 International Journal of Organic Chemistry, 2011, 1, 233-241 doi:10.4236/ijoc.2011.14034 Published Online December 2011 (http://www.SciRP.org/journal/ijoc) Copyright © 2011 SciRes. IJOC 233 Rate Enhancements in the Acetylation and Benzoylation of Certain Aromatic Compounds with Vilsmeier-Haack Reagents Using Acetamide, Benzamide and Oxychlorides under Non-Conventional Conditions Marri Venkateswarlu, Kamatala Chinna Rajanna*, Mukka Satish Kumar, Utkoor Umesh Kumar, Soma Ramgopal, Pondichery Kuppuswamy Saiprakash Department of C hemi st ry , Osmania University, Hyderabad, India E-mail: *kcrajannaou@yahoo.com Received August 8, 2011; revised September 21, 2011; accepted October 3, 2011 Abstract Acetylation and benzoylation reactions of certain aromatic aldehydes, ketones with Vilsmeier-Haack Re- agents using Acetamide and Oxychloride (SOCl2 or POCl3) under conventional (thermal) and non conven- tional [microwave irradiated (MIR), ultrasonic assisted and solvent free mortar pestle (grinding)] conditions. Reactions afforded good to excellent yields of products with both the VH reagents, reaction times were fairly less in the case of [amide/POCl3] than those of [amide/SOCl2] reagent. Reactions are dramatically acceler- ated in under sonicated and microwave irradiations with a trend: MIR (few seconds) >> Sonication (minutes) > Grinding (min) >> thermal (several hrs). Keywords: Acetylation, Benzoylation, Vilsmeier-Haack Reagent (VHR), Acetamide, Benzamide, Micro Wave Irradiation, Grinding, Sonication 1. Introduction Acetylation (or ethanoylation in IUPAC nomenclature) des- cribes a reaction that introduces an acetyl functional group into a chemical compound, while benzoylation introduces a benzoyl group into an organic compound. These reactions are included in the category of the most important transformations in Organic Synthesis [1-3]. A re- action involving the replacement of the hydrogen atom of a hydroxyl group with an acetyl group (CH3 CO) yie- lds a specific ester, the acetate. Acetylation is one of the principal metabolic pathways of the sulfonamides. It is also one of the important synthetic bio-transformations which operate in the metabolism of drugs in which me- tabolites are produced that are more readily excreted than the parent drug. However, dogs are exceptional amongst the domesticated species in that acetylation does not oc- cur in their tissues. Acetylation of the N-terminal alpha- amine of proteins is a widespread modification in eukar- yotes. Forty to fifty percent of yeast proteins and be- tween 80% to 90% of human proteins are modified in this manner. The pattern of modification is found to be the same throughout evolution. Among the various protecting groups used for the hy- droxyl group, acetyl is one of the most common groups, being stable in the acid reaction conditions and also eases of removal by mild alkaline hydrolysis [1,2]. The most commonly used reagent combination for this reaction uses acid anhydride in the presence of acid or base cata- lysts [3]. Various metal salts [4-16] such as CoCl2, TiCl4- AgClO4, TaCl5, TaCl5-SiO2, Ce(III) triflate, Sn(IV) por- phyrine and some metal triflates [17-21] such as Sc (OTf)3, MeSiOTf, In(OTf)3, Cu(OTf)2 and Bi(OTf)3, bis(cyclopentadienyl) zirconium dichloride [22], I2 [23], 1,3-dibromo-5,5-dimethylhydentoin or trichloroisocya- nuric acid [24] have been investigated to meet the de- mand for more efficient and selective methods. Benzoylation reaction is generally carried out using benzoyl chloride with a Lewis acid as the benzoylating agent. In addition to benzoyl chloride [1-2], a number of reagents [25-29] such as, benzoic anhydride, benzoy- ltetrazole, 2-benzoyl-1-methylpyridinium chloride, S-ben- zoic-O,O-diethylphosphoro-dithoic anhydride, benzoyl cyanide, can be used for carrying out this reaction. Though benzoyl chloride may be a health hazard due to its toxic- ity, nevertheless it is widely used because of its ready  M. VENKATESWARLU ET AL. 234 availability and low cost. The reaction is usually cata- lyzed by bases like pyridine, triethylamine and sodium hydroxide [30-31]. Recently Satya Paul et al. [32] de- veloped a rapid, economic and environmentally friendly method for benzoylation of -NH2, -OH and -SH groups using PhCOCl-Py/basic alumina. The developed reagent system was found to a good alternative to classical method since the benzoylation underwent expeditiously with high yields under solvent-free conditions. Vilsmeier-Haack reagent (VH) is one of the most ef- ficient synthetic organic reagents for formylation and acetylation reactions. VH reagents could be prepared fo- rm equimolar mixture of formamide or N, N’-dialkyl for- mamides such as N, N’-dimethyl formamide (DMF), N, N’-diethyl formamide (DEF), along with oxy halides such as POCl3 and SOCl2 at freezing temperatures. Recent reports on Vilsmeier-Haack (VH) reactions revealed that organic compounds in general and hydrocarbons with excess pi-electrons in particular undergo formylation very easily on synthetic scale [33-49]. However, these re- actions are known to afford acetyl derivatives when N, N’-dialkyl formamides are replaced by acetamide or N, N’-dialkyl acetamides such as N, N’-dimethyl acetamide (DMA) and N, N’-diethyl acetamide (DEA) in VH re- agent. However, not many systematic reports are pub- lished in this direction. In view of this the author has em- barked on a systematic synthetic study of certain VH acetylation and benzoylation reactions using (Acetamide + SOCl2)/(Acetamide + POCl3) or (Benzamide + SOCl2)/ (Benzamide + POCl3) as VH regents. A set of organic compounds such as aldehydes and ketones are used in these studies, which have been found their importance in a number of industrially important and biologically im- portant reactions. 2. Experinental Details 2.1. General Procedure for Preparation of Vilsmeier-Haack Reagent The Vilsmeier Haack (VH) adduct is prepared afresh be- fore use from SOCl2 and acetamide (ACTAM). To a chilled (at –50˚C) actamide (ACTAM) in dichloro ethane (DCE) or acetonitrile (ACN), calculated amount of PO- Cl3 was slowly added drop wise to get VH reagent and stored under cold conditions. Similar procedure is ado- pted for the preparation of VH reagent with Benzamide (BNZAM). 2.2. General Procedure for Vilsmeier-Haack Synthesis in Reflux Condition A centimolar (0.01 mol) organic substrate (aromatic al- dehydes, ketones), about 0.015 moles of VH reagent and solvent DCE were taken in a cleaned in a Round bottom flask and refluxed till the reaction is completed. After completion of the reaction, as confirmed by TLC, the reaction mixture is treated with 5% sodium thio sulphate solution, followed by the addition of pet ether. The or- ganic layer was separated, dried over Na2SO4 and evapo- rated under vacuum, purified with column chromatogra- phy using DCE: n-hexane (8:2) as eluent to get pure pro- duct. This methodology has also been successfully used for ACN mediated reactions. This methodology has been successfully is adopted for CAN mediated reactions. The yields of major products are compiled in Tables 1 to 4. 2.3. General Procedure for Vilsmeier-Haack Synthesis under Solvent Free Conditions by Grinding in Mortar with Pestle A centimolar (0.01mol) organic substrate (aldehydes, ke- tones or acetophenone), and about 0.015 moles of VH reagent were taken in a previously cleaned mortar and grounded till the reaction is completed. After completion of the reaction, as checked by TLC, the reaction mixture is treated with 5% sodium thio sulphate solution, fol- lowed by the addition of pet ether. The organic layer was separated, dried over Na2SO4 and evaporated under vac- uum, purified with column chromatography using DCE: n-hexane (8:2) as eluent to get pure product. The yields of major products are given in Tables 1 to 4. 2.4. General Procedure for Sonicated Vilsmeier-Haack Synthesis A centimolar (0.01mol) organic substrate (aldehydes, ke- tones or acetophenone), about 0.015 moles of VH re- agent and solvent DCE were taken in a cleaned in a Round bottom flask and clamped in a sonicator and pro- gress of the reaction is monitored by TLC. After comple- tion of the reaction, as ascertained by TLC, the reaction mixture is treated with 5% sodium thio sulphate solution, followed by a the same work-up procedure as mentioned the above section to get the final product. This method- ology has also been successfully for ACN mediated reac- tions. The yields of major products are shown in Tables 1 to 4. 2.5. General Procedure for MW Irradiated Vilsmeier-Haack Synthesis Methodology adopted for MW assisted VH synthesis is almost similar to that used in the above section. A cen- timolar (0.01 mol) organic substrate (aldehydes, ketones or acetophenone), about 0.015 moles of VH reagent and Copyright © 2011 SciRes. IJOC 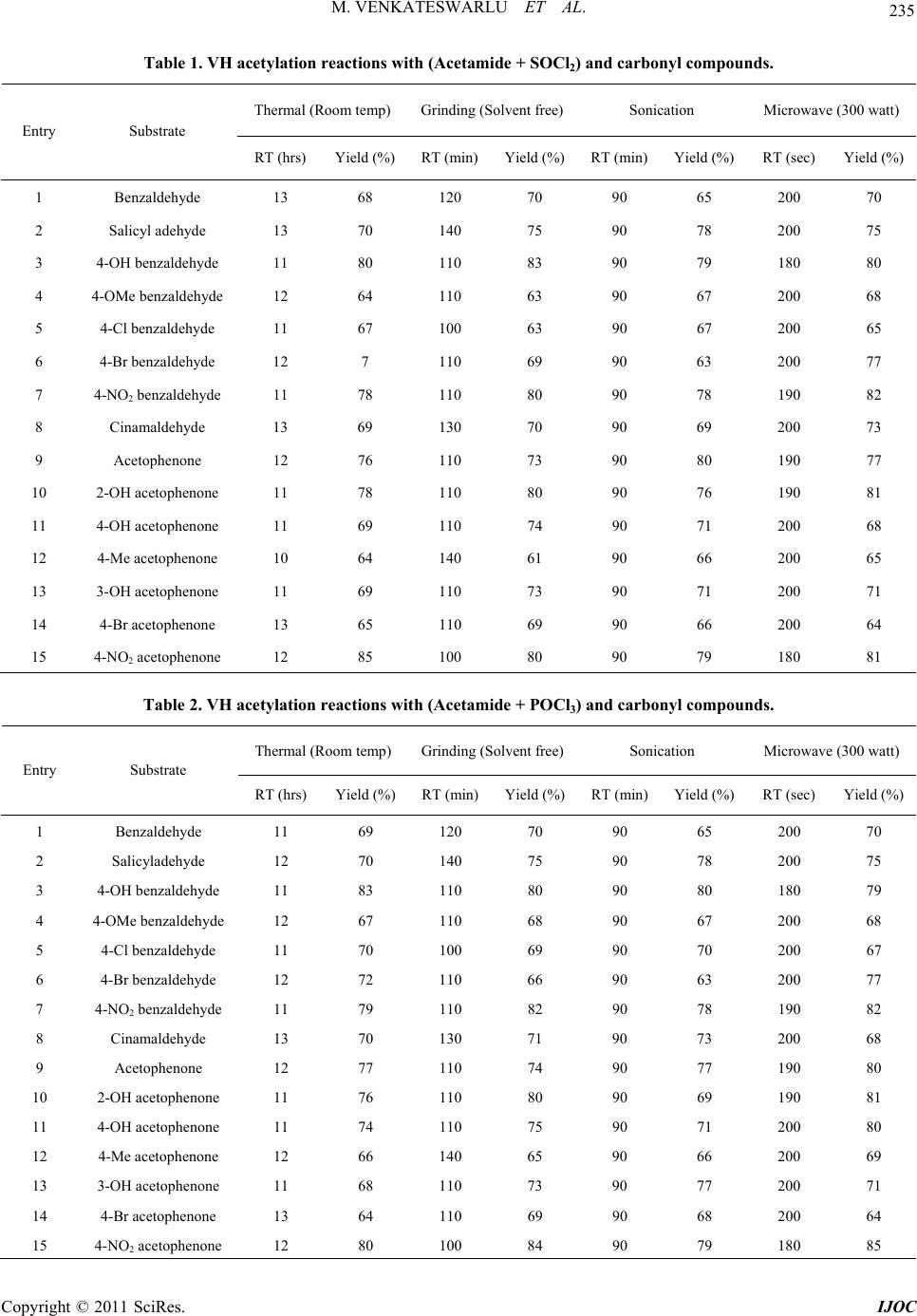 M. VENKATESWARLU ET AL. Copyright © 2011 SciRes. IJOC 235 Table 1. VH acetylation reactions with (Acetamide + SOCl2) and carbonyl compounds. Thermal (Room temp) Grinding (Solvent free)Sonication Microwave (300 watt) Entry Substrate RT (hrs) Yield (%)RT (min)Yield (%)RT (min)Yield (%) RT (sec) Yield (%) 1 Benzaldehyde 13 68 120 70 90 65 200 70 2 Salicyl adehyde 13 70 140 75 90 78 200 75 3 4-OH benzaldehyde 11 80 110 83 90 79 180 80 4 4-OMe benzaldehyde 12 64 110 63 90 67 200 68 5 4-Cl benzaldehyde 11 67 100 63 90 67 200 65 6 4-Br benzaldehyde 12 7 110 69 90 63 200 77 7 4-NO2 benzaldehyde 11 78 110 80 90 78 190 82 8 Cinamaldehyde 13 69 130 70 90 69 200 73 9 Acetophenone 12 76 110 73 90 80 190 77 10 2-OH acetophenone 11 78 110 80 90 76 190 81 11 4-OH acetophenone 11 69 110 74 90 71 200 68 12 4-Me acetophenone 10 64 140 61 90 66 200 65 13 3-OH acetophenone 11 69 110 73 90 71 200 71 14 4-Br acetophenone 13 65 110 69 90 66 200 64 15 4-NO2 acetophenone 12 85 100 80 90 79 180 81 Table 2. VH acetylation reactions with (Acetamide + POCl3) and carbonyl compounds. Thermal (Room temp) Grinding (Solvent free)Sonication Microwave (300 watt) Entry Substrate RT (hrs) Yield (%)RT (min)Yield (%)RT (min)Yield (%) RT (sec) Yield (%) 1 Benzaldehyde 11 69 120 70 90 65 200 70 2 Salicyladehyde 12 70 140 75 90 78 200 75 3 4-OH benzaldehyde 11 83 110 80 90 80 180 79 4 4-OMe benzaldehyde 12 67 110 68 90 67 200 68 5 4-Cl benzaldehyde 11 70 100 69 90 70 200 67 6 4-Br benzaldehyde 12 72 110 66 90 63 200 77 7 4-NO2 benzaldehyde 11 79 110 82 90 78 190 82 8 Cinamaldehyde 13 70 130 71 90 73 200 68 9 Acetophenone 12 77 110 74 90 77 190 80 10 2-OH acetophenone 11 76 110 80 90 69 190 81 11 4-OH acetophenone 11 74 110 75 90 71 200 80 12 4-Me acetophenone 12 66 140 65 90 66 200 69 13 3-OH acetophenone 11 68 110 73 90 77 200 71 14 4-Br acetophenone 13 64 110 69 90 68 200 64 15 4-NO2 acetophenone 12 80 100 84 90 79 180 85  M. VENKATESWARLU ET AL. 236 Table 3. VH benzoylation reactions with (Benzamide + SOCl2) and carbonyl compounds. Thermal (Room temp) Grinding (Solvent free)Sonication Microwave (300 watt) Entry Substrate RT (hrs) Yield (%)RT (min)(Yield) (%)RT (min)Yield (%) RT (sec) Yield (%) 1 Benzaldehyde 12 65 120 67 90 62 200 69 2 Salicyladehyde 12 70 110 75 90 76 200 72 3 4-OH benzaldehyde 11 80 110 81 90 79 190 76 4 4-OMe benzaldehyde 13 67 130 63 90 66 200 68 5 4-Cl benzaldehyde 13 63 130 67 90 65 220 62 6 4-Br benzaldehyde 12 70 130 68 90 63 200 72 7 4-NO2 benzaldehyde 11 81 100 80 90 75 180 82 8 Cinamaldehyde 13 62 110 70 90 67 200 71 9 Acetophenone 12 74 110 76 90 80 190 79 10 2-OH acetophenone 12 80 110 78 90 81 190 76 11 4-OH acetophenone 12 71 110 74 90 69 200 72 12 4-Me acetophenone 11 64 140 61 90 66 200 65 13 3-OH acetophenone 11 69 110 73 90 71 200 68 14 4-Br acetophenone 11 68 110 65 90 63 200 64 15 4-NO2 acetophenone 10 85 100 80 90 79 180 81 Table 4. VH benzoylation reactions with (Benzamide + POCl3) and carbonyl compounds. Thermal (Room temp) Grinding (Solvent free)Sonication Microwave (300 watt) Entry Substrate RT (hrs) Yield (%)RT (min)(Yield) (%)RT (min)Yield (%) RT (sec) Yield (%) 1 Benzaldehyde 11 69 120 70 90 65 200 70 2 Salicyladehyde 12 70 140 75 90 78 200 75 3 4-OH benzaldehyde 11 83 110 80 90 80 180 79 4 4-OMe benzaldehyde 12 67 110 68 90 67 200 68 5 4-Cl benzaldehyde 11 75 100 69 90 76 200 74 6 4-Br benzaldehyde 12 76 110 67 90 65 200 77 7 4-NO2 benzaldehyde 11 79 110 82 90 80 190 82 8 Cinamaldehyde 13 73 130 71 90 73 200 75 9 Acetophenone 12 77 110 79 90 77 190 80 10 2-OH acetophenone 11 76 110 80 90 69 190 81 11 4-OH acetophenone 11 75 110 77 90 73 200 80 12 4-Me acetophenone 12 67 140 65 90 68 200 69 13 3-OH acetophenone 11 70 110 74 90 77 200 73 14 4-Br acetophenone 13 69 110 70 90 68 200 67 15 4-NO2 acetophenone 12 81 100 84 90 79 180 84 Copyright © 2011 SciRes. IJOC 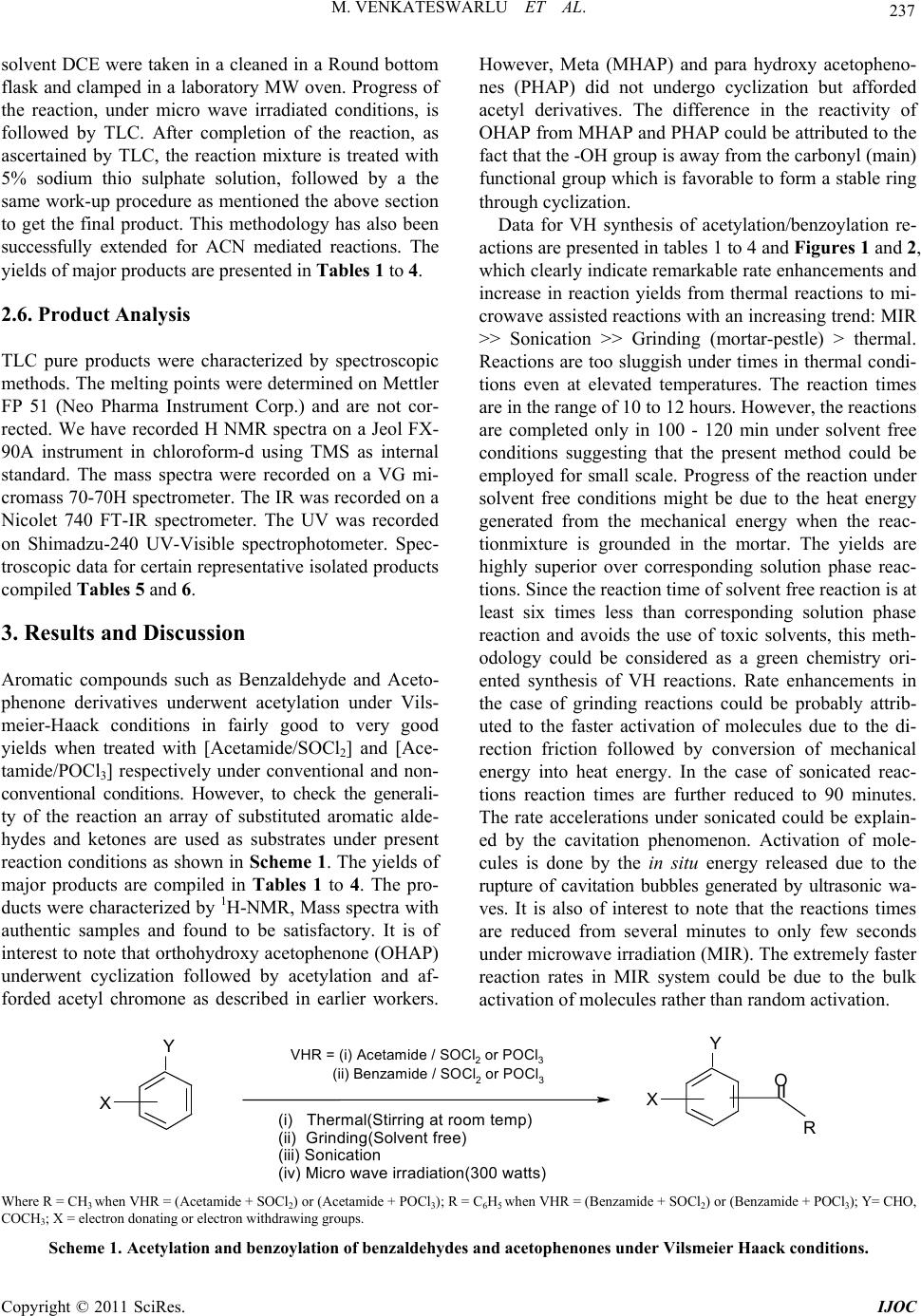 M. VENKATESWARLU ET AL. Copyright © 2011 SciRes. IJOC 237 solvent DCE were taken in a cleaned in a Round bottom flask and clamped in a laboratory MW oven. Progress of the reaction, under micro wave irradiated conditions, is followed by TLC. After completion of the reaction, as ascertained by TLC, the reaction mixture is treated with 5% sodium thio sulphate solution, followed by a the same work-up procedure as mentioned the above section to get the final product. This methodology has also been successfully extended for ACN mediated reactions. The yields of major products are presented in Tables 1 to 4. 2.6. Product Analysis TLC pure products were characterized by spectroscopic methods. The melting points were determined on Mettler FP 51 (Neo Pharma Instrument Corp.) and are not cor- rected. We have recorded H NMR spectra on a Jeol FX- 90A instrument in chloroform-d using TMS as internal standard. The mass spectra were recorded on a VG mi- cromass 70-70H spectrometer. The IR was recorded on a Nicolet 740 FT-IR spectrometer. The UV was recorded on Shimadzu-240 UV-Visible spectrophotometer. Spec- troscopic data for certain representative isolated products compiled Tables 5 and 6. 3. Results and Discussion Aromatic compounds such as Benzaldehyde and Aceto- phenone derivatives underwent acetylation under Vils- meier-Haack conditions in fairly good to very good yields when treated with [Acetamide/SOCl2] and [Ace- tamide/POCl3] respectively under conventional and non- conventional conditions. However, to check the generali- ty of the reaction an array of substituted aromatic alde- hydes and ketones are used as substrates under present reaction conditions as shown in Scheme 1 . The yields of major products are compiled in Tables 1 to 4. The pro- ducts were characterized by 1H-NMR, Mass spectra with authentic samples and found to be satisfactory. It is of interest to note that orthohydroxy acetophenone (OHAP) underwent cyclization followed by acetylation and af- forded acetyl chromone as described in earlier workers. However, Meta (MHAP) and para hydroxy acetopheno- nes (PHAP) did not undergo cyclization but afforded acetyl derivatives. The difference in the reactivity of OHAP from MHAP and PHAP could be attributed to the fact that the -OH group is away from the carbonyl (main) functional group which is favorable to form a stable ring through cyclization. Data for VH synthesis of acetylation/benzoylation re- actions are presented in tables 1 to 4 and Figures 1 and 2, which clearly indicate remarkable rate enhancements and increase in reaction yields from thermal reactions to mi- crowave assisted reactions with an increasing trend: MIR >> Sonication >> Grinding (mortar-pestle) > thermal. Reactions are too sluggish under times in thermal condi- tions even at elevated temperatures. The reaction times are in the range of 10 to 12 hours. However, the reactions are completed only in 100 - 120 min under solvent free conditions suggesting that the present method could be employed for small scale. Progress of the reaction under solvent free conditions might be due to the heat energy generated from the mechanical energy when the reac- tionmixture is grounded in the mortar. The yields are highly superior over corresponding solution phase reac- tions. Since the reaction time of solvent free reaction is at least six times less than corresponding solution phase reaction and avoids the use of toxic solvents, this meth- odology could be considered as a green chemistry ori- ented synthesis of VH reactions. Rate enhancements in the case of grinding reactions could be probably attrib- uted to the faster activation of molecules due to the di- rection friction followed by conversion of mechanical energy into heat energy. In the case of sonicated reac- tions reaction times are further reduced to 90 minutes. The rate accelerations under sonicated could be explain- ed by the cavitation phenomenon. Activation of mole- cules is done by the in situ energy released due to the rupture of cavitation bubbles generated by ultrasonic wa- ves. It is also of interest to note that the reactions times are reduced from several minutes to only few seconds under microwave irradiation (MIR). The extremely faster reaction rates in MIR system could be due to the bulk activation of molecules rather than random activation. X R II Y O VHR = (i) Acetam ide / SOCl2 or POC l3 (ii) Benzamide / SO Cl2 or POCl3 X Y (i) Thermal(Stirring at room temp) (ii) Grinding(Solvent free) (iii) Sonication (iv) Micro wave irradiation(300 watts) Where R = CH3 when VHR = (Acetamide + SOCl2) or (Acetamide + POCl3); R = C6H5 when VHR = (Benzamide + SOCl2) or (Benzamide + POCl3); Y= CHO, COCH3; X = electron donating or electron withdrawing groups. Scheme 1. Acetylation and benzoylation of benzaldehydes and acetophenones unde r Vilsmeier Haack conditions. 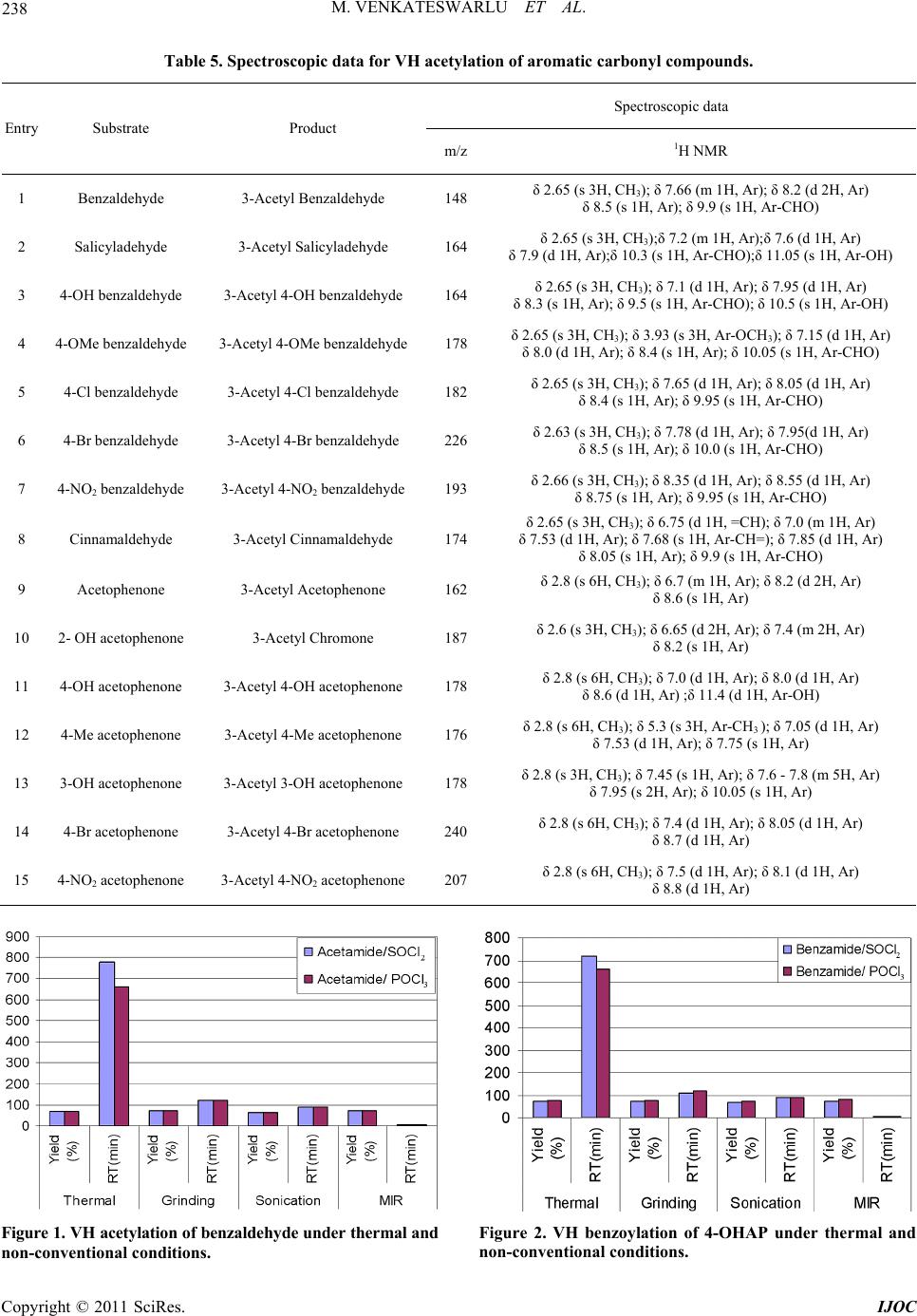 M. VENKATESWARLU ET AL. 238 Table 5. Spectroscopic data for VH acety l ation of ar omatic c arbony l compounds. Spectroscopic data Entry Substrate Product m/z 1H NMR 1 Benzaldehyde 3-Acetyl Benzaldehyde 148 δ 2.65 (s 3H, CH3); δ 7.66 (m 1H, Ar); δ 8.2 (d 2H, Ar) δ 8.5 (s 1H, Ar); δ 9.9 (s 1H, Ar-CHO) 2 Salicyladehyde 3-Acetyl Salicyladehyde 164 δ 2.65 (s 3H, CH3);δ 7.2 (m 1H, Ar);δ 7.6 (d 1H, Ar) δ 7.9 (d 1H, Ar);δ 10.3 (s 1H, Ar-CHO);δ 11.05 (s 1H, Ar-OH) 3 4-OH benzaldehyde 3-Acetyl 4-OH benzaldehyde 164 δ 2.65 (s 3H, CH3); δ 7.1 (d 1H, Ar); δ 7.95 (d 1H, Ar) δ 8.3 (s 1H, Ar); δ 9.5 (s 1H, Ar-CHO); δ 10.5 (s 1H, Ar-OH) 4 4-OMe benzaldehyde 3-Acetyl 4-OMe benzaldehyde 178 δ 2.65 (s 3H, CH3); δ 3.93 (s 3H, Ar-OCH3); δ 7.15 (d 1H, Ar) δ 8.0 (d 1H, Ar); δ 8.4 (s 1H, Ar); δ 10.05 (s 1H, Ar-CHO) 5 4-Cl benzaldehyde 3-Acetyl 4-Cl benzaldehyde 182 δ 2.65 (s 3H, CH3); δ 7.65 (d 1H, Ar); δ 8.05 (d 1H, Ar) δ 8.4 (s 1H, Ar); δ 9.95 (s 1H, Ar-CHO) 6 4-Br benzaldehyde 3-Acetyl 4-Br benzaldehyde 226 δ 2.63 (s 3H, CH3); δ 7.78 (d 1H, Ar); δ 7.95(d 1H, Ar) δ 8.5 (s 1H, Ar); δ 10.0 (s 1H, Ar-CHO) 7 4-NO2 benzaldehyde 3-Acetyl 4-NO2 benzaldehyde 193 δ 2.66 (s 3H, CH3); δ 8.35 (d 1H, Ar); δ 8.55 (d 1H, Ar) δ 8.75 (s 1H, Ar); δ 9.95 (s 1H, Ar-CHO) 8 Cinnamaldehyde 3-Acetyl Cinnamaldehyde 174 δ 2.65 (s 3H, CH3); δ 6.75 (d 1H, =CH); δ 7.0 (m 1H, Ar) δ 7.53 (d 1H, Ar); δ 7.68 (s 1H, Ar-CH=); δ 7.85 (d 1H, Ar) δ 8.05 (s 1H, Ar); δ 9.9 (s 1H, Ar-CHO) 9 Acetophenone 3-Acetyl Acetophenone 162 δ 2.8 (s 6H, CH3); δ 6.7 (m 1H, Ar); δ 8.2 (d 2H, Ar) δ 8.6 (s 1H, Ar) 10 2- OH acetophenone 3-Acetyl Chromone 187 δ 2.6 (s 3H, CH3); δ 6.65 (d 2H, Ar); δ 7.4 (m 2H, Ar) δ 8.2 (s 1H, Ar) 11 4-OH acetophenone 3-Acetyl 4-OH acetophenone 178 δ 2.8 (s 6H, CH3); δ 7.0 (d 1H, Ar); δ 8.0 (d 1H, Ar) δ 8.6 (d 1H, Ar) ;δ 11.4 (d 1H, Ar-OH) 12 4-Me acetophenone 3-Acetyl 4-Me acetophenone 176 δ 2.8 (s 6H, CH3); δ 5.3 (s 3H, Ar-CH3 ); δ 7.05 (d 1H, Ar) δ 7.53 (d 1H, Ar); δ 7.75 (s 1H, Ar) 13 3-OH acetophenone 3-Acetyl 3-OH acetophenone 178 δ 2.8 (s 3H, CH3); δ 7.45 (s 1H, Ar); δ 7.6 - 7.8 (m 5H, Ar) δ 7.95 (s 2H, Ar); δ 10.05 (s 1H, Ar) 14 4-Br acetophenone 3-Acetyl 4-Br acetophenone 240 δ 2.8 (s 6H, CH3); δ 7.4 (d 1H, Ar); δ 8.05 (d 1H, Ar) δ 8.7 (d 1H, Ar) 15 4-NO2 acetophenone 3-Acetyl 4-NO2 acetophenone 207 δ 2.8 (s 6H, CH3); δ 7.5 (d 1H, Ar); δ 8.1 (d 1H, Ar) δ 8.8 (d 1H, Ar) Figure 1. VH acetylation of be nzaldehy de under thermal and non-conventional conditions. Figure 2. VH benzoylation of 4-OHAP under thermal and non-conventional conditions. Copyright © 2011 SciRes. IJOC  239 M. VENKATESWARLU ET AL. Table 6. Spectroscopic data for VH benzoylation of aromatic carbonyl compounds. Spectral data Entry Substrate Product m/z 1H NMR 1 Benzaldehyde 3-Benzoyl Benzaldehyde 210 δ 7.35 (m 4H, Ar); δ 7.65 (d 2H, Ar); δ 8.0 (d 2H, Ar) δ 8.37 (s 1H, Ar); δ 10.05 (s 1H, Ar-CHO) 2 Salicyladehyde 3-Benzoyl Salicyladehyde 258 δ 7.35-7.92 (m 8H, Ar); δ 10.1 (s 1H CHO); δ 10.7 (s 1H, Ar-OH ) 3 4-OH benzaldehyde 3-Benzoyl 4-OH benzaldehyde 258 δ 7.3 (m 4H, Ar); δ 7.6 (d 1H, Ar ); δ 8.0 (d 2H, Ar ) δ 8.3 (s 1H, Ar ); δ 9.95 (s 1H, Ar-CHO ); δ 10.95 (s 2H, Ar-OH ) 4 4-OMe benzaldehyde 3-Benzoyl 4-OMe benzaldehyde240 δ 3.7 (s 3H, OCH3); δ 7.4 (m 4H, , Ar); δ 7.75 (d 1H, Ar) δ 7.9 (d 2H, Ar); δ 7.75 (d 1H, Ar); δ 7.9 (d 2H, Ar) δ 8.2 (d 1H, Ar); δ 10.05 (s 1H, Ar-CHO) 5 4-Cl benzaldehyde 3-Benzoyl 4-Cl benzaldehyde 244 δ 7.4 (m 4H, Ar); δ 7.7 (d 1H, Ar); δ 8.3 (d 2H, Ar) δ 8.45 (s 1H, Ar); δ 10.0 (s 1H, Ar-CHO) 6 4-Br benzaldehyde 3-Benzoyl 4-Br benzaldehyde 288 δ 7.4 (m 4H, Ar ); δ 7.7 (d 1H, Ar); δ 8.0 (d 2H, Ar) δ 8.2 (d 1H, Ar); δ 9.8 (s 1H, Ar-CHO) 7 4-NO2 benzaldehyde 3-Benzoyl 4-NO2 benzaldehyde 255 δ 7.35 (m, 4H Ar); δ 7.65 (d ,1H, Ar ); δ 8.05 (d, 2H, Ar) δ 8.35 (s 1H, Ar); δ 9.95 (s 1H, Ar-CHO) 8 Cinnamaldehyde 3-Benzoyl Cinnamaldehyde 235 δ 6.75 (d ,1H, =CH ); δ 7.4 (m, 1H, Ar); δ 7.45 (d 1H, Ar) δ 7.5-7.8 (m 7H, Ar); δ 8.05 (d 1H, =CH ); δ 9.9 (s 1H, Ar) 9 Acetophenone 3-Benzoyl Acetophenone 224 δ 2.8 (s 3H, CH3); δ 7.8 (m 5H, Ar); δ 7.6 (m 1H, Ar) δ 7.95 (d 2H, Ar); δ 8.45 (s 1H, Ar) 10 2-OH acetophenone 3-Benzoyl Chromone 250 δ 6.34 (s 1H, Ar); δ 7.4 (m 6H, Ar); δ 7.65 (d 2H, Ar) δ 7. 9 (d 1H, Ar) 11 4-OH acetophenone 3-Benzoyl 4-OH acetophenone 240 δ 2.9 (s 3H, CH3); δ 7.3 (m 3H, Ar); δ 7.6 (d 2H, Ar) δ 8.45 (m 2H, Ar); δ 8.8 (s 1H, Ar);δ 10.9 (s 1H, Ar-OH) 12 4-Me acetophenone 3-Benzoyl 4-Me acetophenone 238 δ 2.45 (s 3H, CH3); δ 2.85 (s 3H, CH3-C=O); δ 7.5 (m 3H, Ar) δ 7.7 (d 2H, Ar); δ 8.5 (m 2H, Ar); δ 8.9 (s 1H, Ar) 13 3-OH acetophenone 3-Benzoyl 3-OH acetophenone 240 δ 2.8 (s 3H, CH3); δ 7.45 (s 1H, Ar); δ 7.6 - 7.8 (m 5H, Ar) δ 7.95 (s 2H, Ar); δ 10.05 (s 1H, Ar) 14 4-Br acetophenone 3-Benzoyl 4-Br acetophenone 302 δ 2.65 (s 3H, CH3); δ 7.4 (m 3H, Ar); δ 7.7 (d 2H, Ar) δ 8.5 (m 2H, Ar); δ 8.7 (s 1H, Ar) 15 4-NO2 acetophenone 3-Benzoyl -NO2 acetophenone 269 δ 2.9 (s 3H, CH3); δ 7.3 (m 3H, Ar); δ 7.6 (d 2H, Ar) δ 8.45 (m 2H, Ar); δ 8.8 (s 1H, Ar) 4. Conclusions In conclusion Vilsmeier-Haack Reagents [Acetamide and Oxychloride (SOCl2 or POCl3)] with aromatic aldehydes, ketones afforded acetyl derivatives under conventional (thermal) and non conventional [microwave irradiated (MIR), ultrasonic assisted and solvent free mortar pestle (grinding)] conditions with a trend: MIR (few seconds) >> Sonication (minutes) > Grinding (min) >> thermal (several hrs). Even though both the VH reagents afforded good to excellent yields of products reaction times were fairly less in the case of [Acetamide/POCl3] than those of [Acetamide/SOCl2] reagent. 5. Acknowledgements The authors thank CSIR, New Delhi for financial support in the form of Emeritus Scientist grant awarded to Prof. P. K. Saiprakash. 6. References [1] T. W. Greene and P. G. M Wuts, “Protection Groups in Organic Synthesis,” 3rd Edition, John Wiley, New York, 1999. [2] G. Sartori, R. Ballini, F. Bigi, G. Bosica, R. Maggi and P. Righi, “Protection (and Deprotection) of Functional Groups in Organic Synthesis by Heterogeneous Catalysis,” Che- mical Reviews, Vol. 104, No. 1, 2004, pp. 199-250. [3] A. L. Pearson and W. J. Roush, “Handbook of Reagents for Organic Synthesis; Activating Agents and Protecting Groups,” John Wiley, Chichester, 1999. [4] D. Harton, “Organic Synthesis Collective,” Wiley, New York, 1991. Copyright © 2011 SciRes. IJOC  M. VENKATESWARLU ET AL. 240 [5] R. L. Zhdarov and S. M. Zherodarova, “Chemical Meth- ods of Oligonucleotide Synthesis,” Synthesis, Vol. 1975, No. 4, 1975, pp. 222-245. doi:10.1055/s-1975-23714 [6] G. Hoefle and W. Steglich, “N,N-Dimethyl-4-pyridi- namine, a Very Effective Acylation Catalyst,” Angewandte Chemie International Edition in English, Vol. 8, No. 12, 1969, p. 981. doi:10.1002/anie.196909811 [7] G. Hoefle, W. Steglich and H. Vorbrueggen, “4-Dialkyl- aminopyridines as Highly Active Acylation Catalysts. New Synthetic Method (25),” Angewandte Chemie Inter- national Edition in English, Vol. 17, No. 8, 1978, pp. 569-583. [8] E. F. V. Scriven, “4-Dialkylaminopyridines: Super Acy- lation and Alkylation Catalysts,” Chemical Society Re- views, Vol. 12, No. 2, 1983, pp. 129-161. doi:10.1039/cs9831200129 [9] T. Sano, K. Ohaschi and T. Oreyama, “Remarkably Fast Acylation of Alcohols with Benzoyl Chloride Promoted by TMEDA,” Synthesis, Vol. 7, 1999, pp. 1141-1144. [10] E. Vedejs, N. S. Bennett, L. M. L. Conn, S. T. Diver, M. Gingras, S. Lin, P. A. Oliver and A. L. Peterson, “Tribu- tylphosphine-Catalyzed Acylations of Alcohols: Scope and Related Reactions,” Journal of Organic Chemistry, Vol. 58, No. 25, 1993, pp. 7286-7288. [11] E. Vedejs and S. T. Diver, “Tributylphosphine: A Re- markable Acylation Catalyst,” Journal of the American Chemical Society, Vol. 115, No. 8, 1993, pp. 3358-3359. doi:10.1021/ja00061a056 [12] E. Vedejs, O. Daugulis and S. T. Diver, “Enantioselective Acylations Catalyzed by Chiral Phosphines,” Journal of Organic Chemistry, Vol. 61, No. 2, 1996, pp. 430-431. doi:10.1021/jo951661v [13] A. C. Cope and E. C. Herrich, “Organic Synthesis Col- lective,” Wiley, New York, 1963. [14] S. Chandrasekhar, T. R. Chander and M.Takhi, “Acyla- tion of Alcohols with Acetic Anhydride Catalyzed by TaCl5: Some Implications in Kinetic Resolution,” Tetra- hedron Letters, Vol. 39, No. 20, 1998, pp. 3263-3266. doi:10.1016/S0040-4039(98)00465-1 [15] R. Dalpozzo, A. De Nino, L. Maiuolo, P. A. Procopio, M. Nardi, G. Bartoli and R. Romeo, “Highly Efficient and Versatile Acetylation of Alcohols Catalyzed by Ce- rium(III) Triflate,” Tetrahedron Letters, Vol. 44, No. 30, 2003, pp. 5621-5624. doi:10.1016/S0040-4039(03)01358-3 [16] M. Moghadam, S. Tangestaninejad, V. Mirkhani, I. M. Baltrork and R. Shabani, “Rapid and Efficient Acetyla- tion of Alcohols and Phenols with Acetic Anhydride Cata- lyzed by Electron-Deficient Tin(IV) Porphyrin,” Journal of Moecular Catalysis A: Chemical, Vol. 219, No. 1, 2004, pp. 73-78. doi:10.1016/j.molcata.2004.05.004 [17] K. Ishihara, M. Kubota, H. Kurihara and H. Yamamoto, “Scandium Trifluoromethanesulfonate as an Extremely Active Lewis Acid Catalyst in Acylation of Alcohols with Acid Anhydrides and Mixed Anhydrides,” Journal of Organic Chemistry, Vol. 61, No. 14, 1996, pp. 4560- 4567. doi:10.1021/jo952237x [18] P. A. Procopiou, S. P. D. Baugh, S. S. Flack and G. G. A. Inglis, “An Extremely Powerful Acylation Reaction of Alcohols with Acid Anhydrides Catalyzed by Trimethyl- silyl Trifluoromethanesulfonate,” Journal of Organic Ch- emistry, Vol. 63, No. 7, Vol. 63, 1998, pp. 2342-2347. doi:10.1021/jo980011z [19] K. K. Chauhan, C. G. Frost, I. Love and D. Waite, “In- dium Triflate, an Efficient Catalyst for Acylation Reac- tions,” Synlett, Vol. 11, 1999, pp. 1743-1744. doi:10.1055/s-1999-2941 [20] D. Chandra, P. Saravanan, R. K. Singh and V. K. Singh, “Lewis Acid Catalyzed Acylation Reactions: Scope and Limitations,” Tetrahedron, Vol. 58, No. 7, 2002, pp. 1369- 1374. doi:10.1016/S0040-4020(01)01229-7 [21] A. Orita, G. Tañáis and A. Kakuda, “Highly Efficient and Versatile Acylation of Alcohols with Bi(OTf)3 as Cata- lyst,” Angewandte Chemie International Edition, Vol. 39, No. 16, 2000, pp. 2877-2879. doi:10.1002/1521-3773(20000818)39:16<2877::AID-AN IE2877>3.0.CO;2-V [22] M. L. Kantam, K. Aziz and P. R. Likhar, “Bis(Cyclo- pentadienyl) Zirconium Dichloride Catalyzed Acetylation of Phenols, Alcohols and Amines,” Catalysis Communi- cations, Vol. 7, No. 7, 2006, pp. 484-487. doi:10.1016/j.catcom.2005.10.001 [23] J. W. J. Bosco, A. Agrahari and A. K. Saikia, “Molecular Iodine Catalyzed Selective Acetylation of Alcohols with Vinyl Acetate,” Tetrahedron Letters, Vol. 47, No. 24, 2006, pp. 4065-4068. [24] M. A. Zolfigol, A. Khazaei, A. G. Choghamarani, A. Rostami and M. Hajjami, “Acylation of Alcohols Cata- lyzed by Using 1,3-Dibromo-5,5-dimethylhydentoin or Trichloroisocyanuric Acid,” Catalysis Communications, Vol. 7, No. 6, 2006, pp. 399-402. doi:10.1016/j.catcom.2005.12.004 [25] H. T. Clarke and E. Rahrs, “Organic Syntheses. Collec- tive Volumes,” Coll, Vol. 1, 1941, p. 91. [26] J. Stawinski, T. Hozumi and S. A. Narang, “Ben- zoyltetrazole: A Mild Benzoylating Reagent for Nucleo- sides,” Journal of the Chemical Society, Chemical Com- munications, Vol. 3, 1976, pp. 243-244. doi:10.1039/c39760000243 [27] M. Yamada, Y. Watabe, T. Sakakibara and R. Sudoh, “Preparation of a Water-Soluble Acylating Agent: Ben- zoylation of Acids, Amines, and Phenols with 2-Benzoy- lthio-1-methylpyridinium Chloride in Aqueous Phase,” Journal of the Chemical Society, Chemical Communica- tions, Vol. 4, 1979, pp. 179-180. doi:10.1039/c39790000179 [28] F. A. Carey and K.O. Hodgson, “Efficient Syntheses of Methyl 2-O-Benzoyl- 4, 6- O-benzylidene-α-D-glucopyra- no-side and Methyl 2-O-Benzoyl-4,6-O-benzylidene-α-D- ribo-hexopyranosid-3-ulose,” Carbohydrate Research, Vol. 12, No. 3, 1970, pp. 463-465. doi:10.1016/S0008-6215(00)80628-X [29] T. W. Greene, “Protective Groups in Organic Synthesis,” Wiley, New York, 1981. Copyright © 2011 SciRes. IJOC  M. VENKATESWARLU ET AL. Copyright © 2011 SciRes. IJOC 241 [30] C. B. Reese, “Protective Groups in Organic Chemistry,” Plenum, London, 1973. [31] S. Paul, P. Nanda and R. Gupta, “PhCOCl-Py/Basic Alu- mina as a Versatile Reagent for Benzoylation in Sol- vent-Free Conditions,” Molecules, Vol. 8, 2003, pp. 374- 380. doi:10.3390/80400374 [32] G. A. Olah, St. J. Kuhn, “Friedls Craft’s and Related Reactions,” John Wiley and Sons, New York, 1964. [33] H. Ulrich, “The Chemistry of Imidoyl Halides,” Plenum Press, New York, 1968. [34] R. Bonnet, “The Chemistry of the Carbon Nitrogen Dou- ble Bond,” John Wiley Sons, New York, 1970. doi:10.1002/9780470771204.ch13 [35] E. Compaigne and W. L. Archer, “Organic Synthesis,” John Wiley and Sons, New York, 1953. [36] G. F. Smith, “Indoles. Part I. The Formylation of Indole and Some Reactions of 3-Formylindole,” Journal of the Chemical Society, Vol. 1, 1954, pp. 3842-3846. doi:10.1039/jr9540003842 [37] R. N. Silverstein, C. Ryskiewez, C. Willert and R. C. Kocher, “Improved Synthesis of 2-Pyrrolealdehyde and of N-Methyl-2-Pyrrolealdehyde. Further Studies of Pyr- role Alcohols,” Journal of Organic. Chemistry, Vol. 20, No. 5, 1955, pp. 668-672. doi:10.1021/jo01123a019 [38] A. Lorenz and R. Winzinger, “Über die Vinylenho- mologen der Triphenylmethanfarbstoffe II,” Helvetica Chimica Acta, Vol. 28, No. 1, 1945, pp. 600-612. doi:10.1002/hlca.660280183 [39] H. H. Boshard and H. Zollinger, “Die Synthese von Al- dehyden und Ketonen mit Amidchloriden und Vils- meier-Reagenzien,” Helvetica Chimica Acta, Vol. 42, No. 5, 1959, pp. 1659-1671. [40] S. Alumi, P. Linda, G. Marino, S. Santine and G. Salvelli, “The Mechanism of the Vilsmeier-Haack Reaction. Part II. A Kinetic Study of the Formylation of Thiophen De- rivatives with Dimethylformamide and Phosphorus Oxy- chloride or Carbonyl Chloride in 1,2-Dichloroethane,” Journal of the Chemical Society, Perkin Transactions, Vol. 2, No. 14, 1972, pp. 2070-2073. [41] P. Linda, A. Lucccarelli, G. Marino and G. Savelli, “The Mechanism of the Vilsmeier-Haack Reaction. Part III. Structural and Solvent Effects,” Journal of the Chemical Society, Perkin Transactions, Vol. 2, No. 13, 1974, pp. 1610-1612. [42] Z. Arnold and A. Holy, “Collection Czech,” Chemical Communications, Vol. 27, 1962, pp. 2886-2895. [43] T. D. Smith, “The Reaction of NN-Dimethylformamide with Phosphorus Trichloride,” Journal of the Chemical Society A: Inorganic, Physical, Theoretical, 1966, pp. 841-842. [44] G. J. Martin, S. Poignant, M. L. Filleux and M. T. Que- meneeuer, “Recherches sur la Reaction de Vilsmeier- Haack etude du Mecanisme de Formation du Complexe par des Mesures Cinetiques en Resonance Magnetique Nucleaire,” Tetrahedron Letters, Vol. 11, No. 58, 1970, pp. 5061-5064. [45] G. J. Martin and S. Poignant, “Nuclear Magnetic Reso- nance Investigations of Carbonium Ion Intermediates. Part I. Kinetics and Mechanism of Formation of the Vils- meier-Haack Reagent,” Journal of the Chemical Society, Perkin Transactions, Vol. 2, 1972, pp. 1964-1966. doi:10.1016/S0040-4039(00)96986-7 [46] K. C. Rajanna, F. Soloman, M. M. Ali and P. K. Saip- rakash, “Vilsmeier-Haack Formylation of Coumarin De- rivatives. A Solvent Dependent Kinetic Study,” Interna- tional Journal of Chemical Kinetics, Vol. 28, No. 12, 1996, pp. 865-875. doi:10.1002/(SICI)1097-4601(1996)28:12<865::AID-KI N1>3.0.CO;2-L [47] J. G. Dingwall, D. H. Reid and K. Wade, “Studies of Heterocyclic Compounds. Part VI. NN-Dimethylth- io- form-Amide: A New Reagent in the Vilsmeier Reaction,” Journal of the Chemical Society C: Organic, Vol. 6, 1969, pp. 913-915. [48] T. L. Davis and W. E. Yelland, “Addition of Butylamine to Butyl Isocyanide,” Journal of the American Chemical Society, Vol. 59, No. 10, 1937, pp. 1998-1999. doi:10.1021/ja01289a059 [49] A. Cipiciani, S. Clementi, P. Linda, G. Marino and G. Savelli, “Kinetics and Mechanism of N-Substitution of Indoles and Carbazoles in Vilsmeier-Haack Acetylation,” Journal of the Chemical Society, Perkin Transactions, Vol. 2, 1977, pp. 1284-1287.
|