 Vol.1, No.3, 65-73 (2011) doi:10.4236/oji.2011.13008 C opyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/oji/ Open Journal of Immunology Modulation of human B lymphocyte differentiation by therapeutic immunoglobulins: from protein to mRNA levels Nathalie Dussault1, Nellie Dumont1, Sonia Néron1,2* 1Recherche et Développement, Héma-Québec, Québec, Canada; *Corresponding Author: sonia.neron@hema-quebec.qc.ca 2Département de Biochimie et de Microbiologie, Université Laval, Québec, Qc, Canada. Received 17 August 2011; revised 18 October 2011; accepted 11 November 2011. ABSTRACT Several groups are investigating the mecha- nisms of action of therapeutic immunoglobulins (IVIg) in orde r to improve their use. In vitro mod- els such as CD40-CD154 interaction are neces- sary to study the physiological response of hu- man B lymphocytes to IVIg. Human B lympho- cytes treated with IVIg triggers a rapid phos- phorylation (<1 h) of extracellular-regulatedkin- ases 1 and 2 (ERK1/2), which subsequently re- sults in increased differentiation and decreased proliferation. However, the modulation of human lymphocyte physiology by IVIg is a gradual and cumulative process and requires long-term ex- perimentation. Differentiation of human B lym- phocytes into Ig-secreting cells can be evalu- ated both at the transcription and translation le vels . The secretion of immunoglobulins can be assessed using ELISA or ELISPOTS whereas expression of immunoglobulin genes can be measured using semi-qua ntitative or quantitative PCR methods. We hereby report a comparison of these methods to explain how contradictory observations towards IVIg effects could result from their use. Our results indicate that ELISA and ELISPOTS will provide consistent observa- tions by opposition to real-time PCR quantifica- tion. Besides, the reliability of each of these methods remained dependent on the stimu lation period as well as the preparation of cellular ex- tract s or cell samples following IVIg-treatment. Keywords: Human B Lymphocytes; CD40-CD154; IVIg; Differentiation; Immunomodulation 1. INTRODUCTION Therapeutic immunoglobulins are constituted of IgG (≥98%) and are used in the treatment of several infla- mmatory and autoimmune disorders in which IVIg have been shown to reestablish homeostasis [1]. Following IVIg injection, the concentration of IgG in the patient’s serum increases by 5 to 10 mg/mL [2-4]. In many cases, treatment with IVIg results in the reduction or disap- pearance of pathologic autoimmune antibodies in the patient’s serum for prolonged periods of time. Several studies have proposed that anti-idiotypic antibodies, present in IVIg, could interact with the B-cell receptor (BCR). By doing so, IVIg modulate autoreactive B lymphocytes [1]. According to this hypothesis, we show- ed that IVIg interaction with B lymphocytes results in phosphorylation of ERK1/2, which is downstream to BCR cross-linking [5]. We also showed that IVIg can directly modulate B lymphocytes by inducing secretion of IgG reacting with self- and non-self antigens in cells obtained from healthy individuals [6] as well as from patient with systemic lupus erythematosus [7]. In vitro models are important to study the mechanisms of action of IVIg and several groups are using the CD40-CD154 interaction to delineate their effects on human B lym- phocytes. However, all in vitro models using this inter- action are not equal [8]. Variations in the level of CD40- CD154 interactions can result in phenotypic and func- tional differences in human B lymphocyte responses [9,10] as well as in dendritic cells [11] and macrophages [12]. Cell fate determination toward proliferation or dif- ferentiation is directly proportional to the average num- ber of CD154 molecules per B lymphocyte [9,13]. Es- sentially, low levels of CD40 occupancy (500 to 1000 CD154 molecules per B cell) result in high Ig secretion and low proliferation. Conversely, low Ig secretion and high proliferation is observed when using a high level of interaction (5000 to 10,000 CD154 molecules per B cell) [9,13]. Moreover, sub-populations of human B lympho- cytes will respond distinctively to variable intensity of CD40 stimulation [9,13-15]. Therefore, models based on in vitro CD40-activation of human B lymphocytes may  N. Dussault et al. / Open Journal of Immunology 1 (2011) 6 5-73 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/oji/ 66 lead to very contrasting physiological effects, which are as diverse as the nature of the ligands used to bind CD40 (reviewed in [8]). As a result and on the basis of our pre- vious studies, the strength of IVIg modulation on B lymphocytes will depend on the level of CD40-CD154 interaction [7]. For the present work, we have used low and high levels of CD40-CD154 interaction and compared meth- ods to assess the direct effect of IVIg on those B lym- phocytes. We specifically targeted the differentiation status by using ELISA and ELISPOT methods to deter- mine the secretion rates and the frequency of secreting cells and we used polymerase chain reaction (PCR), semi-quantitative PCR and real-time PCR (Q-PCR) to evaluate immunoglobulins’ expression levels. 2. MATERIALS AND METHODS 2.1. Intravenous Immunoglobulins Commercial preparation of GAMUNEX® IVIg con- taining 9% to 11% of protein and 160 to 240 mM gly- cine, was obtained from Talecris (North Carolina, USA) and albumin from bovine serum (BSA, Cohn fraction V, Sigma-Aldrich Ltd, Oakville, ON, Canada) was prepared at 10% in 10 mM phosphate buffered saline (PBS). IVIg and BSA preparations were dialysed against 10 mM po- tassium/sodium phosphate containing 136 mM NaCl (PBS) (Gibco, Grand Island, NY, USA) and 40 mM gly cine, pH4.5 (Sigma-Aldrich). After dialyse, IgG content in IVIg was mainly monomeric (96% - 98%) and the final concentration was 100 mg/ml [5]. BSA, which does not interfere with B-cell proliferation and IgG secretion [6], was used as a control for protein content. 2.2. Human Peripheral B Lymphocytes This study has been reviewed and approved by Héma- Québec’s Research Ethics Committee. Samples were obtained from healthy individuals after informed consent. Peripheral blood mononuclear cells (PBMNCs) were prepared from leucocytes recovered from leucoreduction system by density centrifugation over Ficoll-Paque (GE Healthcare Bio-Sciences Inc., Baie d’Urfé, QC, Canada) and stored frozen until B-cell purification as described previously [16]. B lymphocytes were purified by nega- tive selection using the Easy Sep CD19 cocktail, ac- cording to the manufacturer’s instructions (Stem Cell Technologies, Vancouver, BC, Canada) and were pure at 90% to 95% CD19+ cells, as determined by flow cy- tometry. 2.3. CD40 Stimulation of B Lymphocytes L4.5 cell line is a genetically modified L929 cell line (CCL-1, American Type Culture Collection, Manassas, VA) [17] expressing 21,000 ± 4000 CD154 molecules per cell [9]. Purified B lymphocytes were seeded in Pri- maria plates (BD Labware) at ~3.75 × 105 cells/mL in the presence of γ-irradiated L4.5 cells expressing CD 154 [18]. L4.5 cells were seeded as to obtain ratios cor- responding to high and low levels of CD40-stimulation, 3 - 5 or 20 - 25 B lymphocytes per L4.5 cell, respectively. B lymphocytes were cultured in a medium based on IMDM supplemented with 10% ultra low IgG FBS con- taining 10 g/mL insulin, 5.5 g/mL transferrin, 6.7 ng/mL sodium selenite, 100 μg/ml streptomycin and 100 U/ml penicillin G (all from Invitrogen, Burlington, ON, Can- ada), 50 U/mL IL-2, 25 U/mL IL-10 (PeproTech, Rocky Hill, NJ, USA) and 100 U/mL IL-4 (R&D Systems, Minneapolis, MN, USA). This complete IMDM medium was used in all assays. B lymphocytes were stimulated with high or low level of CD40-CD154 interaction for 9 days in the presence or absence of 10 mg/ml of IVIg. When indicated, cells were cultured in the presence of 10 mg/mL of BSA or 4 mM glycine pH4.5 as controls for IVIg-addition. Cultures were fed by replacing half of the culture medium every 2 - 3 days, while L4.5 cells were renewed every 4 - 5 days. Cell counts and viability were evaluated in triplicates by trypan blue dye exclu- sion using a hemacytometer. Cultured B lymphocytes were always >96% CD19+ cells. Generation time (Tgen) was calculated according to the formula: 1ln2 t2t1 21 ln 2[N]ln[N]tt and Tgen = 1/. 2.4. ELISA For the determination of IgG and IgM secretion rate, cells were harvested and washed 5 times to remove re- sidual IVIg with PBS containing 2 g/L of glucose (PBS- sglc). To validate that the secretion by the treated cells was de novo protein synthesis, the washed cells were incubated in complete IMDM medium for three hours in the presence or absence of 15 μg/ml cycloheximide (CHX). At this step these 3 h-supernatants were kept and the cells were washed in PBS-glc and seeded at 1 - 2 × 106 cells/mL in IMDM medium alone for 18 to 22 hours. IgG and IgM concentrations were determined in a standard ELISA using plastic-adsorbed goat antibod- ies specific to human γ- and μ-chains and the bound IgG or IgM were both revealed with peroxidase conju- gated goat polyvalent anti-human Ig antibodies. All antibodies were obtained from Jackson Laboratories (Mississauga, Canada). 2.5. ELISPOTS B lymphocytes, untreated or treated with IVIg, were washed 5 times in PBS-glc, transferred in IMDM con- taining 10% FBS and incubated for 3 hours with or 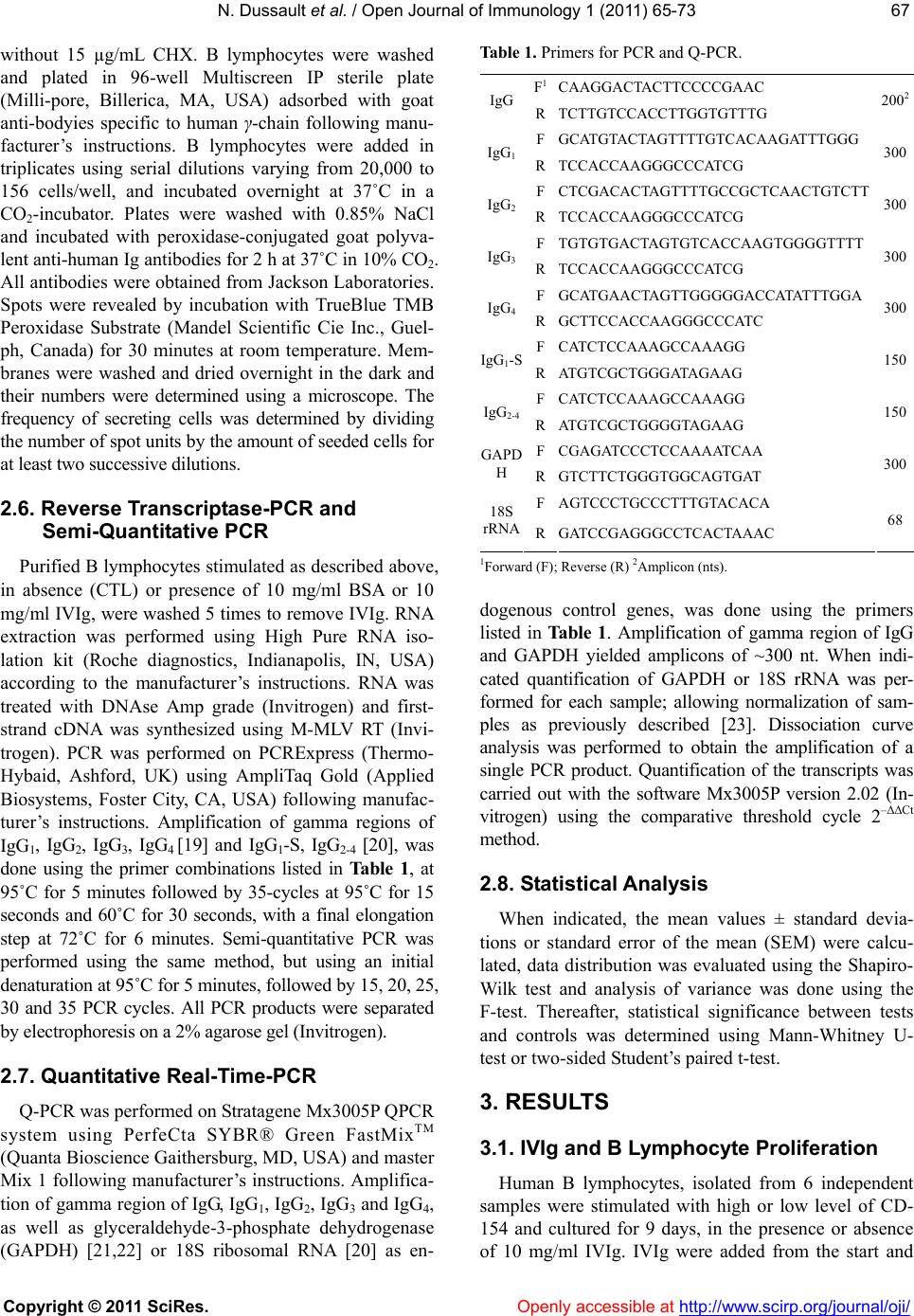 N. Dussault et al. / Open Journal of Immunology 1 (2011) 6 5-73 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/oji/ 6767 without 15 µg/mL CHX. B lymphocytes were washed and plated in 96-well Multiscreen IP sterile plate (Milli-pore, Billerica, MA, USA) adsorbed with goat anti-bodyies specific to human γ-chain following manu- facturer’s instructions. B lymphocytes were added in triplicates using serial dilutions varying from 20,000 to 156 cells/well, and incubated overnight at 37˚C in a CO2-incubator. Plates were washed with 0.85% NaCl and incubated with peroxidase-conjugated goat polyva- lent anti-human Ig antibodies for 2 h at 37˚C in 10% CO2. All antibodies were obtained from Jackson Laboratories. Spots were revealed by incubation with TrueBlue TMB Peroxidase Substrate (Mandel Scientific Cie Inc., Guel- ph, Canada) for 30 minutes at room temperature. Mem- branes were washed and dried overnight in the dark and their numbers were determined using a microscope. The frequency of secreting cells was determined by dividing the number of spot units by the amount of seeded cells for at least two successive dilutions. 2.6. Reverse Transcriptase-PCR and Semi-Quantitative PCR Purified B lymphocytes stimulated as described above, in absence (CTL) or presence of 10 mg/ml BSA or 10 mg/ml IVIg, were washed 5 times to remove IVIg. RNA extraction was performed using High Pure RNA iso- lation kit (Roche diagnostics, Indianapolis, IN, USA) according to the manufacturer’s instructions. RNA was treated with DNAse Amp grade (Invitrogen) and first- strand cDNA was synthesized using M-MLV RT (Invi- trogen). PCR was performed on PCRExpress (Thermo- Hybaid, Ashford, UK) using AmpliTaq Gold (Applied Biosystems, Foster City, CA, USA) following manufac- turer’s instructions. Amplification of gamma regions of IgG1, IgG2, IgG3, IgG4 [19] and IgG1-S, IgG2-4 [20], was done using the primer combinations listed in Tab l e 1 , at 95˚C for 5 minutes followed by 35-cycles at 95˚C for 15 seconds and 60˚C for 30 seconds, with a final elongation step at 72˚C for 6 minutes. Semi-quantitative PCR was performed using the same method, but using an initial denaturation at 95˚C for 5 minutes, followed by 15, 20, 25, 30 and 35 PCR cycles. All PCR products were separated by electrophoresis on a 2% agarose gel (Invitrogen). 2.7. Quantitative Real-Time-PCR Q-PCR was performed on Stratagene Mx3005P QPCR system using PerfeCta SYBR® Green FastMixTM (Quanta Bioscience Gaithersburg, MD, USA) and master Mix 1 following manufacturer’s instructions. Amplifica- tion of gamma region of IgG, IgG1, IgG2, IgG3 and IgG4, as well as glyceraldehyde-3-phosphate dehydrogenase (GAPDH) [21,22] or 18S ribosomal RNA [20] as en- Table 1. Primers for PCR and Q-PCR. F1CAAGGACTACTTCCCCGAAC IgG RTCTTGTCCACCTTGGTGTTTG 2002 FGCATGTACTAGTTTTGTCACAAGATTTGGG IgG1RTCCACCAAGGGCCCATCG 300 F CTCGACACTAGTTTTGCCGCTCAACTGTCTT IgG2RTCCACCAAGGGCCCATCG 300 F TGTGTGACTAGTGTCACCAAGTGGGGTTTT IgG3RTCCACCAAGGGCCCATCG 300 F GCATGAACTAGTTGGGGGACCATATTTGGA IgG4RGCTTCCACCAAGGGCCCATC 300 FCATCTCCAAAGCCAAAGG IgG1-S RATGTCGCTGGGATAGAAG 150 FCATCTCCAAAGCCAAAGG IgG2-4 RATGTCGCTGGGGTAGAAG 150 FCGAGATCCCTCCAAAATCAA GAPD H RGTCTTCTGGGTGGCAGTGAT 300 FAGTCCCTGCCCTTTGTACACA 18S rRNA RGATCCGAGGGCCTCACTAAAC 68 1Forward (F); Reverse (R) 2Amplicon (nts). dogenous control genes, was done using the primers listed in Ta ble 1. Amplification of gamma region of IgG and GAPDH yielded amplicons of ~300 nt. When indi- cated quantification of GAPDH or 18S rRNA was per- formed for each sample; allowing normalization of sam- ples as previously described [23]. Dissociation curve analysis was performed to obtain the amplification of a single PCR product. Quantification of the transcripts was carried out with the software Mx3005P version 2.02 (In- vitrogen) using the comparative threshold cycle 2–ΔΔCt method. 2.8. Statistical Analysis When indicated, the mean values ± standard devia- tions or standard error of the mean (SEM) were calcu- lated, data distribution was evaluated using the Shapiro- Wilk test and analysis of variance was done using the F-test. Thereafter, statistical significance between tests and controls was determined using Mann-Whitney U- test or two-sided Student’s paired t-test. 3. RESULTS 3.1. IVIg and B Lymphocyte Proliferation Human B lymphocytes, isolated from 6 independent samples were stimulated with high or low level of CD- 154 and cultured for 9 days, in the presence or absence of 10 mg/ml IVIg. IVIg were added from the start and 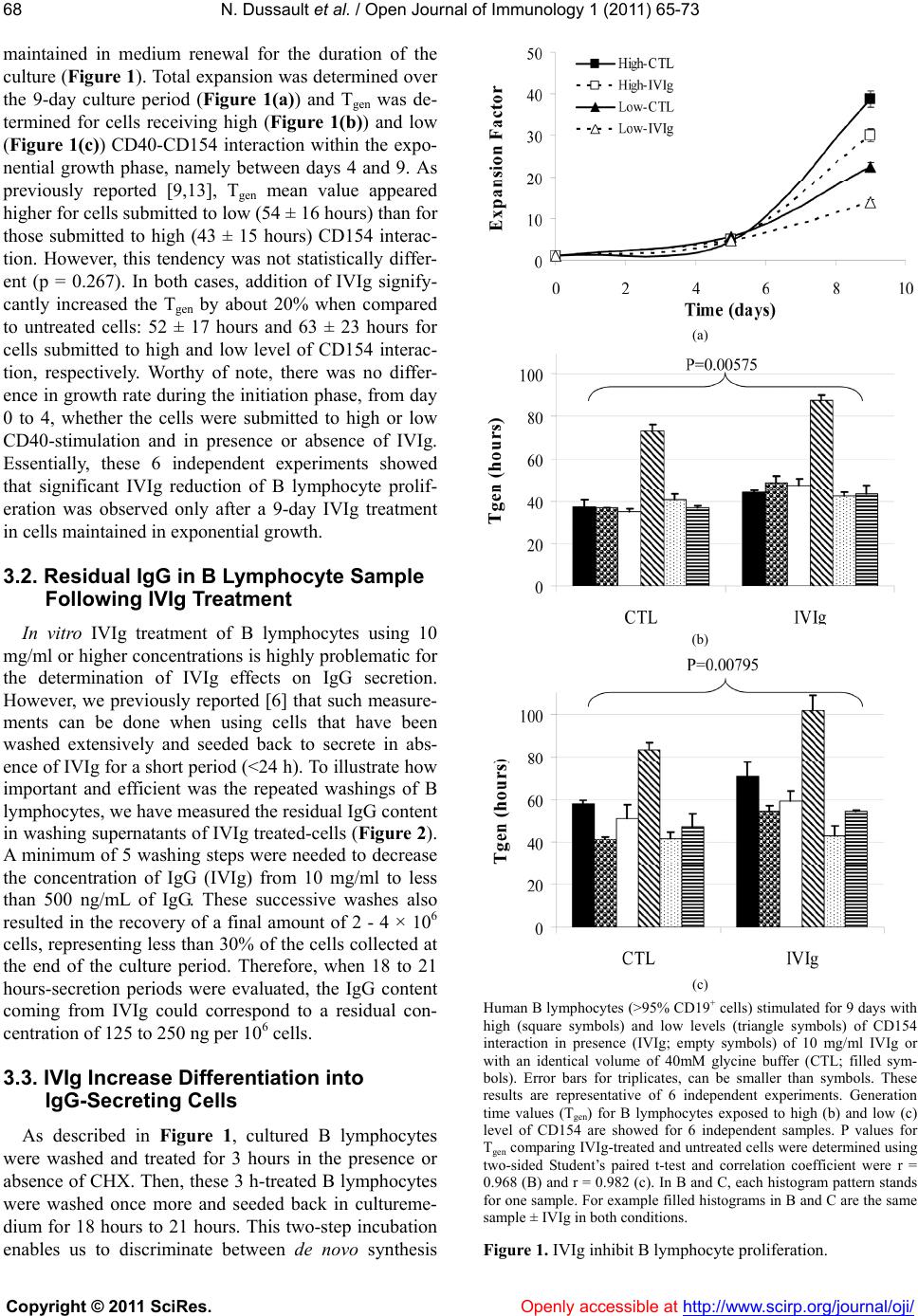 N. Dussault et al. / Open Journal of Immunology 1 (2011) 6 5-73 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/oji/ 68 maintained in medium renewal for the duration of the culture (Figure 1). Total expansion was determined over the 9-day culture period (Figure 1(a)) and Tgen was de- termined for cells receiving high (Figure 1(b)) and low (Figure 1(c)) CD40-CD154 interaction within the expo- nential growth phase, namely between days 4 and 9. As previously reported [9,13], Tgen mean value appeared higher for cells submitted to low (54 ± 16 hours) than for those submitted to high (43 ± 15 hours) CD154 interac- tion. However, this tendency was not statistically differ- ent (p = 0.267). In both cases, addition of IVIg signify- cantly increased the Tgen by about 20% when compared to untreated cells: 52 ± 17 hours and 63 ± 23 hours for cells submitted to high and low level of CD154 interac- tion, respectively. Worthy of note, there was no differ- ence in growth rate during the initiation phase, from day 0 to 4, whether the cells were submitted to high or low CD40-stimulation and in presence or absence of IVIg. Essentially, these 6 independent experiments showed that significant IVIg reduction of B lymphocyte prolif- eration was observed only after a 9-day IVIg treatment in cells maintained in exponential growth. 3.2. Residual IgG in B Lymphocyte Sample Following IVIg Treatment In vitro IVIg treatment of B lymphocytes using 10 mg/ml or higher concentrations is highly problematic for the determination of IVIg effects on IgG secretion. However, we previously reported [6] that such measure- ments can be done when using cells that have been washed extensively and seeded back to secrete in abs- ence of IVIg for a short period (<24 h). To illustrate how important and efficient was the repeated washings of B lymphocytes, we have measured the residual IgG content in washing supernatants of IVIg treated-cells (Figure 2). A minimum of 5 washing steps were needed to decrease the concentration of IgG (IVIg) from 10 mg/ml to less than 500 ng/mL of IgG. These successive washes also resulted in the recovery of a final amount of 2 - 4 × 106 cells, representing less than 30% of the cells collected at the end of the culture period. Therefore, when 18 to 21 hours-secretion periods were evaluated, the IgG content coming from IVIg could correspond to a residual con- centration of 125 to 250 ng per 106 cells. 3.3. IVIg Increase Differentiation into IgG-Secreting Cells As described in Figure 1, cultured B lymphocytes were washed and treated for 3 hours in the presence or absence of CHX. Then, these 3 h-treated B lymphocytes were washed once more and seeded back in cultureme- dium for 18 hours to 21 hours. This two-step incubation enables us to discriminate between de novo synthesis (a) (b) (c) Human B lymphocytes (>95% CD19+ cells) stimulated for 9 days with high (square symbols) and low levels (triangle symbols) of CD154 interaction in presence (IVIg; empty symbols) of 10 mg/ml IVIg or with an identical volume of 40mM glycine buffer (CTL; filled sym- bols). Error bars for triplicates, can be smaller than symbols. These results are representative of 6 independent experiments. Generation time values (Tgen) for B lymphocytes exposed to high (b) and low (c) level of CD154 are showed for 6 independent samples. P values for Tgen comparing IVIg-treated and untreated cells were determined using two-sided Student’s paired t-test and correlation coefficient were r = 0.968 (B) and r = 0.982 (c). In B and C, each histogram pattern stands for one sample. For example filled histograms in B and C are the same sample ± IVIg in both conditions. Figure 1. IVIg inhibit B lymphocyte proliferation. 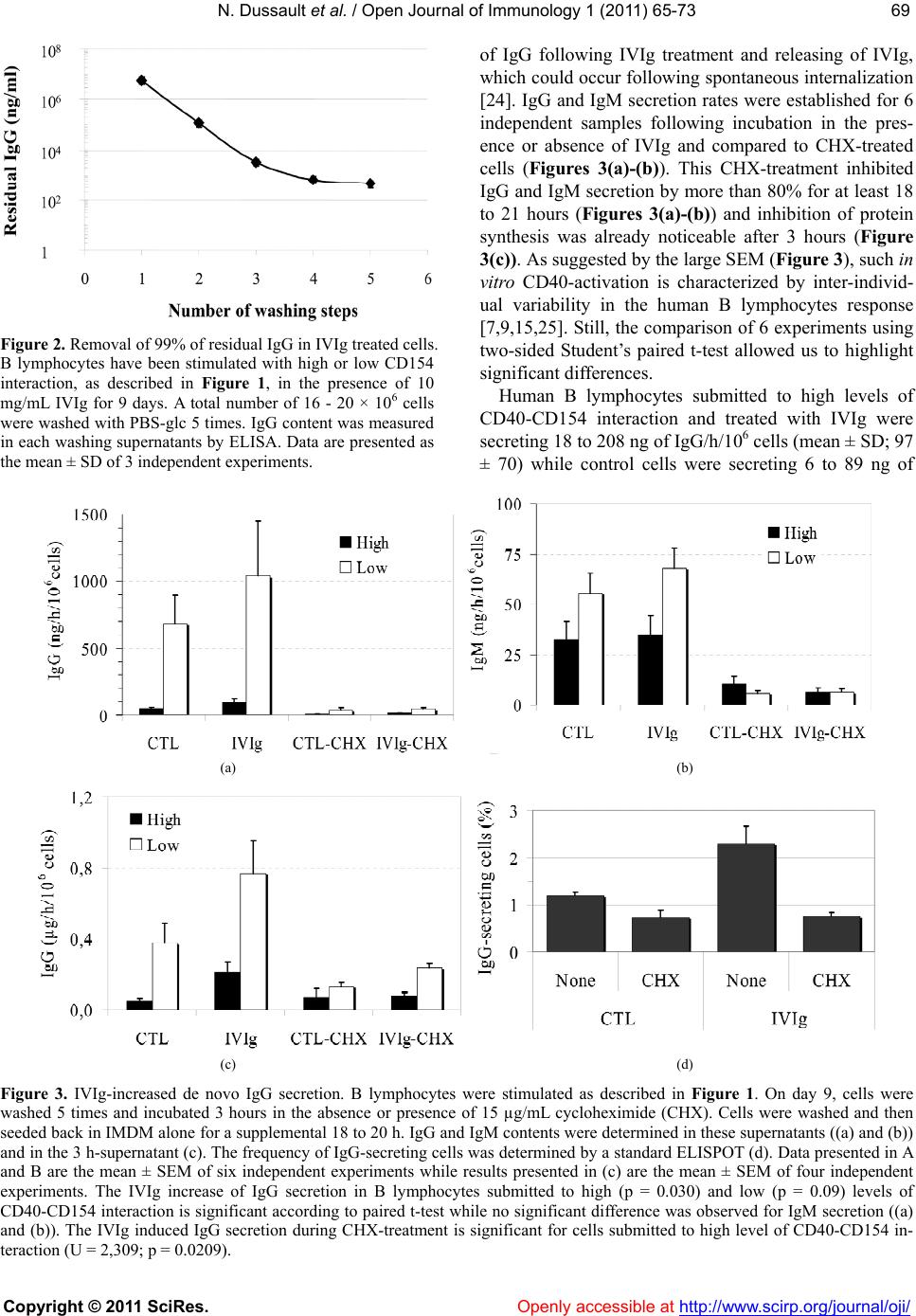 N. Dussault et al. / Open Journal of Immunology 1 (2011) 6 5-73 Copyright © 2011 SciRes. http://www.scirp.org/journal/oji/ 6969 of IgG following IVIg treatment and releasing of IVIg, which could occur following spontaneous internalization [24]. IgG and IgM secretion rates were established for 6 independent samples following incubation in the pres- ence or absence of IVIg and compared to CHX-treated cells (Figures 3(a)-(b)). This CHX-treatment inhibited IgG and IgM secretion by more than 80% for at least 18 to 21 hours (Figures 3(a)-(b)) and inhibition of protein synthesis was already noticeable after 3 hours (Figure 3(c)). As suggested by the large SEM (Figure 3), such in vitro CD40-activation is characterized by inter-individ- ual variability in the human B lymphocytes response [7,9,15,25]. Still, the comparison of 6 experiments using two-sided Student’s paired t-test allowed us to highlight significant differences. Figure 2. Removal of 99% of residual IgG in IVIg treated cells. B lymphocytes have been stimulated with high or low CD154 interaction, as described in Figure 1, in the presence of 10 mg/mL IVIg for 9 days. A total number of 16 - 20 × 106 cells were washed with PBS-glc 5 times. IgG content was measured in each washing supernatants by ELISA. Data are presented as the mean ± SD of 3 independent experiments. Human B lymphocytes submitted to high levels of CD40-CD154 interaction and treated with IVIg were secreting 18 to 208 ng of IgG/h/106 cells (mean ± SD; 97 ± 70) while control cells were secreting 6 to 89 ng of (a) (b) (c) (d) Figure 3. IVIg-increased de novo IgG secretion. B lymphocytes were stimulated as described in Figure 1. On day 9, cells were washed 5 times and incubated 3 hours in the absence or presence of 15 µg/mL cycloheximide (CHX). Cells were washed and then seeded back in IMDM alone for a supplemental 18 to 20 h. IgG and IgM contents were determined in these supernatants ((a) and (b)) and in the 3 h-supernatant (c). The frequency of IgG-secreting cells was determined by a standard ELISPOT (d). Data presented in A and B are the mean ± SEM of six independent experiments while results presented in (c) are the mean ± SEM of four independent experiments. The IVIg increase of IgG secretion in B lymphocytes submitted to high (p = 0.030) and low (p = 0.09) levels of CD40-CD154 interaction is significant according to paired t-test while no significant difference was observed for IgM secretion ((a) and (b)). The IVIg induced IgG secretion during CHX-treatment is significant for cells submitted to high level of CD40-CD154 in- teraction (U = 2,309; p = 0.0209). Openly accessible at  N. Dussault et al. / Open Journal of Immunology 1 (2011) 6 5-73 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/oji/ 70 IgG/h/106 cells (mean ± SD; 50 ± 35). Thus, high levels of CD40-CD154 interaction clearly led to a statistically significant IVIg-increase of IgG secretion (p = 0.0300). On the other hand, human B lymphocytes submitted to low stimulation in the presence of IVIg were secreting 111 to 2951 ng of IgG/h/106 cells (mean ± SD; 1042 ± 1014) while untreated cells were secreting 65 to 1550 ng of IgG/h/106 cells (mean ± SD; 681 ± 544). Such an in- crease of IgG secretion (p = 0.09), namely about 1.5-fold, corresponds to a supplemental secretion of 362 ± 513 ng of IgG/h/106 cells. In contrast, IVIg did not modulate de novo IgM secretion by B lymphocytes submitted to low and high level of CD40-CD154 interaction (Figur e 3(b)). IgG content in the supernatant from CHX-treated cells in 4 independent experiments was also assessed (Figure 3(c)). IVIg increased IgG secretion by 1.8-fold and 4.6- fold for cells submitted to low and high level of CD40- CD154 interaction, respectively. We have also measured IgG de novo synthesis by ELISPOT using CHX treated- cells (Figure 3(d)). On day 9, the frequency of IgG se- creting cells detected by ELISPOT assay was very low (<3%), nonetheless IVIg still appeared to slightly in- crease the frequency of IgG-secreting cells. We have previously shown that IVIg-treatment of human B lymphocytes increases IgG secretion in long- term culture, namely 14 days [5-7]. These results further substantiate that IVIg can increase the de novo IgG se- cretion in CD40-activated B lymphocytes after a 9-day treatment (Figure 1). 3.4. IVIg Modulation and mRNA Synthesis B lymphocytes were cultured for 9 days, as above (Figure 1), in the presence or absence of IVIg. In this case, controls were treated with PBS-40 mM glycine pH 4.5 (CTL) and BSA as indicated methods. RT-PCR was used to verify whether all 5 sets of primers used effi- ciently detected gamma chains as well as subclasses such as IgG1, IgG2 and IgG3 and IgG4 (Figures 4(a) and (b)). Except for the primers used to detect IgG3, all sets of primers used were reliable to detect the various IgG mRNA from controls cells as well as cells treated with BSA or IVIg. IgG3 mRNA was however detected in some samples but showed no increase in IVIg-treated cells (data not shown). For the set of primers used in to detect IgG1 and IgG2 (Figure 4 (b)), these analyses, whi- ch have been done up to 4 times on 3 independent sam- ples, showed an increase in IgG mRNA content follow- ing IVIg treatment. However, these observations were not consistent with the other sets of primers used target- ing the same mRNA. Using semi-quantitative PCR, we evaluated whether IVIg modulation of IgG transcription can be detected (Figure 4 (c)). In this case, the two sets of primers used in panel A target IgG1 (IgG1-S) and IgG2, IgG3 and IgG4 (IgG2-4) [20]. Although the quantitative aspect was im- proved when compared to the RT-PCR experiments, there was no increase in the IgG mRNA levels following IVIg treatment when compared to untreated cells. BSA- treated cells displayed lower expression levels of IgG1 mRNA than untreated cells or IVIg-treated cells. These results did not reveal any significant effect of IVIg and were contradictory with the observed increased of IgG protein, as determined by ELISA, for the same experi- ment and samples. For example, panel A and B corre- spond to a culture where IgG secretion was increased by 2-fold in IVIg treated cells when compared to BSA- treated cells and untreated cells, which were giving iden- tical IgG secretion. Q-PCR analyses were performed to compare the mRNA expression of untreated cells and BSA-treated cells to IVIg-treated cells (Figure 5). Relative IgG mRNA content varied depending on the housekeeping gene used and depending on whether IVIg-cells were compared to untreated or BSA-treated cells. The twofold increase, which is usually considered as a significant difference in Q-PCR analysis, was not observed when IVIg conditions were compared to untreated cells. Once again, IVIg appeared to significantly increase IgG1 and IgG2 expression when compared to the BSA condition. Overall, neither semi-quantitative PCR nor Q-PCR detected an increased IgG secretion in human B lym- phocytes with IVIg. Furthermore, these experiments carried out using 3 independent samples were fluctuating depending on the samples used. (a) (b) (c) Figure 4. IgG mRNA is not influenced by IVIg. B lymphocytes were stimulated for 9 days with low levels of CD40-CD154 interac- tion in the presence or absence of 10 mg/mL bovine serum albumin (BSA) or 10 mg/mL IVIg (IVIg) both dialyzed in 40 mM glycine pH 4.5. For controls, 10% (v/v) 40 mM glycine pH4.5 was added in the culture medium (CTL). IgG mRNA levels were evaluated using PCR method ((a) and (b)) and semi-quantitative PCR (c) with primers (Table 1) specifically targeting IgG, IgG1-s, IgG2-4 , IgG1, IgG2 IgG3 and IgG4 gamma chains, and GAPDH, as indicated at the top of the panels. 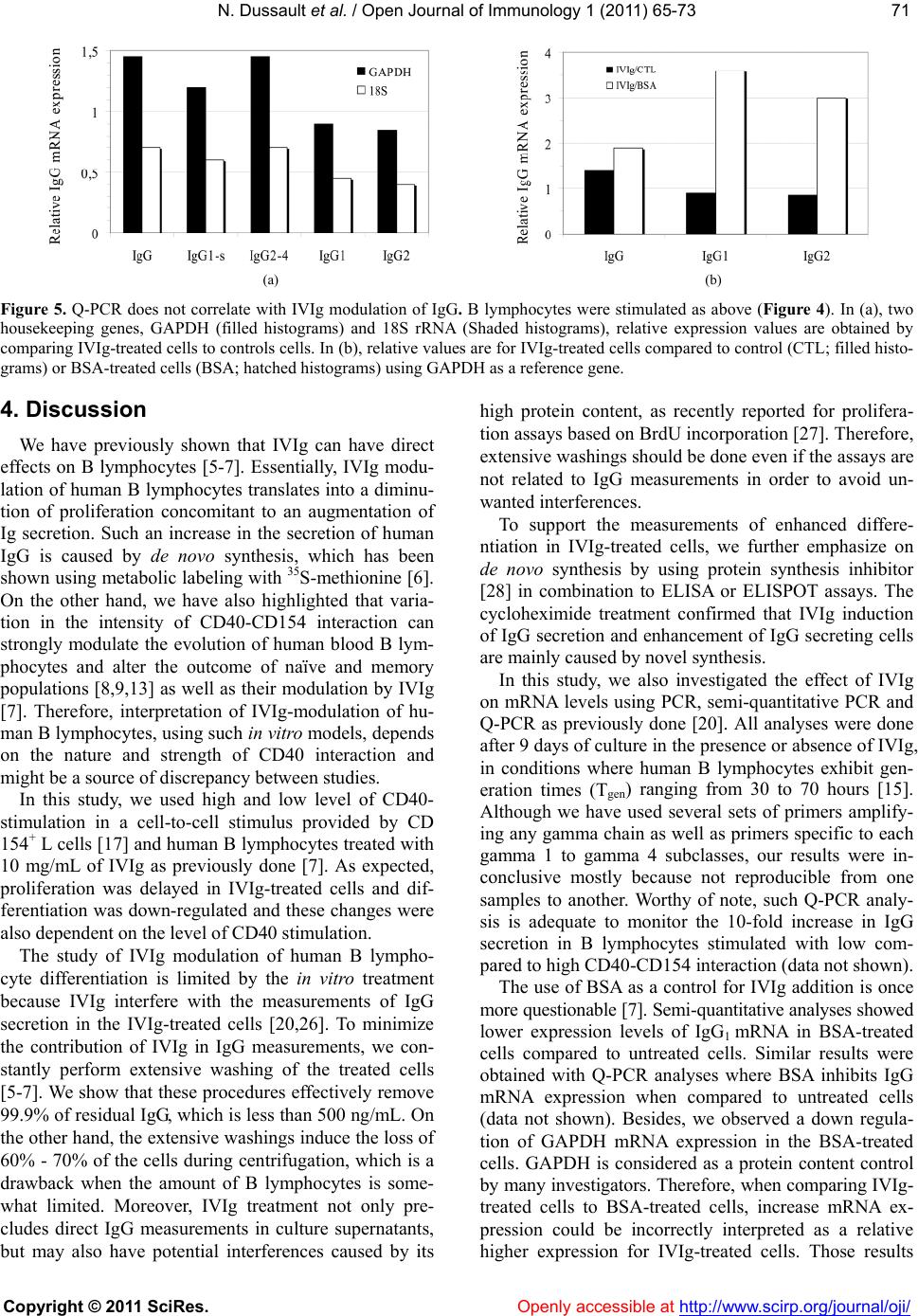 N. Dussault et al. / Open Journal of Immunology 1 (2011) 6 5-73 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/oji/ 7171 (a) (b) Figure 5. Q-PCR does not correlate with IVIg modulation of IgG. B lymphocytes were stimulated as above (Figure 4). In (a), two housekeeping genes, GAPDH (filled histograms) and 18S rRNA (Shaded histograms), relative expression values are obtained by comparing IVIg-treated cells to controls cells. In (b), relative values are for IVIg-treated cells compared to control (CTL; filled histo- grams) or BSA-treated cells (BSA; hatched histograms) using GAPDH as a reference gene. 4. Discussion We have previously shown that IVIg can have direct effects on B lymphocytes [5-7]. Essentially, IVIg modu- lation of human B lymphocytes translates into a diminu- tion of proliferation concomitant to an augmentation of Ig secretion. Such an increase in the secretion of human IgG is caused by de novo synthesis, which has been shown using metabolic labeling with 35S-methionine [6]. On the other hand, we have also highlighted that varia- tion in the intensity of CD40-CD154 interaction can strongly modulate the evolution of human blood B lym- phocytes and alter the outcome of naïve and memory populations [8,9,13] as well as their modulation by IVIg [7]. Therefore, interpretation of IVIg-modulation of hu- man B lymphocytes, using such in vitro models, depends on the nature and strength of CD40 interaction and might be a source of discrepancy between studies. In this study, we used high and low level of CD40- stimulation in a cell-to-cell stimulus provided by CD 154+ L cells [17] and human B lymphocytes treated with 10 mg/mL of IVIg as previously done [7]. As expected, proliferation was delayed in IVIg-treated cells and dif- ferentiation was down-regulated and these changes were also dependent on the level of CD40 stimulation. The study of IVIg modulation of human B lympho- cyte differentiation is limited by the in vitro treatment because IVIg interfere with the measurements of IgG secretion in the IVIg-treated cells [20,26]. To minimize the contribution of IVIg in IgG measurements, we con- stantly perform extensive washing of the treated cells [5-7]. We show that these procedures effectively remove 99.9% of residual IgG, which is less than 500 ng/mL. On the other hand, the extensive washings induce the loss of 60% - 70% of the cells during centrifugation, which is a drawback when the amount of B lymphocytes is some- what limited. Moreover, IVIg treatment not only pre- cludes direct IgG measurements in culture supernatants, but may also have potential interferences caused by its high protein content, as recently reported for prolifera- tion assays based on BrdU incorporation [27]. Therefore, extensive washings should be done even if the assays are not related to IgG measurements in order to avoid un- wanted interferences. To support the measurements of enhanced differe- ntiation in IVIg-treated cells, we further emphasize on de novo synthesis by using protein synthesis inhibitor [28] in combination to ELISA or ELISPOT assays. The cycloheximide treatment confirmed that IVIg induction of IgG secretion and enhancement of IgG secreting cells are mainly caused by novel synthesis. In this study, we also investigated the effect of IVIg on mRNA levels using PCR, semi-quantitative PCR and Q-PCR as previously done [20]. All analyses were done after 9 days of culture in the presence or absence of IVIg, in conditions where human B lymphocytes exhibit gen- eration times (Tgen) ranging from 30 to 70 hours [15]. Although we have used several sets of primers amplify- ing any gamma chain as well as primers specific to each gamma 1 to gamma 4 subclasses, our results were in- conclusive mostly because not reproducible from one samples to another. Worthy of note, such Q-PCR analy- sis is adequate to monitor the 10-fold increase in IgG secretion in B lymphocytes stimulated with low com- pared to high CD40-CD154 interaction (data not shown). The use of BSA as a control for IVIg addition is once more questionable [7]. Semi-quantitative analyses showed lower expression levels of IgG1 mRNA in BSA-treated cells compared to untreated cells. Similar results were obtained with Q-PCR analyses where BSA inhibits IgG mRNA expression when compared to untreated cells (data not shown). Besides, we observed a down regula- tion of GAPDH mRNA expression in the BSA-treated cells. GAPDH is considered as a protein content control by many investigators. Therefore, when comparing IVIg- treated cells to BSA-treated cells, increase mRNA ex- pression could be incorrectly interpreted as a relative higher expression for IVIg-treated cells. Those results  N. Dussault et al. / Open Journal of Immunology 1 (2011) 6 5-73 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/oji/ 72 confirm previous studies showing that BSA have proper- ties that make it unsuitable as a placebo [29]. On the other hand, the use of Q-PCR to monitor IVIg modulation of IgG secretion is questionable, particularly since a maximum of twofold increase secretion is often observed. Besides, these twofold changes are not valid in Q-PCR determination. In fact, the choice of reference “housekeeping” genes can greatly influence the nor- malization results and can vary from one type of cells to another. As example, the choice of 18S rRNA to monitor IVIg treatment [20], which reliability may vary from one tissue to another [21,22,30] and is particularly inconsis- tant in T lymphocytes [31]. Our Q-PCR analyses show a maximum increase of 1.6-fold in IgG mRNA expression following IVIg treatment, which is not considered sig- nificant for this method. In a certain point of view, these results are contradictory with the ELISA conclusion but are in agreement with those of Heidt and collaborators [20] stating that IVIg had no effect on IgG secretion ac- cording Q-PCR analysis of mRNA levels. To avoid IVIg interferences, the quantification of IgM secretion have been previously used to monitor IVIg influence on the immunoglobulin production in common variable immunodeficiency, which was quite significant [26]. However, IgM secretion is restricted to naïve and memory subsets of B lymphocytes and is not representa- tive of switched memory B lymphocytes. Moreover, our results indicated that IVIg effects on IgM secretion was less important and not significant compared to that on IgG synthesis. Therefore, the method described here re- presents an acceptable option to monitor the direct effect of IVIg on B lymphocytes differentiation. Furthermore, variations in the nature and intensity of CD40 binding during in vitro activation of human B lymphocytes will result in the emergence of distinct B-cell populations [9], which might in turn give rise to differential responses to IVIg [7]. In conclusion, by delimiting the strength and weakness in ELISA and PCR methods used to monitor IVIg effects on human B lymphocytes, this study intend to contribute to a better understanding of the mechanisms of action of IVIg from the protein to the mRNA level. 5. ACKNOWLEDGEMENTS We thank all the volunteers who participated in this study and Clau- dine Côté Inf. for scheduling blood sample collection. We are grateful to Marc Cloutier Ph.D. for excellent critical review and manuscript editing. REFERENCES [1] Durandy, A., Kaveri, S. V., Kuijpers, T. W., Basta, M., Miescher, S., Ravetch, J. V. and Rieben, R. (2009) Intra- venous immunoglobulins—Understanding properties and mechanisms. Clinical & Experimental Immunology, 158, 2-13. d oi: 10 .1111 /j. 1365-2249.2009.04022.x [2] Wasserman, R.L., Irani, A.M., Tracy, J., Tsoukas, C., Stark, D., Levy, R., Chen, J., Sorrells, S., Roberts, R. and Gupta, S. (2010) Pharmacokinetics and safety of subcu- taneous immune globulin (human), 10% caprylate/ chro- matography purified in patients with primary immunode- ficiency disease. Clinical & Experimental Immunology, 161, 518-526. doi: 10 .1111 /j. 13 65- 2249.2010.04195.x [3] Kreuz, W., Erdos, M., Rossi, P., Bernatowska, E., Espa- nol, T. and Marodi, L. (2010) A multi-centre study of ef- ficacy and safety of Intratect((R)), a novel intravenous immunoglobulin preparation. Clinical & Experimental Immunology, 101, 512-517. d oi :1 0. 1111/j .1 365 -2249.2010.04187.x [4] Kuitwaard, K., de Gelder, J., Tio-Gillen, A. P., Hop, W.C., van Gelder, T., van Toorenenbergen, A.W., van Doorn, P.A. and Jacobs, B.C. (2009) Pharmacokinetics of intra- venous immunoglobulin and outcome in Guillain-Barre syndrome. Annals of Neurology, 66, 597-603. doi:10.1002/ana.21737 [5] Dussault, N., Ducas, E., Racine, C., Jacques, A., Pare, I., Cote S. and Neron, S. (2008) Immunomodulation of hu- man B cells following treatment with intravenous im- munoglobulins involves increased phosphorylation of extracellular signal-regulated kinases 1 and 2. Interna- tional Immunology, 20, 1369-1379. doi:10.1093/intimm/dxn090 [6] de Grandmont, M.J., Racine, C., Roy, A., Lemieux, R. and Néron, S. (2003) Intravenous immunoglobulins in- duce the in vitro differentiation of human B lymphocytes and the secretion of IgG. Blood, 101, 3065-3073. doi:10.1182/blood-2002-06-1684 [7] Néron, S., Boire, G., Dussault, N., Racine, C., de Brum- Fernandes, A. J., Cote S. and Jacques, A. (2009) CD40- activated B cells from patients with systemic lupus ery- thematosus can be modulated by therapeutic immu- noglobulins in vitro. Archivum Immunologiae et Thera- piae Experimentalis, 57, 447-458. doi:10.1007/s00005-009-0048-3 [8] Néron, S., Nadeau, P.J., Darveau, A. and Leblanc, J.F. (2011) Tuning of CD40-CD154 Interactions in Human B-Lymphocyte Activation: A Broad Array of In Vitro Models for a Complex in vivo Situation. Archivum Im- munologiae et Therapiae Experimentalis, 59, 25-40. [9] Néron, S., Racine, C., Roy, A. and Guérin, M. (2005) Differential responses of human B-lymphocyte subpopu- lations to graded levels of CD40-CD154 interaction. Im- munology, 116, 454-463. doi:10.1007/s00005-010-0108-8 [10] Stewart, R., Wei, W.B., Challa, A., Armitage, R.J., Ar- rand J.R., Rowe, M., Young, L.S., Eliopoulos, A. and Gordon, J. (2007) CD154 tone sets the signaling path- ways and transcriptome generated in model CD40-Plu- ricompetent L3055 Burkitt’s lymphoma cells. Journal of Immunology, 179, 2705-2712. [11] Luft, T., Maraskovsky, E., Schnurr, M., Knebel, K., Kirsch, M., Gorner, M., Skoda, R., Ho, A.D., Nawroth, P. and Bierhaus, A. (2004) Tuning the volume of the im- mune response: Strength and persistence of stimulation determine migration and cytokine secretion of Dendritic  N. Dussault et al. / Open Journal of Immunology 1 (2011) 6 5-73 Copyright © 2011 SciRes. http://www.scirp.org/journal/oji/Openly accessible at 7373 Cells. Blood, 104, 1066-1074. doi:10.1182/blood-2003-12-4146 [12] Mathur, R.K., Awasthi, A., Wadhone, P., Ramanamurthy, B. and Saha, B. (2004) Reciprocal CD40 signals through p38MAPK and ERK-1/2 induce counteracting immune responses. Nature Medicine, 10, 540-544. doi:10.1038/nm1045 [13] Ducas, E., Dussault, N., Roy, A., Dumont, N. and Neron, S. (2009) Estimation of the number of CD154 molecules in membrane extracts used as a source of CD40 stimula- tion of human B lymphocytes. Journal of Immunological Methods, 344, 133-137. doi:10.1016/j.jim.2009.03.009 [14] Fecteau, J.F. and Néron, S. (2003) CD40 stimulation of human peripheral B lymphocytes: Distinct response from naïve and memory cells. Journal of Immunology, 171, 4621-4629. [15] Fecteau, J.F., Roy, A. and Neron, S. (2009) Peripheral blood CD27(+) IgG(+) B cells rapidly proliferate and differentiate into immunoglobulin-secreting cells after exposure to low CD154 interaction. Immunology, 128, e353-e365. doi:1 0.1111 /j .1365-2567.2008.02976.x [16] Néron, S., Thibault, L., Dussault, N., Cote, G., Ducas, E., Pineault, N. and Roy, A. (2007) Characterization of mononuclear cells remaining in the leukoreduction sys- tem chambers of apheresis instruments after routine platelet collection: A new source of viable human blood cells. Transfusion, 47, 1042-1049. d oi :1 0. 1111/j .1 537 -2995.2007.01233.x [17] Néron, S., Pelletier, A., Chevrier, M. C., Monier, G., Le- mieux, R. and Darveau, A. (1996) Induction of LFA-1 independent human B cell proliferation and differentia- tion by binding of CD40 with its ligand. Immunological Investigations, 25, 79-89. doi:10.3109/08820139609059292 [18] Roy, A., Krzykwa, E., Lemieux, R. and Néron, S. (2001) Increased efficiency of gamma-irradiated versus mito- mycin C-treated feeder cells for the expansion of normal human cells in long-term cultures. Journal of Hemato- therapy & Stem Cell Research, 10, 873-880. doi:10.1089/152581601317210962 [19] Barbas, I.C.F., Burton, D.R. and Scott, J.K., Silverman, G.J. (2001) Phage display: A laboratory manual. Cold Spring Harbor Laboratory Press, New-York. [20] Heidt, S., Roelen, D.L., Eijsink, C., Eikmans, M., Claas, F.H.J. and Mulder, A. (2009) Intravenous immunoglobu- lin preparations have no direct effect on B cell prolifera- tion and immunoglobulin production. Clinical & Ex- perimental Immunology, 158, 99-105. d oi :1 0. 1111/j .1 365 -2249.2009.03996.x [21] de Kok, J.B., Roelofs, R.W., Giesendorf, B.A., Pennings, J.L., Waas, E.T., Feuth, T., Swinkels, D.W. and Span, P.N. (2005) Normalization of gene expression measurements in tumor tissues: Comparison of 13 endogenous control genes. Laboratory Investigation, 85, 154-159. [22] Giricz, O., Lauer-Fields, J.L. and Fields, G.B. (2008) The normalization of gene expression data in melanoma: in- vestigating the use of glyceraldehyde 3-phosphate dehy- drogenase and 18S ribosomal RNA as internal reference genes for quantitative real-time PCR. Analytical Bio- chemistry, 380, 137-139. doi:10.1016/j.ab.2008.05.024 [23] Livak, K.J. and Schmittgen, T.D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods, 25, 402-408. [24] Proulx, D.P., Aubin, E., Lemieux, R. and Bazin, R. (2009) Spontaneous internalization of IVIg in activated B cells. Immunology Letters, 124, 18-26. doi:10.1016/j.imlet.2009.03.012 [25] Fecteau, J.F. and Néron, S. (2004) Characterization of naïve and memory B cell differentiation toward Plasma cells following low CD40 stimulation. Immunology Col- lection of Free Papers. Medimond Srl, Bologna, 303-307. [26] Bayry, J., Fournier, E.M., Maddur, M.S., Vani, J., Wootla, B., Siberil, S., Dimitrov, J.D., Lacroix-Desmazes, S., Ber- dah, M., Crabol, Y., Oksenhendler, E., Levy, Y., Mouthon, L., Sautes-Fridman, C., Hermine, O. and Kaveri, S.V. (2011) Intravenous immunoglobulin induces proliferation and immunoglobulin synthesis from B cells of patients with common variable immunodeficiency: A mechanism underlying the beneficial effect of IVIg in primary im- munodeficiencies. Journal of Autoimmunity, 36, 9-15. doi:10.1016/j.jaut.2010.09.006 [27] Padet, L., St-Amour, I., Aubin, E., Proulx, D. P., Bazin, R. and Lemieux, R. (2009) Dose-dependent inhibition of BrdU detection in the cell proliferation ELISA by culture medium proteins. Journal of Immunoassay and Immu- nochemistry, 30, 348-357. doi:10.1080/15321810903084863 [28] van Laar, J.M., Melchers, M., Teng, Y.K.O., van der Zouwen, B., Mohammadi, R., Fischer, R., Margolis, L., Fitzgerald, W., Grivel, J.C., Breedveld, F.C., Lipsky, P.E. and Grammer, A.C. (2007) Sustained secretion of im- munoglobulin by long-lived human tonsil plasma cells. The American Journal of Pathology, 171, 917-927. doi:10.2353/ajpath.2007.070005 [29] Hommes, O.R., Haas, J., Soelberg-Sorenson, P. and Frie- drichs, M. (2009) IVIG trials in MS. Is albumin a pla- cebo? Journal of Neurology, 256, 268-270. doi:10.1007/s00415-009-0893-3 [30] Ho-Pun-Cheung, A., Cellier, D. and Lopez-Crapez, E. (2008) Considerations for normalisation of RT-qPCR in oncology. Ann Biol Clin (Paris), 66, 121-129. [31] Mane, V.P., Heuer, M.A., Hillyer, P., Navarro, M.B. and Rabin, R.L. (2008) Systematic method for determining an ideal housekeeping gene for real-time PCR analysis. J Biomol Tech, 19, 342-347.
|