Paper Menu >>
Journal Menu >>
 Vol.2, No.4, 300-305 (2010) Health doi:10.4236/health.2010.24044 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ S tress cardiomyopathy: clinical features and outcomes Lilian Hamity 1, Franco Ramel lo1, Leandro Marani 1, Alejandro Moyano1, Eduard o Moreyr a2, Luis E. Alday1 1Hospital Aeronáutico Córdoba, Córdoba, Argentina; lealday@arnet.com.ar 2Professor of Medicine, Universidad Nacional de Córdoba, Córdoba, Argentina Received 6 January 2010; revised 25 January 2010; accepted 29 January 2010. ABSTRACT Objectives: To establish the prevalence, clinical features, and outcomes of the recently recog- nized stress cardiomyopathy whose physiopa- thology is still not completely clarified. Material and methods: The prevalence and clinical find- ings of stress cardiomy op athy were assessed in a group of 378 patients undergoing cinecoro- nariography for acute coronary syndromes dur- ing a 7-year period. The inclusion criteria were severe chest pain, ischemic electrocardiogra- phic changes, reversible left ventricular dys- hypokinesia, and normal coronary arteries. Eight patients, 7 female (2.1% of all patients and 5.0% of the women), with a mean age of 65.3 ± 8.5 years fulfilled the requirements. Results: The precipitating factor was severe stress in all of them. Cardi ac enzymes were slightly raised. Th ere was apical le ft ven tric ular dy skin esi a i n 6 patients, midventricular in another, and diffuse hypokine- sia in the remaining. One patient showed mod- erate mitral regurgitation. The response to con- ventional treatment and patient outcomes were favorable in all cases with prompt reversal of the left ventricular dyskinesia as assessed by echocardiography. There were 4 recurrences, 2 requiring readmission to hospital, despite con- tinuous treatment with combined alfa and beta adrenergic blockers and calcium antagonists. Conclusions: In our hospital, stress cardiomyo- pathy had a prevalence of 2.1% in all patients with acute coronary syndromes and 5.1% in women and should be considered in their dif- ferential diagnosis, especially in middle aged female p atient s with a history of sev ere previous stress. There was a favorable outcome but re- currences may occur despite uninterrupted me- dical treatment following discharge. Keywords: Stress Cardiomyopathy; Takotsubo Syndrome; Myocardial Ischemia; Cardiomyopathy 1. INTRODUCTION The recently described stress cardiomyopathy (SCM), resembles an acute coronary syndrome (ACS) presenting with severe chest pain, left ventricular dys- or hypokine- sia not following a determined pattern corresponding to a single involved artery as it occurs in acute myocardial infarction. It was first described in Japan in the early 90’s as “ta- kotsubo syndrome” (from tako: octopus, tsubo: trap) for the similarity with the narrow necked and large rounded bottom fishing pots used in Japan to trap octopus, with the angiographic left ventricular systolic appearance in affected patients [1]. Other names proposed for this syndrome are “apical ballooning syndrome”, “broken heart”, “transient myocardial stunning”, and “transient apical dyskinesia” [2-8]. The syndrome occurs almost exclusively in middle aged women with a clinical onset usually following an episode of severe emotional stress although it may occur during the course of an illness, after some kind of surgery, or even without any provok- ing factor [3,9]. There is a varying severity, with risk of arrhythmias, congestive heart failure, shock and even death. The left ventricular kinetic ab normalities are tran- sient with full recovery of the contractility in about 2 months along with normalization of the electrocardio- graphic ischemic changes in a variable period of time. The incidence of recurrences has been estimated around 11% in a group of 100 patients in a 4-year follow-up period [10]. In this study we analyze the features and frequency of SCM in a group of consecutive patients with ACS re- quiring selective coronarographic study (SCG) during a 7-year period in a general hospital. 2. METHODS We performed a retrospective-prospective, observational study to determine the frequency of SCM, as already defined, in all patients studied by SCG for ACS from March 2002 to February 2009. In clusion cr iteria were: a) 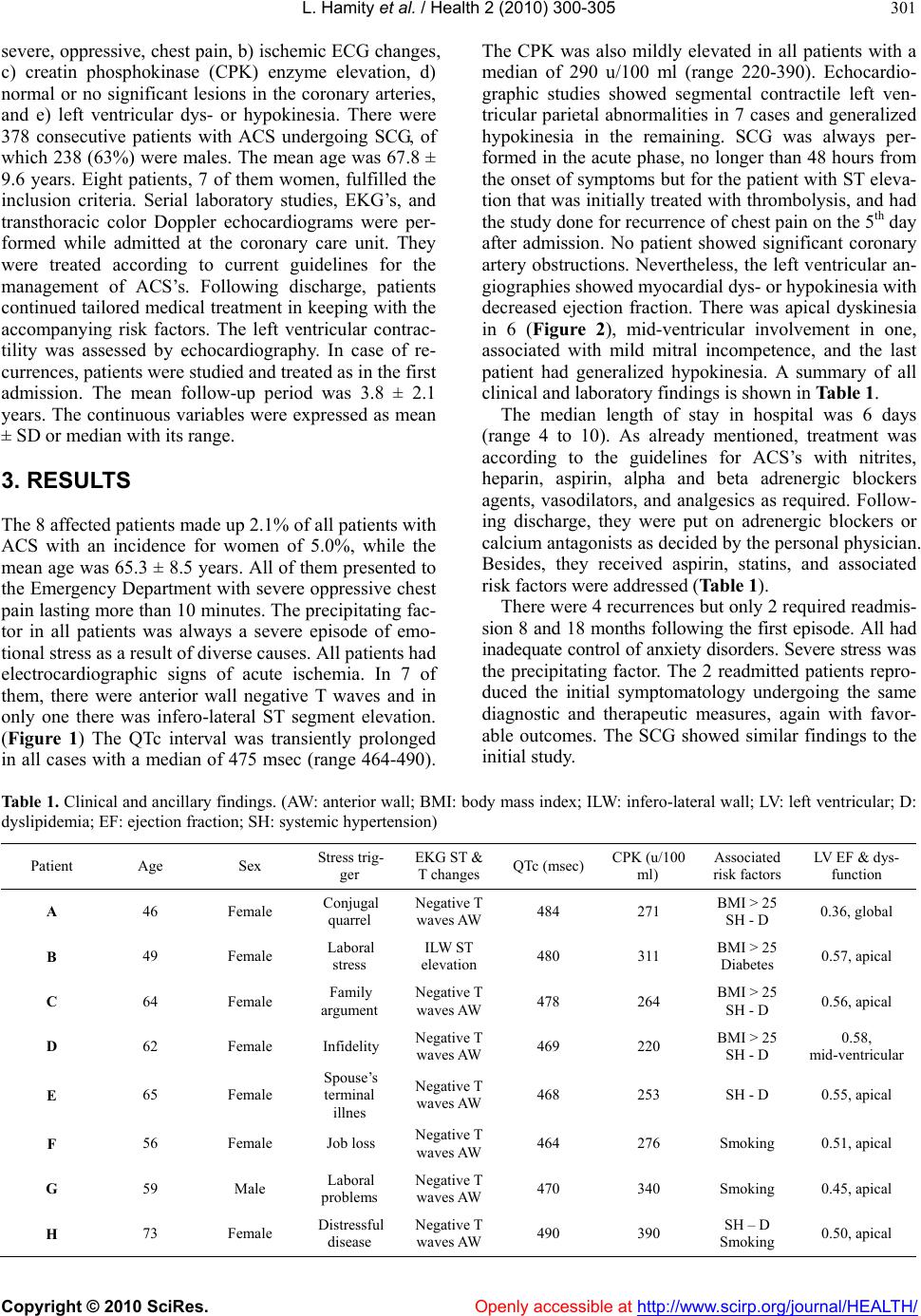 L. Hamity et al. / Health 2 (2010) 300-305 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 301 severe, oppressive, chest pain, b) ischemic ECG changes, c) creatin phosphokinase (CPK) enzyme elevation, d) normal or no significant lesions in the coronary arteries, and e) left ventricular dys- or hypokinesia. There were 378 consecutive patients with ACS undergoing SCG, of which 238 (63%) were males . The mean age was 67.8 ± 9.6 years. Eight patients, 7 of them women, fulfilled the inclusion criteria. Serial laboratory studies, EKG’s, and transthoracic color Doppler echocardiograms were per- formed while admitted at the coronary care unit. They were treated according to current guidelines for the management of ACS’s. Following discharge, patients continued tailored medical treatment in k eeping with the accompanying risk factors. The left ventricular contrac- tility was assessed by echocardiography. In case of re- currences, patients were studied and treated as in the first admission. The mean follow-up period was 3.8 ± 2.1 years. The continuous variables were expressed as mean ± SD or median with its range. 3. RESULTS The 8 affected patients made up 2.1% of all patients with ACS with an incidence for women of 5.0%, while the mean age was 65.3 ± 8.5 years. All of them presented to the Emergency Department with severe oppressive chest pain lasting more than 10 min utes. The precip itating fac- tor in all patients was always a severe episode of emo- tional stress as a result of diverse causes. All patients had electrocardiographic signs of acute ischemia. In 7 of them, there were anterior wall negative T waves and in only one there was infero-lateral ST segment elevation. (Figure 1) The QTc interval was transiently prolonged in all cases with a median of 475 msec (range 464-490). The CPK was also mildly elevated in all patients with a median of 290 u/100 ml (range 220-390). Echocardio- graphic studies showed segmental contractile left ven- tricular parietal abnormalities in 7 cases and generalized hypokinesia in the remaining. SCG was always per- formed in the acute phase, no longer than 48 hou rs from the onset of symptoms but for the patient with ST eleva- tion that was initially treated with thrombolysis, and had the study done for recurrence of chest pain on the 5 th day after admission. No patient showed significant coronary artery obstructions. Nevertheless, the left ventricular an- giographies showed myocardial dys- or hypokinesia with decreased ejection fraction. There was apical dyskinesia in 6 (Figure 2), mid-ventricular involvement in one, associated with mild mitral incompetence, and the last patient had generalized hypokinesia. A summary of all clinical and laboratory findings is shown in Table 1. The median length of stay in hospital was 6 days (range 4 to 10). As already mentioned, treatment was according to the guidelines for ACS’s with nitrites, heparin, aspirin, alpha and beta adrenergic blockers agents, vasodilators, and analgesics as required. Follow- ing discharge, they were put on adrenergic blockers or calcium antagonists as decided by the personal physician. Besides, they received aspirin, statins, and associated risk factors were addressed (Table 1). There were 4 recurrences but only 2 required readmis- sion 8 and 18 months following the first episode. All had inadequate control of anxiety disorders. Severe stress was the precipitating factor. The 2 readmitted patients repro- duced the initial symptomatology undergoing the same diagnostic and therapeutic measures, again with favor- able outcomes. The SCG showed similar findings to the initial study. Table 1. Clinical and ancillary findings. (AW: anterior wall; BMI: body mass index; ILW: infero-lateral wall; LV: left ventricular; D: dyslipidemia; EF: ejection fraction; SH: systemic hypertension) Patient Age Sex Stress trig- ger EKG ST & T changes QTc (msec)CPK (u/100 ml) Associated risk factors LV EF & dys- function A 46 Female Conjugal quarrel Negative T waves AW 484 271 BMI > 25 SH - D 0.36, global B 49 Female Laboral stress ILW ST elevation 480 311 BMI > 25 Diabetes 0.57, apical C 64 Female Family argument Negative T waves AW 478 264 BMI > 25 SH - D 0.56, apical D 62 Female Infidelity Negative T waves AW 469 220 BMI > 25 SH - D 0.58, mid-ventricular E 65 Female Spouse’s terminal illnes Negative T waves AW 468 253 SH - D 0.55, apical F 56 Female Job loss Negative T waves AW 464 276 Smoking 0.51, apical G 59 Male Laboral problems Negative T waves AW 470 340 Smoking 0.45, apical H 73 Female Distressful disease Negative T waves AW 490 390 SH – D Smoking 0.50, apical  L. Hamity et al. / Health 2 (2010) 300-305 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 302 (a) Admission EKG (patient E) (b) EKG of same patient at discharge Figure 1. (a) Admission EKG of patient E showing deeply negative T waves in the anterior wall and prolonged QT interval; (b) EKG of same patient at discharge with normalization of repolarization abnormalities. Figure 2. Left ventriculography of patient G in systole and diastole showing typical apical bal- looning.  L. Hamity et al. / Health 2 (2010) 300-305 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 303 4. DISCUSSION The findings in our study in pa tients with SCM mirrored those described in papers following the original descrip- tion of the syndrome in Japan in the early 90’s [1]. The involved populatio n was mainly middle aged females [2]. The chest pain at presentation was indistinguishable from that occurring in ACS and always precipitated b y a severe stress episode [9]. The EKG showed acute ische- mic changes, usually anterior wall T wave inversion with lengthening of the QTc interval, accompanied by small rise of the myocardial markers (the CPK enzyme in our study) [2,10]. We did not find abnormal Q waves on the EKG though they may also transiently happen [2]. SCG ruled out the presence of significant coronary stenoses while apical left ventricular dyskinesia oc- curred in 7 patients with extension to the midventricu- lar region in one of them. In the remaining patient there was generalized hypokinesia [12]. One patient also had moderate mitral regurgitation, which with the left ven- tricular contraction abnormalities were transient [13]. Response to ACS treatment was favorable without major complications resulting in short length of stay. Among them, arrhythmias, heart failure, shock, and very rarely inhospital death have been described [2,9, 12]. Several risk factors were present in all cases and were addressed according to current recommendations. Despite continuous medical treatment following dis- charge, there were 4 mid-term recurrences, 2 requiring readmission to hospital, with similar course to the ini- tial presentation. Anxiety disorders were more difficult to control in patients with recurrences as compared to those without them. In one study, the frequency of re- currences was around 11%. Patients at risk could not be identified and adrenergic beta blocker therapy did not prevent them [10]. Prevalence of SCM has been estimated in about 2% of patients presenting with ACS and 3 times higher if just women are considered. We had a somewhat similar occurrence (2.1 and 5.0%, respectively). According to these figures the diagnosis of SCM should be borne in mind in patients with ACS in any of both situations. [5,9,14]. Increased recognition of this syndrome is re- flected in the higher number of publications in recent years. Since the physiopathogenesis of SCM has still not been elucidated several theories have been proposed. There is widespread agreement that an excess of cir- culating catecholamines would cause the observed de- rangements through different mechanisms. [4] The oc- currence of spasm of the epicardial coronary arteries was initially propo sed and th en microcircu latory dysfun ction. [1,9] However, both were discarded since the dyskinetic territory was more extensive than that corresponding to the perfusion of just one artery and it was not clear whe- ther the microcirculatory dysfunction was the cause or the consequence of SCM. [16] On the contrary, other authors suggested, based on laboratory studies showing higher levels of catecholamines in SCM than in similar control patients with myocardial infarction, a direct my- ocardial damage. Furthermore, patients undergoing en- domyocardial biopsy, showed monocyte inflammatory infiltrates and myocytolysis similar to those occurring in excess catecholamine myocardial damage. [17] More recently, it has been proposed that high level circulating catecholamines would trigger an intracellular signaling pathway that would change the stimulating protein G function to inhibiting protein G in beta 2 receptors. Al- though this change would serve as a protecting mecha- nism against cell apoptosis mediated by intensive activa- tion of beta 1 receptors, it would also produce a negative inotropic effect in cardiomyocytes. The higher apical left ventricular adrenergic receptor density would explain why this region is the most frequently affected in SCM while the basal segments are usually spared. Adreno- receptor distribution individual phenotypes in SCM pa- tients would explain the different left ventricular re- gional involvement. [7] Accordingly, less frequent left ventricular hypo-dyskinesias like the midventricular, generalized, and the inverted “takotsubo” affecting basal contractility but sparing the apex might occur [18,19]. On the other hand, in a recent review of all types of SCM, Bybee and Prasad proposed on the basis of expe ri- mental studies, that excessive stress would activate the right cortical insula and the ipsilateral cerebral amigdala stimulating the autonomic nervous system, producing a local excess of catecholamines. At their tu rn, the y would activate the calcium channels increasing the cytosolic and mitochondrial calcium levels with release of free radicals and lipid membrane peroxidation., resulting in cell death and band contraction necrosis producing EKG abnormalities and arrhythmias [12]. Some authors, though accepting the role of increased catecholamine secretion as a triggering factor, suggest that SCM would be instead an aborted “myocardial in- farction”. It is hypothesized that coronary artery lesions, unidentified by SCG because of their very small size, would produce plaque accidents with spontaneous reso- lution through endogenous fibrinolysis preventing an- giographic recognition due to the time elapsed since on- set of symptoms and performance of the study. SCM would then be the consequence of previous myocardial ischemia. Radioisotope studies using metabolic markers have shown altered fatty acid handling and glycose transportation probably caused by catecholamine excess and calcium intracellular overload resulting in “myocar- dial metabolic stunning” [20-22]. The cause of the more frequent occurrence of SCM in menopausic women has not been elucidated. A gender difference has been suggested or else, microcirculatory  L. Hamity et al. / Health 2 (2010) 300-305 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 304 endothelial dysfunction facilitated by a decrease of es- trogenic stimulation [5]. Dynamic left ventricular outflow tract obstruction has also been described in SCM patients. [23] It is still not clear if the gradient is the cause or a consequence of the syndrome [16]. Finally, Akashi et al., based on experimental studies in rats, propose that sudden, unexpected, stress, would ac- tivate autonomic nervous system neurons with estro- genic receptors causing higher release of catecholamines that would stimulate vascular and cardiac adrenorecep- tors resulting in systemic hypertension and increased inotropism. The aforementioned higher expression of apical adrenoreceptors, would be responsible of the dys- kinesia at that level through the toxic effect on the car- diomyocytes. The dynamic left ventricular outflow tract obstruction present in some patients with SCM would be caused by the basal myocardial hypercontractility. The loss of estrogenic protection in the nervous system and myocardium following menopause would exaggerate all these disarrang ements [15]. The previous discussion shows that the origin and fa- cilitating triggering factors of SCM are still under debate. Since SCM description and better identification are re- cent, it is still not clear which is the ideal treatment for these patients in the acute phase and after discharge to prevent recurrences. Nevertheless, supported by labo- ratory animal experimental studies, Akashi et al., rec- ommend combined alfa and beta adrenergic blockers instead of individual administration. Calcium channel antagonists and nitrites would also be effective [15]. 5. CONCLUSIONS The prevalence of SCM in a 7-year period in our hospi- tal was 2.1% of all patients with ACS requiring SCG and 5.0% if just women were considered. There was an ini- tial favorable outcome with satisfactory response to conventional medical treatment though recurrences oc- curred in patients with inad equate control in anx iety dis- orders and 2 of them required readmission to hospital. REFERENCES [1] Dote, K., Sato, H., Tateishi, H., Uchida, T. and Ishihara, M. (1991) Myocardial stunning due to simultaneous mul- tivessel coronary spasms: A review of 5 cases. Journal of Cardiology, 21, 203-214. [2] Abe, Y. and Kondo, M. (2003) Apical ballooning of the left ventricle: A distinct entity? Heart, 89, 974-976. [3] Bybee, K.A., Kara, T., Prasad, A., Lerman, A., Barsness, G.W., Wright, S., et al. (2004) Systematic review: Tran- sient left ventricular apical ballooning: A syndrome that mimics ST-segment elevation myocardial infarction. An- nals of Internal Medicine, 141, 858-865. [4] Brandspiegel, H.Z., Marinchak, R.A., Rials, S.J. and Kowey, P.R. (1998) A broken heart. Circulation, 98, 1349. [5] Pavin, D., Le Breton, H. and Daubert, C. (1997) Human stress cardiomyopathy mimicking acute myocardial syn- drome. Heart, 78, 509-511. [6] Sharkey, S.W., Lesser, J.R., Zenovich, A. G., Maron, M.S., Lindberg, J., Longe, T.F., et al. (2005) Acute and reverse- ble cardiomyopathy provoked by stress in women from the United States. Circulation, 111, 472-479. [7] Lyon, A.R., Rees, P.S.C., Prasad, S., Poole-Wilson, P.A. and Harding, S.E. (2008) Stress (Takotsubo) cardio- myopathy—a novel pathophysiological hypothesis to ex- plain cathecolamine—induced myocardial stunning. Na- ture Clinical Practice Car diovascular Medicine, 5, 2 2-29. [8] Juri, J.E., Smith, F.J., Bahamonde, L.A., De Rosa, A. And Salzberg, S. (2005) Discinesia apical transitoria. Rev Argent Cardiol, 73, 64-67. [9] Gianni, M., Denta l i, F., Grandi, A.M., Sumner, G., Hira lal, R. and Lonn, E. (2006) Apical ballooning syndrome or Takotsubo cardiomyopathy: A systematic review. Euro- pean Heart Journal, 27, 1523-1529. [10] Elesber, A.A., Prasad, A., Lennon, R.J., Wright, R. S., Ler- man, A. and Rihal, C.S. (2007) Four-year recurrence rate and prognosis of the apical ballooning syndrome. Journal of the American College of Cardiology, 50, 4 4 8 -452. [11] Tsuchihashi, K., Ueshima, K., Uchida, T., Oh-mura, N., Kimura, K., Owa, M., et al. (2001) Transient left ven- tricular apical ballooning without coronary artery stenosis: A novel heart syndrome mimicking acute myocardial in- farction. Journal of the American College of Cardiology, 38, 11-18. [12] Bybee, K.A. and Prasad, A. (2008) Stress-related car- diomyopathy syndromes. Circulation, 118, 397-409. [13] Parodi, G., Del Pace, S., Salvadori, C., Carrabba, N., Olivotto, I. and Gensini, G.F. (2007) Tuscani registry of Tako-Tsubo cardiomyopathy: Left ventricular apical bal- looning syndrome as a novel cause of acute mitral regur- gitation. Journal of the American College of Cardiology, 50, 647-649. [14] Elian, D., Osherou, A., Matetzky, S., Hod, H., Guetta, V., Feinberg, M.S., et al. (2006) Left ventricular apical bal- looning syndrome: Not an uncommon variant of acute myocardial infarction in women. Clinical Cardiology, 29, 9-13. [15] Akashi, Y.J., Goldstein, D.S., Barbaro, G. and Ueyama, T. (2008) Takotsubo cardiomyopathy: A new form of acute reversible heart failure. Circulation, 118, 2754-2762. [16] Nef, H.M., Mollmann, H. and Elsasser, A. (2007) Tako- tsubo cardiomyopathy (apical ballooning). Heart, 93, 1309-1315. [17] Wittstein, I.S., Thiemann, D.R., Lima, J.A.C., Baughman, K.L., Shulman, S.P., Gerstenblith, G., et al. (2005) Neu- rohumoral features of myocardial stunning due to sudden emotional stress. The New England Journal of Medicine, 352, 539-548. [18] Hurst, R.T., Askew, J.W., Reuss, C.S., Lee, R.W., Swen- ney, J.P., Fortuin, F.D., et al. (2006) Transient midven- tricular ballooning: A new variant. Journal of the Ameri- can College of Cardiology, 48, 579-583 [19] Sanchez-Recalde, A., Costero, O., Oliver, J.M., Iborra, C., Ruiz, E. and Sobrino, J.A. (2006) Pheochromocytoma- related cardiomyopathy inverted takotsubo contractile pattern. Circulation, 113, e738-e739. [20] Khallafi, H., Chacko, V., Varveralis, N. and Elmi, F. (2008) “Broken heart syndrome”: Catecholamine surge  L. Hamity et al. / Health 2 (2010) 300-305 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 305 or aborted myocardial infarction? The Journal of Inva- sive Cardiology, 20, e9-e13 [21] Kurisu, S., Inohue, I., Kawagoe, T., Ishihara, M., Shimatani, I., Nishioka, K., et al. (2003) Myocardial perfusion and fatty acid metabolism in patients with tako-tsubo-like left ventricular dysfunction. Journal of the American College of Cardiology, 41, 743-748. [22] Bybee, K.A., Murphy, J., Wright, R.S., Prasad, A., Rihal, C.S. and Chareontaitawee, P. (2006) Acute impairment of regional myocardial glucose utilization in the apical bal- looning (Takotsubo) syndrome. Journal of Nuclear Cardiol- ogy, 13(2), 244-250. [23] Villareal, R.P., Achari, A., Wilansky, S. and Wilson, J.M.l. (2001) Anteroapical stunning and left ventricular outflow tract obstruction. Mayo Clinic Proceeding, 76, 79-83. |

