852119 E + 02 11 (3d'') 12 (3p'') 13 (3s) 14 (5d') 15 (4f') 4.096055 E − 01 4.437983 E − 01 4.506598 E − 01 7.279785 E − 01 1.052722 E + 00 − 5.832648 E + 01 − 2.280348 E + 01 − 6.515035 E + 00 − 1.298346 E + 00 − 1.298943 E + 00 1.234953 E + 02 7.900383 E + 01 7.420106 E + 01 4.903137 E + 01 2.527371 E + 01 16 (4s) 17 (4p'') 18 (4d'') 19 (4f'') 1.308684 E + 00 1.393838 E + 00 1.641369 E + 00 3.158166 E + 00 − 1.409128 E + 00 − 1.196100 E + 00 − 6.671165 E − 01 − 1.263770 E − 01 1.484975 E + 01 1.131803 E + 01 8.915607 E + 00 3.000450 E + 00 20 (5s) 21 (5p'') 22 (5d'') 23 (6s) 4.287157 E + 00 5.330777 E + 00 1.062715 E + 01 1.819478 E + 01 − 1.619138 E − 02 − 1.013193 E − 02 − 6.761963 E − 04 − 7.926851 E − 05 9.792991 E − 01 5.038921 E − 01 1.234208 E − 01 9.712115 E − 03 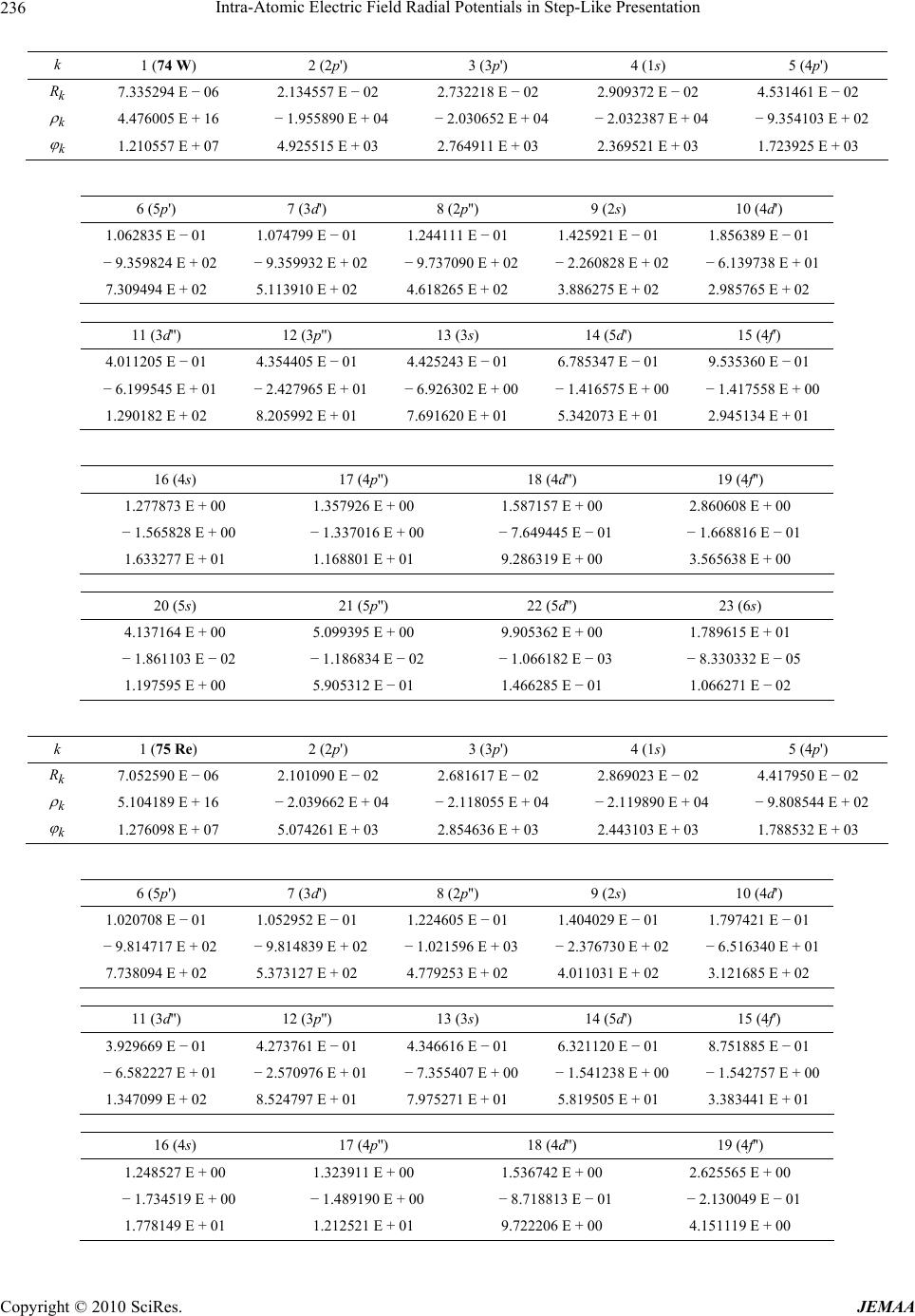 Intra-Atomic Electric Field Radial Potentials in Step-Like Presentation Copyright © 2010 SciRes. JEMAA 236 k 1 (74 W) 2 (2p') 3 (3p') 4 (1s) 5 (4p') k R 7.335294 E − 06 2.134557 E − 02 2.732218 E − 02 2.909372 E − 02 4.531461 E − 02 k 4.476005 E + 16 − 1.955890 E + 04 − 2.030652 E + 04 − 2.032387 E + 04 − 9.354103 E + 02 k 1.210557 E + 07 4.925515 E + 03 2.764911 E + 03 2.369521 E + 03 1.723925 E + 03 6 (5p') 7 (3d') 8 (2p'') 9 (2s) 10 (4d') 1.062835 E − 01 1.074799 E − 01 1.244111 E − 01 1.425921 E − 01 1.856389 E − 01 − 9.359824 E + 02 − 9.359932 E + 02 − 9.737090 E + 02 − 2.260828 E + 02 − 6.139738 E + 01 7.309494 E + 02 5.113910 E + 02 4.618265 E + 02 3.886275 E + 02 2.985765 E + 02 11 (3d'') 12 (3p'') 13 (3s) 14 (5d') 15 (4f') 4.011205 E − 01 4.354405 E − 01 4.425243 E − 01 6.785347 E − 01 9.535360 E − 01 − 6.199545 E + 01 − 2.427965 E + 01 − 6.926302 E + 00 − 1.416575 E + 00 − 1.417558 E + 00 1.290182 E + 02 8.205992 E + 01 7.691620 E + 01 5.342073 E + 01 2.945134 E + 01 16 (4s) 17 (4p'') 18 (4d'') 19 (4f'') 1.277873 E + 00 1.357926 E + 00 1.587157 E + 00 2.860608 E + 00 − 1.565828 E + 00 − 1.337016 E + 00 − 7.649445 E − 01 − 1.668816 E − 01 1.633277 E + 01 1.168801 E + 01 9.286319 E + 00 3.565638 E + 00 20 (5s) 21 (5p'') 22 (5d'') 23 (6s) 4.137164 E + 00 5.099395 E + 00 9.905362 E + 00 1.789615 E + 01 − 1.861103 E − 02 − 1.186834 E − 02 − 1.066182 E − 03 − 8.330332 E − 05 1.197595 E + 00 5.905312 E − 01 1.466285 E − 01 1.066271 E − 02 k 1 (75 Re) 2 (2p') 3 (3p') 4 (1s) 5 (4p') k R 7.052590 E − 06 2.101090 E − 02 2.681617 E − 02 2.869023 E − 02 4.417950 E − 02 k 5.104189 E + 16 − 2.039662 E + 04 − 2.118055 E + 04 − 2.119890 E + 04 − 9.808544 E + 02 k 1.276098 E + 07 5.074261 E + 03 2.854636 E + 03 2.443103 E + 03 1.788532 E + 03 6 (5p') 7 (3d') 8 (2p'') 9 (2s) 10 (4d') 1.020708 E − 01 1.052952 E − 01 1.224605 E − 01 1.404029 E − 01 1.797421 E − 01 − 9.814717 E + 02 − 9.814839 E + 02 − 1.021596 E + 03 − 2.376730 E + 02 − 6.516340 E + 01 7.738094 E + 02 5.373127 E + 02 4.779253 E + 02 4.011031 E + 02 3.121685 E + 02 11 (3d'') 12 (3p'') 13 (3s) 14 (5d') 15 (4f') 3.929669 E − 01 4.273761 E − 01 4.346616 E − 01 6.321120 E − 01 8.751885 E − 01 − 6.582227 E + 01 − 2.570976 E + 01 − 7.355407 E + 00 − 1.541238 E + 00 − 1.542757 E + 00 1.347099 E + 02 8.524797 E + 01 7.975271 E + 01 5.819505 E + 01 3.383441 E + 01 16 (4s) 17 (4p'') 18 (4d'') 19 (4f'') 1.248527 E + 00 1.323911 E + 00 1.536742 E + 00 2.625565 E + 00 − 1.734519 E + 00 − 1.489190 E + 00 − 8.718813 E − 01 − 2.130049 E − 01 1.778149 E + 01 1.212521 E + 01 9.722206 E + 00 4.151119 E + 00 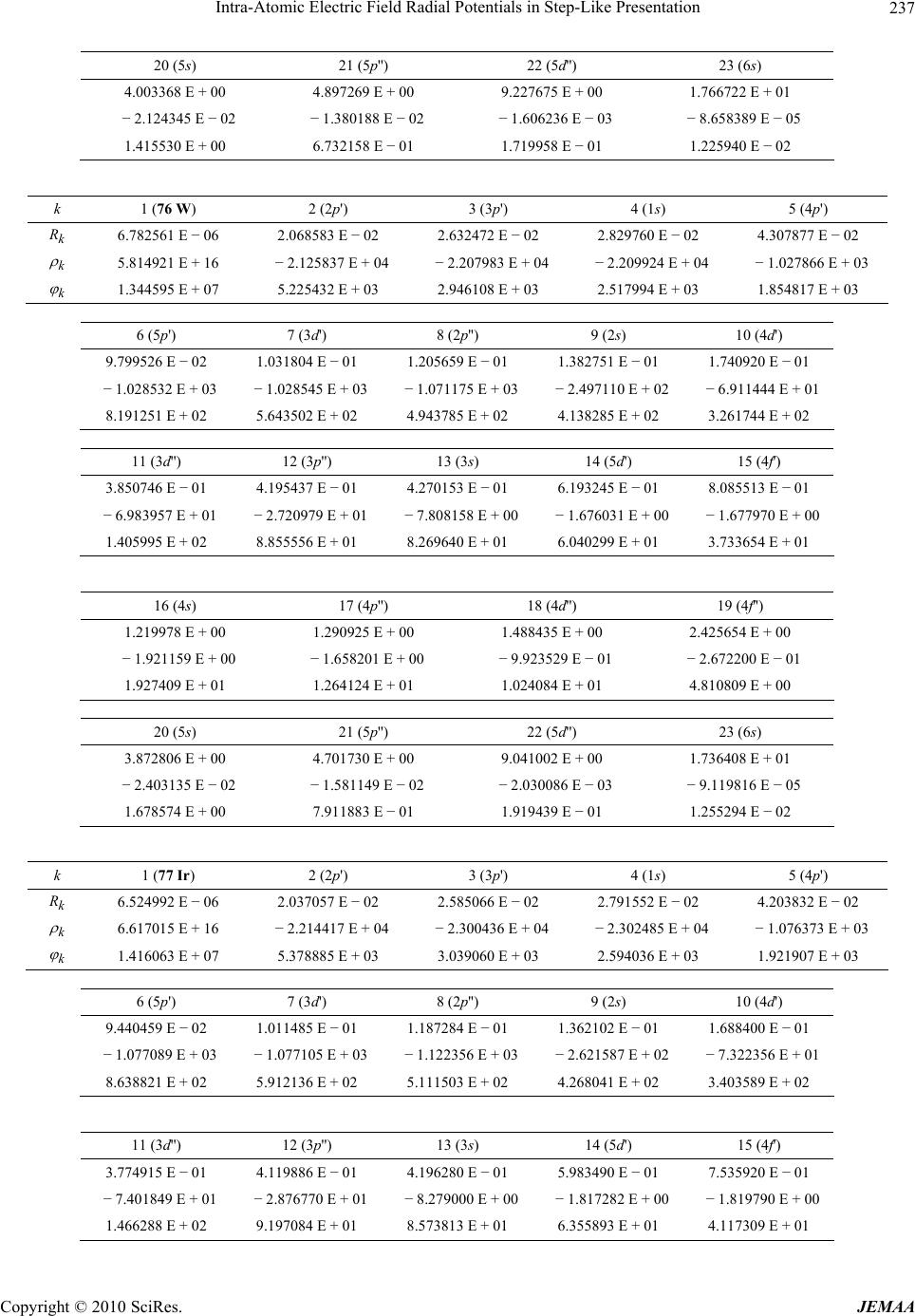 Intra-Atomic Electric Field Radial Potentials in Step-Like Presentation Copyright © 2010 SciRes. JEMAA 237 20 (5s) 21 (5p'') 22 (5d'') 23 (6s) 4.003368 E + 00 4.897269 E + 00 9.227675 E + 00 1.766722 E + 01 − 2.124345 E − 02 − 1.380188 E − 02 − 1.606236 E − 03 − 8.658389 E − 05 1.415530 E + 00 6.732158 E − 01 1.719958 E − 01 1.225940 E − 02 k 1 (76 W) 2 (2p') 3 (3p') 4 (1s) 5 (4p') k R 6.782561 E − 06 2.068583 E − 02 2.632472 E − 02 2.829760 E − 02 4.307877 E − 02 k 5.814921 E + 16 − 2.125837 E + 04 − 2.207983 E + 04 − 2.209924 E + 04 − 1.027866 E + 03 k 1.344595 E + 07 5.225432 E + 03 2.946108 E + 03 2.517994 E + 03 1.854817 E + 03 6 (5p') 7 (3d') 8 (2p'') 9 (2s) 10 (4d') 9.799526 E − 02 1.031804 E − 01 1.205659 E − 01 1.382751 E − 01 1.740920 E − 01 − 1.028532 E + 03 − 1.028545 E + 03 − 1.071175 E + 03 − 2.497110 E + 02 − 6.911444 E + 01 8.191251 E + 02 5.643502 E + 02 4.943785 E + 02 4.138285 E + 02 3.261744 E + 02 11 (3d'') 12 (3p'') 13 (3s) 14 (5d') 15 (4f') 3.850746 E − 01 4.195437 E − 01 4.270153 E − 01 6.193245 E − 01 8.085513 E − 01 − 6.983957 E + 01 − 2.720979 E + 01 − 7.808158 E + 00 − 1.676031 E + 00 − 1.677970 E + 00 1.405995 E + 02 8.855556 E + 01 8.269640 E + 01 6.040299 E + 01 3.733654 E + 01 16 (4s) 17 (4p'') 18 (4d'') 19 (4f'') 1.219978 E + 00 1.290925 E + 00 1.488435 E + 00 2.425654 E + 00 − 1.921159 E + 00 − 1.658201 E + 00 − 9.923529 E − 01 − 2.672200 E − 01 1.927409 E + 01 1.264124 E + 01 1.024084 E + 01 4.810809 E + 00 20 (5s) 21 (5p'') 22 (5d'') 23 (6s) 3.872806 E + 00 4.701730 E + 00 9.041002 E + 00 1.736408 E + 01 − 2.403135 E − 02 − 1.581149 E − 02 − 2.030086 E − 03 − 9.119816 E − 05 1.678574 E + 00 7.911883 E − 01 1.919439 E − 01 1.255294 E − 02 k 1 (77 Ir) 2 (2p') 3 (3p') 4 (1s) 5 (4p') k R 6.524992 E − 06 2.037057 E − 02 2.585066 E − 02 2.791552 E − 02 4.203832 E − 02 k 6.617015 E + 16 − 2.214417 E + 04 − 2.300436 E + 04 − 2.302485 E + 04 − 1.076373 E + 03 k 1.416063 E + 07 5.378885 E + 03 3.039060 E + 03 2.594036 E + 03 1.921907 E + 03 6 (5p') 7 (3d') 8 (2p'') 9 (2s) 10 (4d') 9.440459 E − 02 1.011485 E − 01 1.187284 E − 01 1.362102 E − 01 1.688400 E − 01 − 1.077089 E + 03 − 1.077105 E + 03 − 1.122356 E + 03 − 2.621587 E + 02 − 7.322356 E + 01 8.638821 E + 02 5.912136 E + 02 5.111503 E + 02 4.268041 E + 02 3.403589 E + 02 11 (3d'') 12 (3p'') 13 (3s) 14 (5d') 15 (4f') 3.774915 E − 01 4.119886 E − 01 4.196280 E − 01 5.983490 E − 01 7.535920 E − 01 − 7.401849 E + 01 − 2.876770 E + 01 − 8.279000 E + 00 − 1.817282 E + 00 − 1.819790 E + 00 1.466288 E + 02 9.197084 E + 01 8.573813 E + 01 6.355893 E + 01 4.117309 E + 01 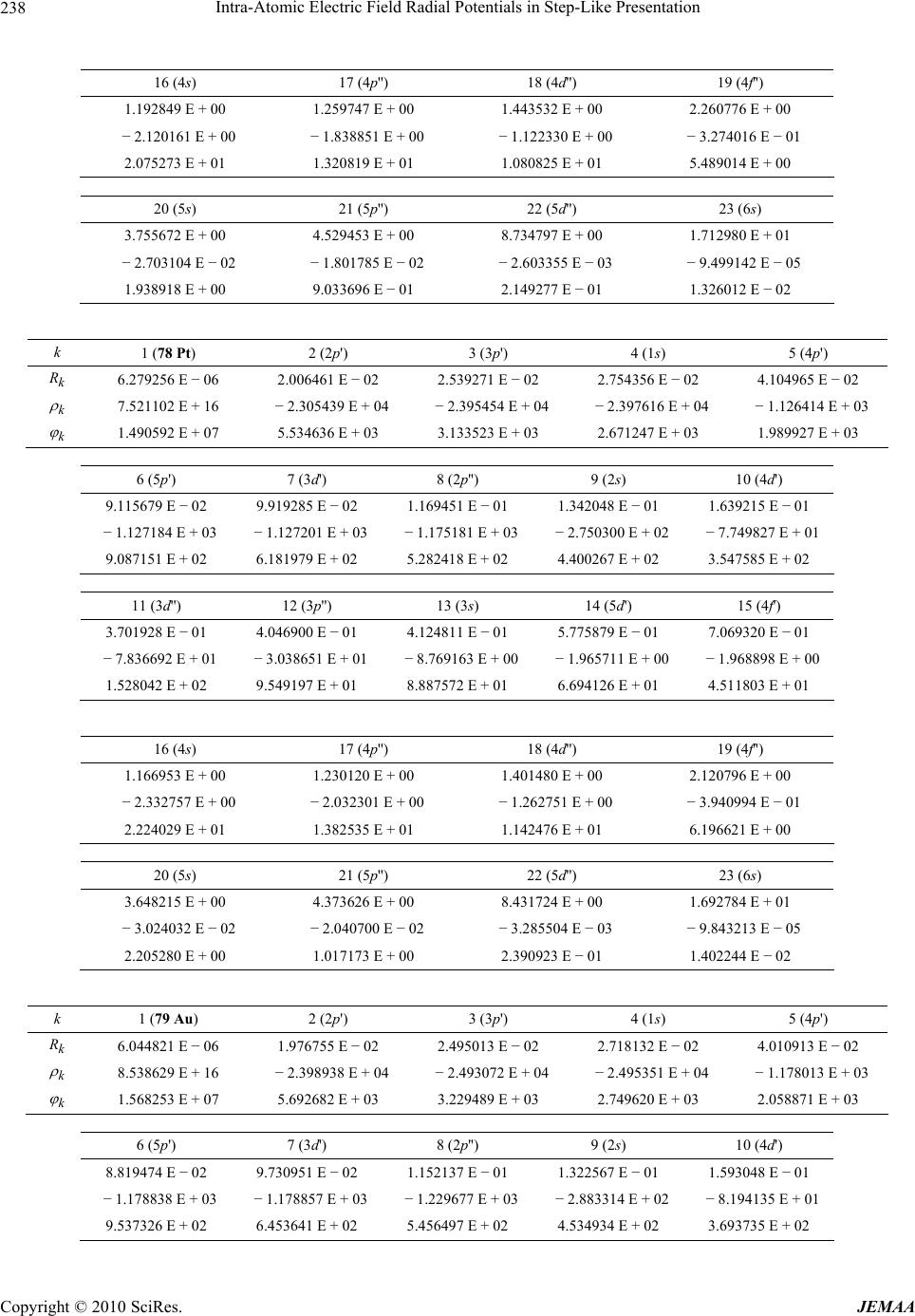 Intra-Atomic Electric Field Radial Potentials in Step-Like Presentation Copyright © 2010 SciRes. JEMAA 238 16 (4s) 17 (4p'') 18 (4d'') 19 (4f'') 1.192849 E + 00 1.259747 E + 00 1.443532 E + 00 2.260776 E + 00 − 2.120161 E + 00 − 1.838851 E + 00 − 1.122330 E + 00 − 3.274016 E − 01 2.075273 E + 01 1.320819 E + 01 1.080825 E + 01 5.489014 E + 00 20 (5s) 21 (5p'') 22 (5d'') 23 (6s) 3.755672 E + 00 4.529453 E + 00 8.734797 E + 00 1.712980 E + 01 − 2.703104 E − 02 − 1.801785 E − 02 − 2.603355 E − 03 − 9.499142 E − 05 1.938918 E + 00 9.033696 E − 01 2.149277 E − 01 1.326012 E − 02 k 1 (78 Pt) 2 (2p') 3 (3p') 4 (1s) 5 (4p') k R 6.279256 E − 06 2.006461 E − 02 2.539271 E − 02 2.754356 E − 02 4.104965 E − 02 k 7.521102 E + 16 − 2.305439 E + 04 − 2.395454 E + 04 − 2.397616 E + 04 − 1.126414 E + 03 k 1.490592 E + 07 5.534636 E + 03 3.133523 E + 03 2.671247 E + 03 1.989927 E + 03 6 (5p') 7 (3d') 8 (2p'') 9 (2s) 10 (4d') 9.115679 E − 02 9.919285 E − 02 1.169451 E − 01 1.342048 E − 01 1.639215 E − 01 − 1.127184 E + 03 − 1.127201 E + 03 − 1.175181 E + 03 − 2.750300 E + 02 − 7.749827 E + 01 9.087151 E + 02 6.181979 E + 02 5.282418 E + 02 4.400267 E + 02 3.547585 E + 02 11 (3d'') 12 (3p'') 13 (3s) 14 (5d') 15 (4f') 3.701928 E − 01 4.046900 E − 01 4.124811 E − 01 5.775879 E − 01 7.069320 E − 01 − 7.836692 E + 01 − 3.038651 E + 01 − 8.769163 E + 00 − 1.965711 E + 00 − 1.968898 E + 00 1.528042 E + 02 9.549197 E + 01 8.887572 E + 01 6.694126 E + 01 4.511803 E + 01 16 (4s) 17 (4p'') 18 (4d'') 19 (4f'') 1.166953 E + 00 1.230120 E + 00 1.401480 E + 00 2.120796 E + 00 − 2.332757 E + 00 − 2.032301 E + 00 − 1.262751 E + 00 − 3.940994 E − 01 2.224029 E + 01 1.382535 E + 01 1.142476 E + 01 6.196621 E + 00 20 (5s) 21 (5p'') 22 (5d'') 23 (6s) 3.648215 E + 00 4.373626 E + 00 8.431724 E + 00 1.692784 E + 01 − 3.024032 E − 02 − 2.040700 E − 02 − 3.285504 E − 03 − 9.843213 E − 05 2.205280 E + 00 1.017173 E + 00 2.390923 E − 01 1.402244 E − 02 k 1 (79 Au) 2 (2p') 3 (3p') 4 (1s) 5 (4p') k R 6.044821 E − 06 1.976755 E − 02 2.495013 E − 02 2.718132 E − 02 4.010913 E − 02 k 8.538629 E + 16 − 2.398938 E + 04 − 2.493072 E + 04 − 2.495351 E + 04 − 1.178013 E + 03 k 1.568253 E + 07 5.692682 E + 03 3.229489 E + 03 2.749620 E + 03 2.058871 E + 03 6 (5p') 7 (3d') 8 (2p'') 9 (2s) 10 (4d') 8.819474 E − 02 9.730951 E − 02 1.152137 E − 01 1.322567 E − 01 1.593048 E − 01 − 1.178838 E + 03 − 1.178857 E + 03 − 1.229677 E + 03 − 2.883314 E + 02 − 8.194135 E + 01 9.537326 E + 02 6.453641 E + 02 5.456497 E + 02 4.534934 E + 02 3.693735 E + 02 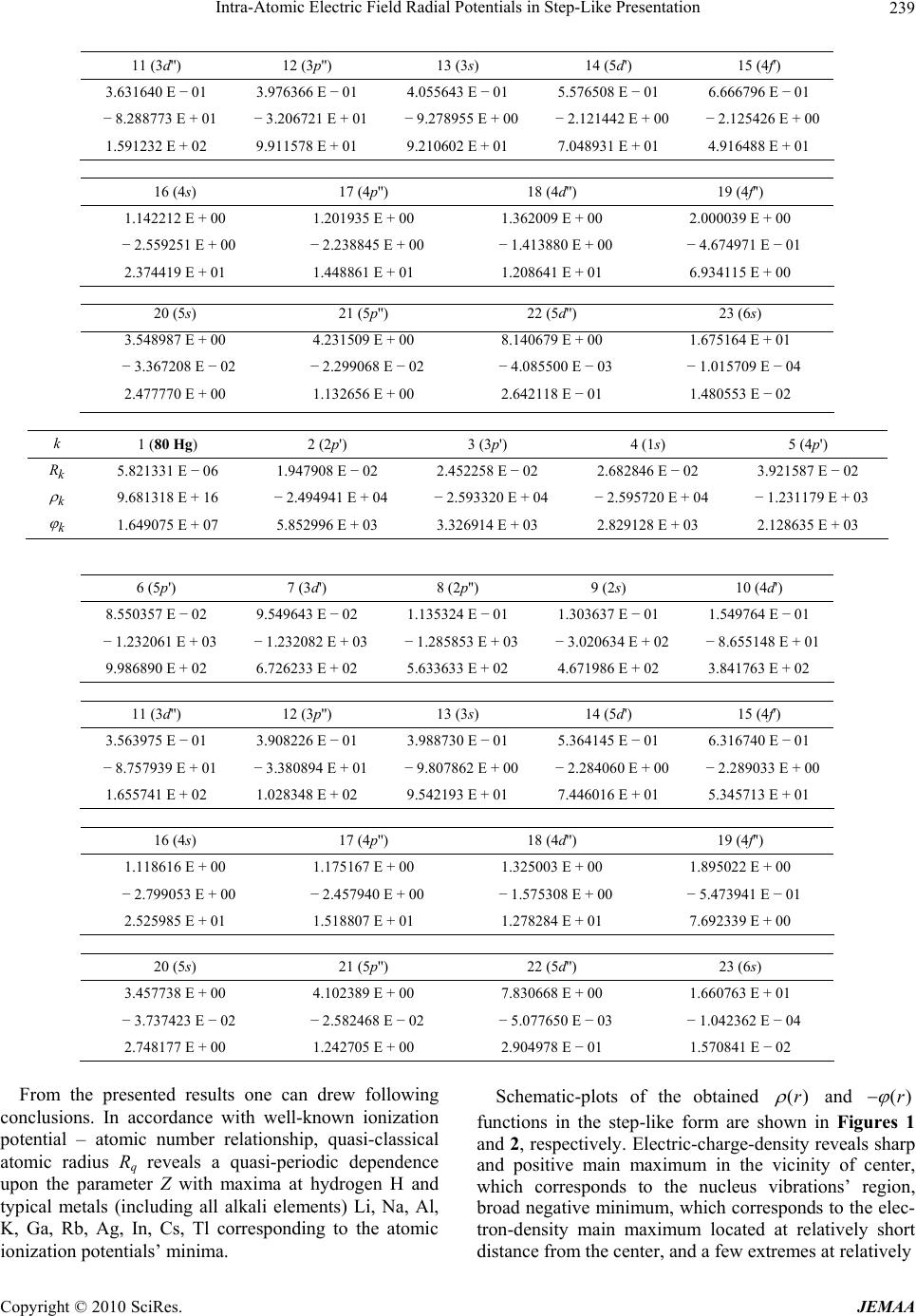 Intra-Atomic Electric Field Radial Potentials in Step-Like Presentation Copyright © 2010 SciRes. JEMAA 239 11 (3d'') 12 (3p'') 13 (3s) 14 (5d') 15 (4f') 3.631640 E − 01 3.976366 E − 01 4.055643 E − 01 5.576508 E − 01 6.666796 E − 01 − 8.288773 E + 01 − 3.206721 E + 01 − 9.278955 E + 00 − 2.121442 E + 00 − 2.125426 E + 00 1.591232 E + 02 9.911578 E + 01 9.210602 E + 01 7.048931 E + 01 4.916488 E + 01 16 (4s) 17 (4p'') 18 (4d'') 19 (4f'') 1.142212 E + 00 1.201935 E + 00 1.362009 E + 00 2.000039 E + 00 − 2.559251 E + 00 − 2.238845 E + 00 − 1.413880 E + 00 − 4.674971 E − 01 2.374419 E + 01 1.448861 E + 01 1.208641 E + 01 6.934115 E + 00 20 (5s) 21 (5p'') 22 (5d'') 23 (6s) 3.548987 E + 00 4.231509 E + 00 8.140679 E + 00 1.675164 E + 01 − 3.367208 E − 02 − 2.299068 E − 02 − 4.085500 E − 03 − 1.015709 E − 04 2.477770 E + 00 1.132656 E + 00 2.642118 E − 01 1.480553 E − 02 k 1 (80 Hg) 2 (2p') 3 (3p') 4 (1s) 5 (4p') k R 5.821331 E − 06 1.947908 E − 02 2.452258 E − 02 2.682846 E − 02 3.921587 E − 02 k 9.681318 E + 16 − 2.494941 E + 04 − 2.593320 E + 04 − 2.595720 E + 04 − 1.231179 E + 03 k 1.649075 E + 07 5.852996 E + 03 3.326914 E + 03 2.829128 E + 03 2.128635 E + 03 6 (5p') 7 (3d') 8 (2p'') 9 (2s) 10 (4d') 8.550357 E − 02 9.549643 E − 02 1.135324 E − 01 1.303637 E − 01 1.549764 E − 01 − 1.232061 E + 03 − 1.232082 E + 03 − 1.285853 E + 03 − 3.020634 E + 02 − 8.655148 E + 01 9.986890 E + 02 6.726233 E + 02 5.633633 E + 02 4.671986 E + 02 3.841763 E + 02 11 (3d'') 12 (3p'') 13 (3s) 14 (5d') 15 (4f') 3.563975 E − 01 3.908226 E − 01 3.988730 E − 01 5.364145 E − 01 6.316740 E − 01 − 8.757939 E + 01 − 3.380894 E + 01 − 9.807862 E + 00 − 2.284060 E + 00 − 2.289033 E + 00 1.655741 E + 02 1.028348 E + 02 9.542193 E + 01 7.446016 E + 01 5.345713 E + 01 16 (4s) 17 (4p'') 18 (4d'') 19 (4f'') 1.118616 E + 00 1.175167 E + 00 1.325003 E + 00 1.895022 E + 00 − 2.799053 E + 00 − 2.457940 E + 00 − 1.575308 E + 00 − 5.473941 E − 01 2.525985 E + 01 1.518807 E + 01 1.278284 E + 01 7.692339 E + 00 20 (5s) 21 (5p'') 22 (5d'') 23 (6s) 3.457738 E + 00 4.102389 E + 00 7.830668 E + 00 1.660763 E + 01 − 3.737423 E − 02 − 2.582468 E − 02 − 5.077650 E − 03 − 1.042362 E − 04 2.748177 E + 00 1.242705 E + 00 2.904978 E − 01 1.570841 E − 02 From the presented results one can drew following conclusions. In accordance with well-known ionization potential – atomic number relationship, quasi-classical atomic radius Rq reveals a quasi-periodic dependence upon the parameter Z with maxima at hydrogen H and typical metals (including all alkali elements) Li, Na, Al, K, Ga, Rb, Ag, In, Cs, Tl corresponding to the atomic ionization potentials’ minima. Schematic-plots of the obtained )(r and )( r functions in the step-like form are shown in Figures 1 and 2, respectively. Electric-charge-density reveals sharp and positive main maximum in the vicinity of center, which corresponds to the nucleus vibrations’ region, broad negative minimum, which corresponds to the elec- tron-density main maximum located at relatively short distance from the center, and a few extremes at relatively  Intra-Atomic Electric Field Radial Potentials in Step-Like Presentation Copyright © 2010 SciRes. JEMAA 240 r (r) Figure 1. Schematic-plot of the electric-charge-density step- like radial distribution in atoms r (r) Figure 2. Schematic-plot of the electron-potential-energy step-like radial distribution in atoms long distance, which are characteristic for the shell- structure of atoms. As for the electron-potential-energy- function, anywhere it is negative and monotonously rises. Thus, it posses only minimum at the center (an additional minimum may be revealed in effective-potential-func- tion). Of course, behind the atomic radius both )( r and )(r functions in the step-like presentation are identically zero. 5. Accuracy of Binding-Energy and Electronic-Structure Calculations Based on Radial Step-Like Atomic Potentials It is not out of place to consider accuracy of the binding energy and electronic-structure calculations carried out within the semiclassical approximation, i.e. on the basis of above introduced radial step-like atomic potentials. It is most convenient to estimate the method accuracy for Thomas-Fermi (TF) statistical semiclassical atomic mod- el starting from the only analytical solution F r r 4 2 8 81 )( (17) of the TF equation. Here F is the Fermi-energy for intra-atomic electron gas, i.e. higher occupied electron level. Corresponding electron charge density is expressed by the function 6 8 243 )( r r (18) As electron charge equals to 1 its potential energy in atom 4 /1)()( rrrU when 0r. Then, inner turning point radius 0 r for any electron bound in TF “atom”. As for the outer turning point radius r of the electron with energy F E, it can be found as only real positive root of the equation )( rUE eff , where 2 2/)1()()( rllrUrUeff is the effective po- tential energy of the electron with orbital quantum num- ber l, i.e. 24 2 2 )1( 8 81 r ll r FE (19) Because differences between semiclassical electron energies are negligible if compared with their depth, one can suppose that approximately all of them coincide with Fermi-energy, F E . In that case, the product )1( ll also should be substituted for its standard semiclassical expression 2 )2/1( l. As a result, we get 12 9 l r (20) Consequently, averaged partial charge density of a l-electron-subshell equals to 4 3 3972 )12( 3/4 1 )( l r r l (21) and 0, respectively, inside and outside the r -sphere. As is known, when summation over the principal and orbital quantum numbers n and l characterizing elec- tron motion in central-symmetric electric field is substi- tuted for semiclassical integration the combinations 2/1 n and 2/1 l serve as integration vari- ables. As 1 nl the limit of integration over should be taken equal to 2/11n. As for the de- generacy factor, it equals to 4)12(2 l. Note that partial electron charge density takes on a nonzero value 43 243/2 if 2/9 rr . Consequently,  Intra-Atomic Electric Field Radial Potentials in Step-Like Presentation Copyright © 2010 SciRes. JEMAA 241 r2/9 . But, maxmax and, then, the ratio r2/9 should serve as the limit of integration over too. Now we can found total electron charge density by means of semiclassical integration: r calSemiclassi r ddr 2 9 00 6 2 4 3 80 729 243 2 4)( (22) It yields semiclassical atomic potential in following form F r r calSemiclassi 4 3/122 80 )90(81 )( (23) Variable parts of the obtained semiclassical expres- sions reveal same radial dependences (6 /1~ r and 6 /1~ r, respectively, for electron charge density and potential) as corresponding exact analytical solutions, but differ from them by the multipliers 10/3 and 3/2 )10/3( . Therefore, semiclassically determined struc- tural and energy parameters are expected to be dis- tinguished from their exact values by the multipliers of order of magnitude 1~02.1)3/10(~ 3/1 and 1~96.0)10/3(~ 3/2 , respectively. Thus the estimated errors of the semiclassical approach make up a few per- cent. This conclusion is actually proved by the above cited calculations performed for some one-, two-, and three-dimensional real polyatomic structures. 6. Conclusions Obtained results, numerically reflected in presented ta- bles, vividly show that an effective method of parame- terization of the intra-atomic electric field can by based on semiclassical approach. Such possibility follows from the Maslov criterion, according to which the exact and semiclassical atomic electron-energy spectra should be similar to each other irrespective of the atomic poten- tials’ smoothness properties. Within the semiclassical approximation, intra-atomic eclectic charge density and electric field potential distributions can be presented by the step-like radial functions, where nucleus and elec- tron-states classical turning point radii play role of the steps’ limits, while charge density and potential inside a step are substituted for their volume averaged values. Superposition of the semiclassical atomic step-like radial potentials can serve as an initial approximation for the substance inner potential. Binding energy and electronic structure calculations based on such potential allow de- termining of the substance structural and energy parame- ters with relative accuracy making up a few percent, what is quite sufficient for materials science purposes. 7. Acknowledgment Authors are very grateful to the Georgia National Sci- ence Foundation (GNSF) for the financial assistance (Grant #GNSF/ST08/4-411). REFERENCES [1] L. S. Chkhartishvili, “Selection of Equilibrium Configu- rations for Crystalline and Molecular Structures Based on Quasi-Classical Inter-Atomic Potential,” Transactions of the Global Transaction Unit, Vol. 3(427), 1999, pp. 13-19. [2] L. Chkhartishvili, D. Lezhava, O. Tsagareishvili and D. Gulua, “Ground State Parameters of B2, BC, BN and BO Diatomic Molecules,” Transactions of the AMIAG, Vol. 1, 2004, pp. 295-300. [3] L. Chkhartishvili, D. Lezhava and O. Tsagareishvili, “Quasi-Classical Determination of Electronic Energies and Vibration Frequencies in Boron Compounds,” Jour- nal of Solid State Chemistry, Vol. 154, 2000, pp. 148-152. [4] L. Chkhartishvili and D. Lezhava, “Zero-Point Vibration Effect on Crystal Binding Energy: Quasi-Classical Cal- culation for Laminated Boron Nitride,” Transactions of the Global Transaction Unit, Vol. 6(439), 2001, pp. 87-90. [5] L. Chkhartishvili, “Ground State Parameters of Wurtzite Boron Nitride: Quasi-Classical Estimations,” Proceedings of the 1st International Boron Symposium, 2002, pp. 139-143. [6] L. Chkhartishvili, “Quasi-Classical Approach: Electronic Structure of Cubic Boron Nitride Crystals,” Journal of Solid State Chemistry, Vol. 177, 2004, pp. 395-399. [7] L. S. Chkhartishvili, “Quasi-Classical Estimates of the Lattice Constant and Band Gap of a Crystal: Two-Dimensional Boron Nitride,” Physics of the Solid State, Vol. 46, 2004, pp. 2126-2133. [8] L. Chkhartishvili, Quasi-Classical Analysis of Bo- ron-Nitride Binding,” Proceedings of the 2nd Interna- tional Boron Symposium, 2004, pp. 165-171. [9] L. Chkhartishvili, “Quasi-Classical Analysis of Electron Bandwidths in Wurtzite-Like Boron Nitride,” Transac- tions of the IChTU, Vol. 1, 2005, pp. 296-314. [10] L. S. Chkhartishvili, “Analytical Optimization of the Lat- tice Parameter Using the Binding Energy Calculated in the Quasi-Classical Approximation,” Physics of the Solid State, Vol. 48, 2006, pp. 846-853. [11] L. Chkhartishvili, “Density of Electron States in Wurtz- ite-Like Boron Nitride: A Quasi-Classical Calculation,” Materials Science: An Indian Journal, Vol. 2, 2006, pp. 18-23. [12] L. Chkhartishvili, “Zero-Point Vibration Energy within Quasi-Classical Approximation: Boron Nitrides,” Pro- ceedings of the Javakhishvili TSU (Physics), Vol. 40, 2006, pp. 130-138. [13] L. S. Chkhartishvili, “Equilibrium Geometry of the Boron Nitride Ultra-Small-Radius Nanotubes,” Material Science  Intra-Atomic Electric Field Radial Potentials in Step-Like Presentation Copyright © 2010 SciRes. JEMAA 242 of Nanostructures, Vol. 1, 2009, pp. 33-44. [14] L. Chkhartishvili, “Boron Nitride Nanosystems of Regu- lar Geometry,” Journal of Physics: Conference Series, Vol. 176, 2009, p. 17. [15] L. Chkhartishvili, “Equilibrium Geometries of the Boron Nitride Layered Systems,” Proceedings of the 4th Inter- national Boron Symposium, 2009, pp. 161-170. [16] L. Chkhartishvili, “Boron Nitride Nanosystems,” In L. Chkhartishvili (Ed.), Boron Based Solids, Research Signpost, Trivandrum, 2010, in Press. [17] L. Chkhartishvili, On Quasi-Classical Estimations of Boron Nanotubes Ground-State Parameters,” Journal of Physics: Conference Series, Vol. 176, 2009, p. 9. [18] L. Chkhartishvili, “Molar Binding Energy of the Boron Nanosystems,” Proceedings of the 4th International Bo- ron Symposium, 2009, pp. 153-160. [19] L. Chkhartishvili, Nanotubular Boron: Ground-State Es- timates,” Georgian International Journal of Science and Technology, 2010, in Press. [20] L. S. Chkhartishvili, D. L. Gabunia and O. A. Tsaga- reishvili, “Estimation of the Isotopic Effect on the Melt- ing Parameters of Boron,” Inorganic Materials, Vol. 43, 2007, pp. 594-596. [21] L. S. Chkhartishvili, D. L. Gabunia and O. A. Tsaga- reishvili, “Effect of the Isotopic Composition on the Lat- tice Parameter of Boron,” Powder Metallurgy & Metal Ceramics, Vol. 47, 2008, 616-621. [22] D. Gabunia, O. Tsagareishvili, L. Chkhartishvili and L. Gabunia, “Isotopic Composition Dependences of Lattice Constant and Thermal Expansion of -Rhombohedral Boron,” Journal of Physics: Conference Series, Vol. 176, 2009. [23] L. Chkhartishvili, “Isotopic Effects of Boron,” Trends in Inorganic Chemistry, 2010, in Press. [24] N. Bohr, “On the Constitution of Atoms and Molecules,” Philosophical Magazine, Vol. 26, 1913, pp. 1-25. [25] K. G. Kay, “Exact Wave Functions for Coulomb Problem from Classical Orbits,” Physical Review Letters, Vol. 83, 1999, pp. 5190-5193. [26] S. I. Nikitin and V. N. Ostrovskij “Problem of the Vibra- tion-Rotation States in Two-Electron Atom,” In V. N. Ostrovskij (Ed.), Perturbation Theory in Atomic Calcula- tions, Nauka, Moscow, 1985, pp. 146-165. [27] B. Baghchi and P. Holody, “An Interesting Application of Bohr Theory,” American Journal of Physics, Vol. 56, 1988, pp. 746-747. [28] T. Yamamoto and K. Kaneko, “Exploring a Classical Model of the Helium Atom,” Progress in Theoretical Physics, Vol. 100, 1998, pp. 1089-1100. [29] G. Tanner, K. Richter and J.-M. Rost, “The Theory of Two-Electron Atoms: Between Ground State and Com- plete Fragmentation,” Reviews of Modern Physics, Vol. 72, 2000, pp. 497-544. [30] K. M. Magomedov and P. M. Omarova, “Quasi-Classical Calculation of Electronic Systems,” Dagestan Academy of Sciences Press, Makhachkala, 1989. [31] M. Casas, A. Plastino and A. Puente, “Alternative Ap- proach to the Semiclassical Description of N-Fermions Systems,” Physical Review A, Vol. 49, 1994, pp. 2312- 2317. [32] D. Vrinceanu and M. R. Flannery, “Classical Atomic form Factor,” Physical Review Letters, Vol. 82, 1999, pp. 3412-3415. [33] A. Jaroń, J. Z. Kamiński and F. Ehlotzky, “Bohr’s Corre- spondence Principle and X-Ray Generation by Laser- Stimulated Electron–Ion Recombination,” Physical Re- view A, Vol. 63, 2001, p. 4. [34] M. I. Petrashen, “On Semiclassical Method of Solving of the Wave Equation,” Proceedings of the Leningrad State University (Series of Physical Sciences), Vol. 7, 1949, pp. 59-78. [35] R. Gaspar, I. I. Glembotskij, I. Y. Petkyavichyus and A. P. Yutsis, “Quantum Numbers and Universal Potential,” In A. P. Yutsis (Ed.), Theory of Atoms and Atomic Spectra, I., Latvian State University, Riga, 1974, pp. 54-58. [36] B. Rohwedder and B.-G. Englert, “Semiclassical Quanti- zation in Momentum Space,” Physical Review A, Vol. 49, 1994, pp. 2340-2346. [37] M. G. Veselov and L. N. Labzovskij, “Atomic Theory, Electron Shell Structure,” Nauka, Moscow, 1986. [38] R. Sakenshaft and L. Spruch, “Semiclassical Evaluation of Sums of Squares of Hydrogenic Bound-State Wave Functions,” Journal of Physics B, Vol. 18, 1985, pp. 1919-1925. [39] J. Tao and G. Li, “Approximate Bound to the Average Electron Momentum Density for Atomic Systems,” Phy- sica Scripta, Vol. 58, 1998, pp. 193-195. [40] K. M. Magomedov, “On Quasi-Classical Self-Consistent Atomic Field,” Proceedings of the USSR Academy of Sciences, Vol. 285, 1985, pp. 1110-1115. [41] A.-N. Popa, “Accurate Bohr-Type Semiclassical Model for Atomic and Molecular Systems,” Reports of the Insti- tute of Atomic Physics, Vol. E12, 1991, pp. 1-90. [42] J. N. L. Connor, “Semiclassical Theory of Elastic Scat- tering,” In M. S. Child (Ed.), Semiclassical Methods in Molecular Scattering and Spectroscopy, Reidel Publish- ing, Dordrecht–Boston–London, 1979, pp. 45-107. [43] R. J. le Roy, “Applications of Bohr Quantization in Dia- tomic Molecular Spectroscopy,” In M. S. Child (Ed.), Semiclassical Methods in Molecular Scattering and Spectroscopy, Reidel Publishing, Dordrecht–Boston– London, 1979, pp. 109-126. [44] H. Kuratsuji, “Semiclassical Asymptotics for Fermions Systems Based on the Coherent-State Path Integral,” Physics Letters A, Vol. 108, 1985, pp. 139-143. [45] J.-T. Kim, “Semiclassical Wentzel-Kramers-Brillouin (WKB) Method for Generating the Potential Energy Curve and the Therm Energies of Diatomic K2 Mole- cule,” The Journal of the Korean Physical Society, Vol. 35, 1999, pp. 168-170.  Intra-Atomic Electric Field Radial Potentials in Step-Like Presentation Copyright © 2010 SciRes. JEMAA 243 [46] A. G. Magner, S. N. Fedotkin, F. A. Ivanyuk, P. Meier and M. Brack, “Shells and Periodic Orbits in Fermions Systems,” Czechoslovak Journal of Physics, Vol. 48, 1998, pp. 845-852. [47] J. Marañon, “Path Integral’s Semiclassical Quantifica- tion,” Quantum Chemistry, Vol. 52, 1994, pp. 609-616. [48] K. Sohlberg, R. E. Tuzun, B. G. Sumpter and D. W. Noid, Full Three-Body Primitive Semiclassical Treatment of H2 + ,” Physical Review A, Vol. 57, 1998, pp. 906-913. [49] F. Remacle and R. D. Levine, “On the Classic Limit for Electronic Structure and Dynamics in the Orbital Ap- proximation,” Journal of Chemical Physics, Vol. 113, 2000, pp. 4515-4523. [50] W. A. Fedak and J. J. Prentis, “Quantum Jumps and Clas- sical Harmonics,” American Journal of Physics, Vol. 70, 2002, pp. 332-344. [51] A. G. Chirkov and I. V. Kazanets, “Classical Physics and Electron Spin,” Technical Physics, Vol. 45, 2000, pp. 1110-1114. [52] M. Madhusoodanan and K. G. Kay, “Globally Uniform Semiclassical Wave-Function for Multidimensional Sys- tems,” Journal of Chemical Physics, Vol. 109, 1998, pp. 2644-2655. [53] G. V. Shpatakovskaya, “Quasi-Classical Description of Electronic Super-Shells in Simple Metal Clusters,” JETP Letters, Vol. 70, 1999, pp. 334-339. [54] G. V. Shpatakovskaya, “Quasi-Classical Analysis of the Spectra of Two Groups of Central Potentials,” JETP Let- ters, Vol. 73, 2001, pp. 268-270. [55] M. Brack, “The Physics of Simple Metal Clusters: Self- Consistent Jellium Model and Semiclassical Approach- es,” Reviews of Modern Physics, Vol. 65-I, 1993, pp. 677-732. [56] V. P. Maslov, “Perturbation Theory and Asymptotic Me- thods,” Moscow University Press, Moscow, 1965. [57] L. Chkhartishvili, “Quasi-Classical Theory of Substance Ground State,” Technical University Press, Tbilisi, 2004. [58] C. Froese–Fischer, “The Hartree–Fock Method for Atoms, A numerical approach,” Wiley, New York, 1977. |

