 International Journal of Organic Chemistry, 2011, 1, 218-223 doi:10.4236/ijoc.2011.14032 Published Online December 2011 (http://www.SciRP.org/journal/ijoc) Copyright © 2011 SciRes. IJOC Heterosynthesis Using Nitriles: Novel Pyrrolo[2,3-b]pyridines Fathy M. Abdelrazek1*, Ahmed A. Fadda2, Samar S. Mohamed1 1Chemistry Department, Faculty of Science, Cairo University, Giza, Egypt 2Chemistry Department, Faculty of Science, Mansoura University, Mansoura, Egypt E-mail: *prof.fmrazek@gmail.com Received August 2, 2011; revised September 13, 2011; accepted September 21, 2011 Abstract 2-Amino-4-benzoyl-1-arylpyrrole-3-carbonitriles react with arylidene malonodinitriles, β-ketoesters and β- diketones to afford pyrrolo[2,3-b]pyridine derivatives. Keywords: N-Arylpyrroles, Cinnamonitriles, Pyrrolo[2,3-b]py r i dine s 1. Introduction Pyrroles and their fused derivatives represent an impor- tant class of heterocyclic compounds due to their diverse biological and pharmaceutical activities [1-4]. In the last two decades we were involved in a program aiming to develop new simple routes for the synthesis of heterocyclic compounds of anticipated biological activity to be evaluated as biodegradable agrochemicals [5-8]. We have reported the synthesis of a variety of pyrrole, N-substituted pyrrole and fused-pyrrole derivatives of biological interest [9-16]. Recently some pyrrolo[2,3-b] pyridine derivatives were shown to be effective as inhi- bitors of tumor necrosis factor alpha [17]. This encour- aged us to prepare some new N-substituted pyrrolo-py- ridine derivatives for biological evaluation study. The pyrrole derivatives 2a-d (Scheme 1; obtained recently by us) [18] seemed suitable precursors to fulfill this object- tive through exploring the presence of the enaminonitrile moiety [19] as a site of reaction. 2. Results and Discussion Pyrrole derivatives 2a-d (Scheme 1; available from ena- minone 1 in reaction with aromatic amines) [18] were reacted with the (hetero) arylidenes of malonodinitrile 3a-c to afford yellow to brown colored products. Based on their elemental analyses as well as mass and spectral data structures 6a-l were assigned to these prod- ucts. These new compounds are assumed to be formed via nucleophilic addition of the -NH2 of 2a-d to the acti- vated double bond in 3 to produce firstly the intermedi- ates 4a-l which subsequently undergo cyclization via the active methyne protons to yield intermediates 5a-l, whi- ch in role loses HCN to afford the isolable products 6a-l (Scheme 1). Next, compounds 2a-d were treated with β-ketoesters 7a and 7b to yield pyrrolo-[2,3-b]-pyridine derivatives 9a- h via, most likely, the intermediates 8a-h which were formed by trans amidation followed by an intramolecular addition of the active methylene to the cyano group. Ele- mental analyses and spectral data were in complete agre- ement with the proposed structures 9a-h. Compounds 2a-d also react with β-diketones 10a and 10b to give highly colored products. It is assumed that this third type of cyclocondensation reaction occurred via the intermediates 11a-h which then undergo addition of the active methylene to the CN group to yield the pyrrolo [2,3-b]pyridine derivatives 12a-h. Elemental analyses and spectral data were fully consistent with the proposed structures 12a-h. 3. Experimental Melting points were measured on an Electrothermal (9100) apparatus and are uncorrected. IR spectra were recorded as KBr pellets on a Perkin Elmer 1430 spectrophotome- ter. The 1H NMR and 13C NMR spectra were carried out on a Varian Gemini 300 MHz spectrometer in DMSO-d6 using TMS as internal standard and chemical shifts are expressed in δ ppm values. Assignments were made by correlation of the off-resonance decoupled 13C-NMR spectra and determination of the 1H-chemical shifts. Ma- ss spectra were recorded on a Shimadzu GCMS-GB 1000 PX (70 ev). Elemental analyses were carried out at the Micro-analytical Center at Cairo University. 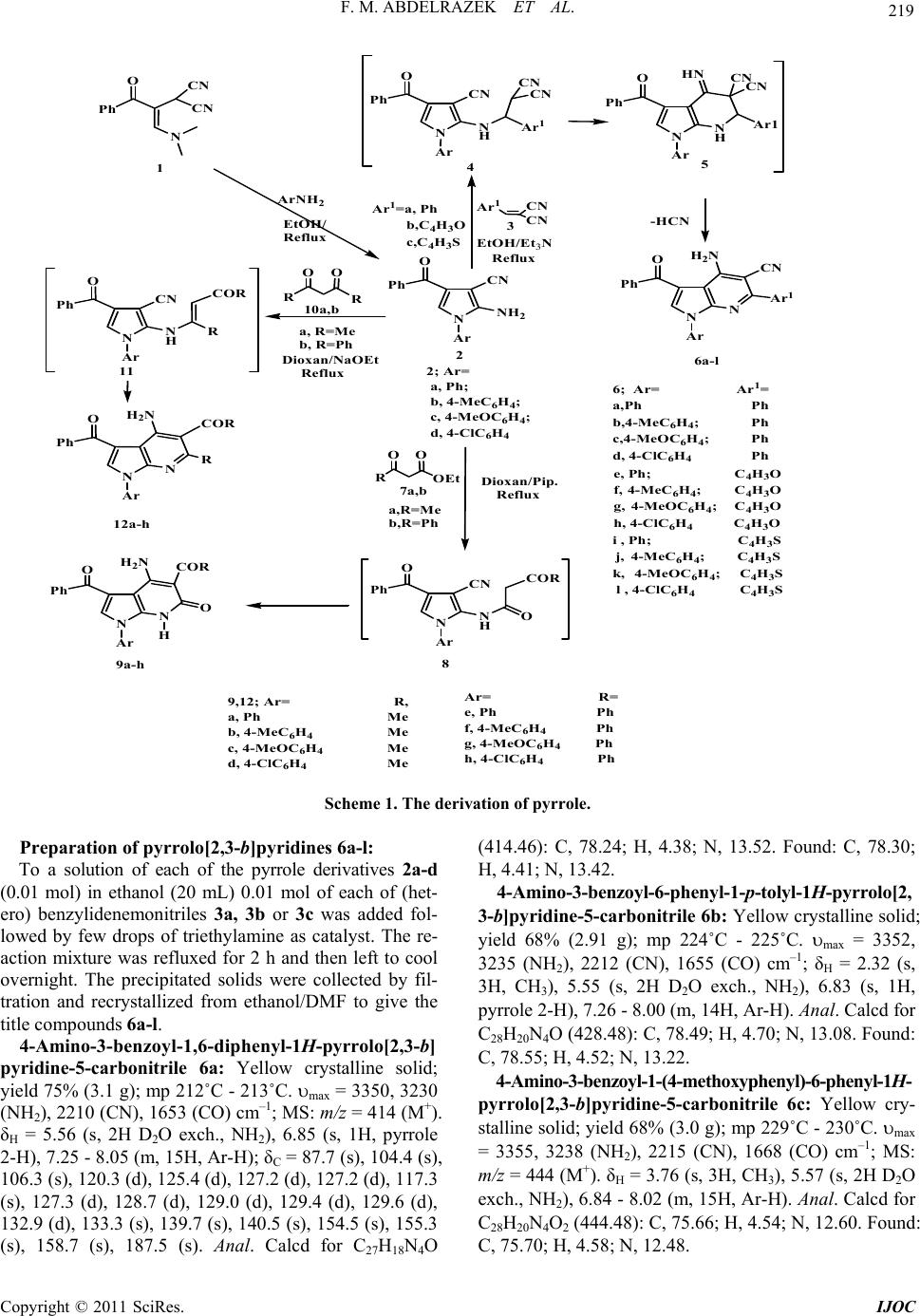 219 F. M. ABDELRAZEK ET AL. NN H O CN Ph Ar CN CN Ar 1 N O Ph Ar HN N H CN CN Ar1 NNH 2 O CN Ph Ar CN CN Ar 1 N O Ph Ar H 2 N N CN Ar 1 6a-l 2 EtOH/Et 3 N Reflux 45 2; Ar= a, Ph; b, 4-MeC 6 H 4 ; c, 4-MeOC 6 H 4 ; d, 4-ClC 6 H 4 6; Ar= Ar 1 = a,Ph Ph b,4-MeC 6 H 4 ;Ph c,4-MeOC 6 H 4 ;Ph d, 4-ClC 6 H 4 Ph -HCN Ar 1 =a, Ph b,C 4 H 3 O c,C 4 H 3 S e, Ph; C 4 H 3 O f, 4-MeC 6 H 4 ;C 4 H 3 O g, 4-MeOC 6 H 4 ;C 4 H 3 O h, 4-ClC 6 H 4 C 4 H 3 O i,Ph; C 4 H 3 S j, 4-MeC 6 H 4 ;C 4 H 3 S k, 4-MeOC 6 H 4 ;C 4 H 3 S l , 4-ClC 6 H 4 C 4 H 3 S NN H CN Ph Ar O COR R OO OEt 7a,b 8 O N Ph Ar H 2 N NO COR 9a-h O a,R=Me b,R=Ph NN H O CN Ph Ar COR R R OO R N O Ph Ar H 2 N NR COR 11 10a,b 12a-h a, R=Me b, R=Ph 9,12; Ar=R, a, Ph Me b, 4-MeC 6 H 4 Me c, 4-MeOC 6 H 4 Me d, 4-ClC 6 H 4 Me O CN CN Ph N 1 ArNH 2 Dioxan/Pip. Reflux Dioxan/NaOEt Reflux EtOH/ Reflux 3 H Ar= R= e, Ph Ph f, 4-MeC 6 H 4 Ph g, 4-MeOC 6 H 4 Ph h, 4-ClC 6 H 4 Ph Scheme 1. The derivation of pyrr ole. Preparation of pyrr ol o[ 2,3-b]pyridines 6a-l: To a solution of each of the pyrrole derivatives 2a-d (0.01 mol) in ethanol (20 mL) 0.01 mol of each of (het- ero) benzylidenemonitriles 3a, 3b or 3c was added fol- lowed by few drops of triethylamine as catalyst. The re- action mixture was refluxed for 2 h and then left to cool overnight. The precipitated solids were collected by fil- tration and recrystallized from ethanol/DMF to give the title compounds 6a-l. 4-Amino-3-benzoyl-1,6-diphenyl-1H-pyrrolo[2,3-b] pyridine-5-carbonitrile 6a: Yellow crystalline solid; yield 75% (3.1 g); mp 212˚C - 213˚C. max = 3350, 3230 (NH2), 2210 (CN), 1653 (CO) cm–1; MS: m/z = 414 (M+). δH = 5.56 (s, 2H D2O exch., NH2), 6.85 (s, 1H, pyrrole 2-H), 7.25 - 8.05 (m, 15H, Ar-H); δC = 87.7 (s), 104.4 (s), 106.3 (s), 120.3 (d), 125.4 (d), 127.2 (d), 127.2 (d), 117.3 (s), 127.3 (d), 128.7 (d), 129.0 (d), 129.4 (d), 129.6 (d), 132.9 (d), 133.3 (s), 139.7 (s), 140.5 (s), 154.5 (s), 155.3 (s), 158.7 (s), 187.5 (s). Anal. Calcd for C27H18N4O (414.46): C, 78.24; H, 4.38; N, 13.52. Found: C, 78.30; H, 4.41; N, 13.42. 4-Amino-3-benzoyl-6-phenyl-1-p-tolyl-1H-pyrrolo[2, 3-b]pyridine-5-carbonitrile 6b: Yellow crystalline solid; yield 68% (2.91 g); mp 224˚C - 225˚C. max = 3352, 3235 (NH2), 2212 (CN), 1655 (CO) cm–1; δH = 2.32 (s, 3H, CH3), 5.55 (s, 2H D2O exch., NH2), 6.83 (s, 1H, pyrrole 2-H), 7.26 - 8.00 (m, 14H, Ar-H). Anal. Calcd for C28H20N4O (428.48): C, 78.49; H, 4.70; N, 13.08. Found: C, 78.55; H, 4.52; N, 13.22. 4-Amino-3-benzoyl-1-(4-methoxyphenyl)-6-phenyl-1H- pyrrolo[2,3-b]pyridine-5-carbonitrile 6c: Yellow cry- stalline solid; yield 68% (3.0 g); mp 229˚C - 230˚C. max = 3355, 3238 (NH2), 2215 (CN), 1668 (CO) cm–1; MS: m/z = 444 (M+). δH = 3.76 (s, 3H, CH3), 5.57 (s, 2H D2O exch., NH2), 6.84 - 8.02 (m, 15H, Ar-H). Anal. Calcd for C28H20N4O2 (444.48): C, 75.66; H, 4.54; N, 12.60. Found: C, 75.70; H, 4.58; N, 12.48. Copyright © 2011 SciRes. IJOC  F. M. ABDELRAZEK ET AL. 220 4-Amino-3-benzoyl-1-(4-chlorophenyl)-6-phenyl-1 H-pyrrolo[2,3-b]pyridine-5-carbonitrile 6d: Yellowish green crystalline solid; yield 78% (3.5 g); mp 235˚C - 237˚C. max = 3353, 3235 (NH2), 2213 (CN), 1663 (CO) cm–1; δH = 5.54 (s, 2H D2O exch., NH2), 6.88 (s, 1H, pyrrole 2-H), 7.25 - 8.05 (m, 14H, Ar-H). Anal. Calcd for C27H17ClN4O (448.90): C, 72.24; H, 3.82; N, 12.48. Found: C, 72.30; H, 3.85; N, 12.55. 4-Amino-3-benzoyl-6-(furan-2-yl)-1-phenyl-1H-pyr rolo[2,3-b]pyridine-5-carbonitrile 6e: Yellowish brown powder; yield 65% (2.63 g); mp 169˚C - 170˚C. max = 3328, 3230 (NH2), 2213 (CN), 1658 (CO) cm–1; MS: m/z = 404 (M+). δH = 5.57 (s, 2H D2O exch., NH2), 6.35 - 7.85 (m, 14H, Ar-H). Anal. Calcd for C25H16N4O2 (404.42): C, 74.25; H, 3.99; N, 13.85. Found: C, 74.32; H, 3.95; N, 13.65. 4-Amino-3-benzoyl-6-(furan-2-yl)-1-p-tolyl-1H-pyr rolo[2,3-b]pyridine-5-carbonitrile 6f: Brownish pow- der; yield 63% (2.63 g); mp 173˚C - 175˚C. max = 3330, 3232 (NH2), 2213 (CN), 1660 (CO) cm–1; δH = 2.33 (s, 3H, CH3), 5.62 (s, 2H D2O exch., NH2), 6.32 - 7.82 (m, 13H, Ar-H). Anal. Calcd for C26H18- N4O2 (418.45): C, 74.63; H, 4.34; N, 13.34. Found: C, 74.52; H, 4.45; N, 13.55. 4-Amino-3-benzoyl-1-(4-methoxyphenyl)-6-(furan-2- yl)-1H-pyrrolo[2,3-b]pyridine-5-carbonitrile 6g: Brown powder; yield 67% (2.91 g); mp 178˚C - 179˚C. max = 3335, 3228 (NH2), 2211 (CN), 1663 (CO) cm–1; MS: m/z = 434 (M+). δH = 3.83 (s, 3H, CH3), 5.63 (s, 2H D2O exch., NH2), 6.35 - 7.85 (m, 13H, Ar-H). Anal. Calcd for C26H18N4O3 (434.45): C, 71.88; H, 4.18; N, 12.90. Found: C, 71.92; H, 4.25; N, 13.05. 4-Amino-3-benzoyl-1-(4-chlorophenyl)-6-(furan-2-y l)-1H-pyrrolo[2,3-b]pyridine-5-carbonitrile 6h: Green- ish brown powder; yield 69% (3.0 g); mp 192˚C - 193˚C. max = 3342, 3235 (NH2), 2217 (CN), 1668 (CO) cm–1; δH = 5.65 (s, 2H D2O exch., NH2), 6.37 - 7.88 (m, 13H, Ar-H). Anal. Calcd for C25H15ClN4O2 (438.87): C 68.42; H 3.45; Cl 8.08; N 12.77. Found: C 68.45; H 3.52; Cl 8.18; N 12.85. 4-Amino-3-benzoyl-1-phenyl-6-(thiophen-2-yl)-1H-p yrrolo[2,3-b]pyridine-5-carbonitrile 6i: Yellowish crys- talline solid; yield 72% (3.0 g); mp 174˚C - 175˚C. max = 3329, 3232 (NH2), 2218 (CN), 1654 (CO) cm–1; δH = 5.55 (s, 2H D2O exch., NH2), 6.95 - 7.84 (m, 14H, Ar-H). Anal. Calcd for C25H16N4OS (420.49): C, 71.41; H, 3.84; N, 13.32. Found: C 71.48; H 3.92; N 13.45. 4-Amino-3-benzoyl-6-(thiophen-2-yl)-1-p-tolyl-1H-p yrrolo[2,3-b]pyridine-5-carbonitrile 6j: Brownish fine crystals; yield 68% (2.95 g); mp 181˚C - 182˚C.max = 3333, 3235 (NH2), 2220 (CN), 1667 (CO) cm–1; MS: m/z = 434 (M+). δH = 2.34 (s, 3H, CH3), 5.63 (s, 2H D2O exch., NH2), 6.92 - 7.82 (m, 13H, Ar-H). Anal. Calcd for C26H18N4OS (434.51): C, 71.87; H, 4.18; N, 12.89; S, 7.38. Found: C, 71.95; H, 4.25; N, 12.62; S, 7.52. 4-Amino-3-benzoyl-1-(4-methoxyphenyl)-6-(thiophen -2-yl)-1H-pyrrolo[2,3-b]pyridine-5-carbonitrile 6k: Br- own fine crystals; yield 66% (2.97 g); mp 205˚C - 206˚C. max = 3340, 3238 (NH2), 2215 (CN), 1670 (CO) cm–1; MS: m/z = 450 (M+). δH = 3.78 (s, 3H, CH3), 5.62 (s, 2H D2O exch., NH2), 6.85 - 7.84 (m, 13H, Ar-H). Anal. Calcd for C26H18N4O2S (450.51): C, 69.32; H, 4.03; N, 12.44; S, 7.12. Found: C, 69.38; H, 4.13; N, 12.55; S, 7.25. 4-Amino-3-benzoyl-1-(4-chlorophenyl)-6-(thiophen -2-yl)-1H-pyrrolo[2,3-b]pyridine-5-carbonitrile 6l: Pale greenish brown powder; yield 73% (3.3 g); mp 209˚C - 210˚C. max = 3344, 3238 (NH2), 2221 (CN), 1672 (CO) cm–1; δH = 5.66 (s, 2H D2O exch., NH2), 6.95 - 7.79 (m, 13H, Ar-H). Anal. Calcd for C25H15ClN4OS (454.93): C, 66.00; H, 3.32; Cl, 7.79; N, 12.32; S, 7.05. Found: C, 66.10; H, 3.38; Cl, 8.00; N, 12.45; S, 7.23. Preparation of pyrr ol o[ 2,3-b]pyridines 9a-h: To a solution of each of the pyrrole derivatives 2a-d (0.01 mol) in dioxane (20 mL) 0.01 mol of β-ketoesters 7a or 7b was added followed by few drops of piperidine catalyst. The reaction mixture was refluxed for 2 h then left to cool overnight. The precipitated solids were col- lected by filtration and recrystallized from dioxane to afford the title compounds 9a-h. 5-Acetyl-4-amino-3-benzoyl-1-phenyl-1,7-dihydro- pyrrolo[2,3-b]pyridine-6-one 9a: Yellow crystals; yield 75% (2.78 g); mp 207˚C - 208˚C. max = 3420 - 2235 (br. NH & NH2), 1645, 1653 & 1678 (3CO) cm–1; MS: m/z = 371 (M+). δH = 2.35 (s, 3H, CH3), 5.56 (s, 2H D2O exch., NH2), 6.93 (s, 1H, pyrrole 2-H), 7.28 - 7.85 (m, 10H, Ar-H), 8.22 (s, 1H, NH); δC = 22.4 (q), 111.2 (s), 112.3 (s), 112.4 (s), 119.9 (s), 120.2 (d), 120.6 (s), 125.4 (d), 128.6 (d), 129.2 (d), 129.5 (d), 132.4 (d), 133.3 (s), 140.5 (s), 163.7 (s), 167.4 (s), 186.7 (s), 196.5 (s). Anal. Calcd for C22H17N3O3 (371.39): C, 71.15; H, 4.61; N, 11.31. Found: C, 71.20; H, 4.68; N, 11.50. 5-Acetyl-4-amino-3-benzoyl-1-(p-t olyl)-1,7-dihydro- pyrrolo[2,3-b]pyridine-6-one 9b: Yellow crystals; yield 73% (2.8 g); mp 210˚C - 212˚C. max = 3425 - 2232 (br. NH & NH2), 1646, 1650 & 1673 (3CO) cm–1; MS: m/z = 385 (M+). δH = 2.33 (s, 3H, CH3), 2.36 (s, 3H, CH3), 5.55 (s, 2H D2O exch., NH2), 6.91 (s, 1H, pyrrole 2-H), 7.15 - 7.84 (m, 9H, Ar-H), 8.14 (s, 1H, NH); Anal. Calcd for C23H19N3O3 (385.42): C 71.67; H 4.97; N 10.90. Found: C 71.62; H 5.05; N 10.58. 5-Acetyl-4-amino-3-benzoyl-1-(4-methoxyphenyl)-1, 7-dihydropyrrolo[2,3-b]pyridine-6-one 9c: Yellow cry- stals; yield 74% (2.96 g); mp 199˚C - 201˚C. max = 3428 - 2233 (br. NH & NH2), 1644, 1652 & 1674 (3CO) cm–1; δH = 2.32 (s, 3H, CH3), 3.80 (s, 3H, CH3), 5.57 (s, 2H Copyright © 2011 SciRes. IJOC  221 F. M. ABDELRAZEK ET AL. D2O exch., NH2), 6.84 - 7.82 (m, 10H, Ar-H), 8.58 (s, 1H, NH); Anal. Calcd for C23H19N3O4 (401.41): C, 68.82; H 4.77; N 10.47. Found: C 68.60; H 5.00; N 10.65. 5-Acetyl-4-amino-3-benzoyl-1-(4-chlorophenyl)-1,7- dihydropyrrolo[2,3-b]pyridine-6-one 9d: Greenish yel- low crystalline solid; yield 77% (3.12 g); mp 215˚C - 216˚C. max = 3430 - 2235 (br. NH & NH2), 1640, 1654 & 1673 (3CO) cm–1; δH = 2.34 (s, 3H, CH3), 5.58 (s, 2H D2O exch., NH2), 6.95 (s, 1H, pyrrole 2-H), 7.18 - 7.80 (m, 9H, Ar-H), 8.65 (s, 1H, NH); Anal. Calcd for C22H16ClN3O3 (405.83): C 65.11; H 3.97; Cl 8.74; N 10.35. Found: C 65.20; H 4.07; Cl 8.65; N 10.25. 4-Amino-3,5-dibenzoyl-1-phenyl-1,7-dihydropyrrol o[2,3-b]pyridine-6-one 9e: Dark yellow crystals; yield 75% (3.25 g); mp 237˚C - 238˚C. max = 3460 - 3235 (br. NH & NH2), 1642, 1653 & 1655 (3CO) cm–1; MS: m/z = 433 (M+). δH = 6.95 (s, 1H, pyrrole 2-H), 7.25 - 7.84 (m, 15H, Ar-H), 8.15 (s, 2H D2O exch., NH2), 10.4 (s, 1H D2O exch., NH); Anal. Calcd for C27H19N3O3 (433.46: C, 74.81; H, 4.42; N, 9.69. Found: C, 74.70; H, 4.45; N, 9.55. 4-Amino-3,5-dibenzoyl-1-(p-tolyl)-1,7-dihydropyr- rolo[2,3-b]pyridine-6-one 9f: Brownish yellow powder; yield 65% (2.90 g); mp 220˚C - 222˚C. max = 3455 - 3228 (br. NH & NH2), 1647, 1655 & 1658 (3CO) cm–1; MS: m/z = 447 (M+). δH = 2.36 (s, 3H, CH3), 6.94 (s, 1H, pyrrole 2-H), 7.12 - 7.84 (m, 14H, Ar-H), 8.18 (s, 2H D2O exch., NH2), 10.6 (s, 1H D2O exch., NH); Anal. Calcd for C28H21N3O3 (447.48): C, 75.15; H, 4.73; N, 9.39. Found: C, 74.90; H, 4.50; N, 9.52. 4-Amino-3,5-dibenz oyl-1-(4-methoxyphenyl )-1,7-di- hydropyrrolo[2,3-b]pyridine-6-one 9g: Brownish yel- low powder; yield 64% (2.96 g); mp 210˚C - 212˚C. max = 3455 - 3228 (br. NH & NH2), 1644, 1655 & 1658 (3CO) cm–1; δH = 2.36 (s, 3H, CH3), 6.94 (s, 1H, pyrrole 2-H), 7.12 - 7.84 (m, 14H, Ar-H), 8.18 (s, 2H D2O exch., NH2), 10.6 (s, 1H D2O exch., NH); Ana l. Calcd for C28H21N3O4 (463.48): C, 72.56; H, 4.57; N, 9.07. Found: C, 72.72; H, 4.52; N, 9.22. 4-Amino-3,5-dibenzoyl-1-(4-chlorophenyl)-1,7-dihyd- ropyrrolo[2,3-b]pyridine-6-one 9h: Greenish yellow powder; yield 66% (3.1 g); mp 227˚C - 229˚C. max = 3455 - 3228 (br. NH & NH2), 1646, 1654 & 1660 (3CO) cm–1; δH = 2.34 (s, 3H, CH3), 6.94 (s, 1H, pyrrole 2-H), 7.16 - 7.83 (m, 14H, Ar-H), 8.18 (s, 2H D2O exch., NH2), 10.65 (s, 1H D2O exch., NH); Anal. Calcd for C27H18- ClN3O3 (467.90): C 69.31; H 3.88; Cl 7.58; N 8.98. Found: C 69.35; H 3.95; Cl 7.74; N 9.08. Synthesis of pyrrolo[2,3-b]pyridines 12a-h: To a solution of each of the pyrrole derivatives 2a-d (0.01 mol) in dioxane (20 mL) 0.01 mol of each of β-diketones 10a or 10b was added followed by a cata- lytic amount of sodium ethoxide. The reaction mixture was refluxed for 3 h and then left to cool to room tem- perature. The reaction mixture was then poured on ice cold water and neutralized with few drops of conc. HCl (pH 7). The precipitated solids were collected by filtra- tion and recrystallized from ethanol/DMF to afford the title compounds 12a-h. 5-Acetyl-4-amino-3-benzoyl-6-methyl-1-pheny l-1H- pyrrolo[2,3-b]pyridine 12a: Yellow flakes; yield 72% (2.66 g); mp 230˚C - 231˚C. max = 3355 & 3228 (NH2), 1655 & 1662 (2CO) cm–1; MS: m/z = 369 (M+). δH = 2.44 (s, 3H, CH3), 2.50 (s, 3H, CH3), 6.92 (s, 1H, pyrrole 2-H), 7.25 - 7.85 (m, 10H, Ar-H), 8.22 (s, 2H D2O exch., NH2); δC = 14.3(q), 22.6 (q), 102.7(s), 107.2 (s), 111.2(s), 120.3(d), 125.3(d), 128.3(d), 128.7(d), 129.2(d), 129.6(d), 132.5(d), 133.4(s), 140.5(s), 147.2(s), 148.7(s), 153.8(s), 185.3(s), 195.5(s). Anal. Calcd for C23H19N3O2 (369.42): C, 74.78; H, 5.18; N, 11.37. Found: C, 74.85; H, 5.22; N, 11.50. 5-Acetyl-4-amino-3-benzoyl-6-methyl-1-(p-tolyl)-1 H-pyrrolo[2,3-b]pyridine 12b: Dark yellow powder; yield 68% (2.6 g); mp 237˚C - 239˚C. max = 3358 & 3232 (NH2), 1656 & 1665 (2CO) cm–1; δH = 2.37 (s, 3H, CH3), 2.46 (s, 3H, CH3), 2.52 (s, 3H, CH3), 6.88 (s, 1H, pyrrole 2-H), 7.05 - 7.82 (m, 9H, Ar-H), 8.25 (s, 2H D2O exch., NH2); Anal. Calcd for C24H21N3O2 (383.44): C, 75.18; H, 5.52; N, 10.96. Found: C, 75.35; H, 5.28; N, 10.75. 5-Acetyl-4-amino-3-benzoyl-1-(4-methoxyphenyl) -6 -methyl-1H-pyrrolo[2,3-b]pyridine 12c: Brownish ye- llow powder; yield 66% (2.63 g); mp 245˚C - 246˚C. max = 3354 & 3222 (NH2), 1653 & 1660 (2CO) cm–1; MS: m/z = 399 (M+). δH = 2.45 (s, 3H, CH3), 2.50 (s, 3H, CH3), 3.77 (s, 3H, CH3), 6.79 - 7.84 (m, 10H, Ar-H), 8.35 (s, 2H D2O exch., NH2); Anal. Calcd for C24H21- N3O3 (399.44): C, 72.16; H, 5.30; N, 10.52. Found: C, 72.32; H, 5.25; N, 10.70. 5-Acetyl-4-amino-3-benzoyl-1-(4-chlorophenyl)-6- methyl-1H-pyrrolo[2,3-b]pyridine 12d: Lemon yellow powder; yield 70% (2.82 g); mp 257˚C - 259˚C. max = 3355 & 3226 (NH2), 1648 & 1656 (2CO) cm–1; δH = 2.48 (s, 3H, CH3), 2.52 (s, 3H, CH3), 6.93 (s, 1H, pyrrole 2-H), 7.15 - 7.84 (m, 9H, Ar-H), 8.24 (s, 2H D2O exch., NH2); Anal. Calcd for C23H18ClN3O2 (403.86): C, 68.40; H, 4.49; Cl, 8.78; N, 10.40. Found: C, 68.48; H, 4.55; Cl, 8.85; N, 10.50. 4-Amino-3,5-dibenzoy l-1,6-diphenyl-1H-pyrrolo[2, 3-b]pyridine 12e: Reddish yellow powder; yield 76% (3.75 g); mp 272˚C - 273˚C. max = 3345 & 3186 (NH2), 1642 & 1650 (2CO) cm–1; MS: m/z = 493 (M+). δH = 6.92 (s, 1H, pyrrole 2-H), 7.18 - 7.85 (m, 20H, Ar-H), 8.44 (s, 2H D2O exch., NH2); Anal. Calcd for C33H23N3O2 (493.55): C, 80.31; H, 4.70; N, 8.51. Found: C, 80.35; H, 4.78; N, 8.65. Copyright © 2011 SciRes. IJOC  F. M. ABDELRAZEK ET AL. 222 4-Amino-3,5-dibenzoyl-6-phenyl-1-p-tolyl-1H-pyrr- olo[2,3-b]pyridine 12f: Red powder; yield 72% (3.65 g); mp 279˚C - 280˚C. max = 3346 & 3192 (NH2), 1640 & 1652 (2CO) cm–1; δH = 2.30 (s, 3H, CH3), 6.95 (s, 1H, pyrrole 2-H), 7.15 - 7.82 (m, 19H, Ar-H), 8.39 (s, 2H D2O exch., NH2); Anal. Cal- cd for C34H25N3O2 (507.58): C, 80.45; H, 4.96; N, 8.28. Found: C, 80.36; H, 4.80; N, 8.60. 4-Amino-3,5-dibenzoyl-1-(4-methoxyphenyl)-6-phen yl-1H-pyrrolo[2,3-b]pyridine 12g: Red powder; yield 72% (3.76 g); mp 290˚C - 292˚C. max = 3348 & 3222 (NH2), 1641 & 1655 (2CO) cm–1; δH = 3.80 (s, 3H, CH3), 6.78 - 7.83 (m, 20H, Ar-H), 8.22 (s, 2H D2O exch., NH2); Anal. Calcd for C34H25N3O3 (523.58): C, 77.99; H, 4.81; N, 8.03. Found: C, 80.76; H, 4.80; N, 8.20. 4-Amino-3,5-dibenzoyl-1-(4-chlorophenyl)-6-pheny l-1H-pyrrolo[2,3-b]pyridine 12h: Crimson red powder; yield 73% (3.85 g); mp 305˚C - 307˚C. max = 3354 & 3229 (NH2), 1652 & 1658 (2CO) cm–1; δH = 6.91 (s, 1H, pyrrole 2-H), 7.18 - 7.83 (m, 19H, Ar-H), 8.36 (s, 2H D2O exch., NH2); Anal. Calcd for C33H22ClN3O2 (528.00): C, 75.07; H, 4.20; Cl, 6.71; N, 7.96. Found: C, 75.00; H, 4.25; Cl, 6.78; N, 7.75. 4. Conclusions A rapid and concise synthesis of three new series of highly functionalized pyrrolo[2,3-b]pyridines was de- scribed as occurring with satisfactory to good yields. They were offset by the simplicity of our strategy to- gether with the structural profits gained. The envisaged biological activity of compounds is in progress. 5. Acknowledgements The continuous help and support of the Alexander von Humboldt-Foundation (Germany) to F. M. Abdelrazek through granting short research fellowships is greatly acknowledged. The kind hospitality of Professor Peter Metz, Institut für Organische Chemie, TU Dresden is also highly appreciated. 6. References [1] J. J. Kulagowski, H. B. Broughton, N. R. Curtis, I. M. Mawer, M. P. Ridgill, R. Baker, F. Emms, S. B. Freed- man, R. Marwood, S. Patel, C. I. Ragan and P. D. Leeson, “3[4-(4-Chlorophenyl)-piperazin-1-yl]-1H-pyrrolo[2,3-b] Pyridine: An Antagonist with High Affinity and Selectiv- ity for the Human Dopamine D-4 Receptor,” Journal of Medicinal Chemistry, Vol. 39, No. 10, 1996, pp. 1941- 1942. doi:10.1021/jm9600712 [2] J. R. Henry, K. C. Rupert, J. H. Dodd, I. J. Turchi, S. A. Wadsworth, D. E. Cavender, B. Fahmy, G. C. Olini, J. E. Davis, J. L. P. Genesy, P. H. Schafer and J. J. Siekierka, “6-Amino-2-(4-fluorophen-yl)-4-metho-xy-3-(4-pyridyl)- 1H-pyrrolo[2,3-b]pyridine (RNJ 68354): A Potent and Selective P-38 Kinase Inhibitor,” Journal of Medicinal Chemistry, Vol. 41, No. 22, 1998, pp. 4196-4198. doi:10.1021/jm980497b [3] I. A. Schepetkin, A. I. Khlebnikov and M. T. Quinn, “N- Benzoylpyrazoles Are Novel Small-Molecule Inhibitors of Human Neutrophil Elastase,” Journal of Medicinal Chemistry, Vol. 50, No. 20, 2007, pp. 4928-4938. doi:10.1021/jm070600+ [4] A. Miszke, H. Foks, A. Kedazia, E. Kwapisz and Z. Zwolska, “The Synthesis and Microbiological Activity of 2-Mercapto-4-(pyrrolidin-1-yl)pyridine Derivatives,” He- terocycles, Vol. 75, No. 9, 2008, pp. 2251-2261. doi:10.3987/COM-08-11390 [5] F. M. Abdelrazek, P. Metz, N. H. Metwally and S. F. El-Mahrouky, “Synthesis and Molluscicidal Activity of New Cinnoline and Pyrano[2,3-c]pyrazole Derivatives,” Archiv der Pharmazie, Vol. 339, No. 8, 2006, pp. 456- 460. [6] F. M. Abdelrazek, P. Metz, O. Kataeva, A. Jaeger and S. F. El-Mahrouky, “Synthesis and Molluscicidal Activity of Some New Chromene, Pyrano[2,3-c]pyrazole Deriva- tives,” Archiv der Pharmazie, Vol. 340, No. 10, 2007, pp. 543-548. doi:10.1002/ardp.200700157 [7] A. A. Fadda, F. M. Abdelrazek, K. S. Mohamed, H. M. M. Ghieth and H. A. Etman, “Synthetic Applications of Benzothiazole Containing Cyanoacetyl Group,” Euro- pean Journal of Chemistry, Vol. 1, No. 2, 2010, pp. 90-95. doi:10.5155/eurjchem.1.2.90-95.32 [8] F. M. Abdelrazek, A. A. Fadda and A. N. Elsayed, “A Novel Synthesis of Some New Pyridazine and Pyridaz- ino[4,5-d]pyridazine Derivatives,” Synthetic Communica- tions, Vol. 41, No. 8, 2011, pp. 1119-1126. doi:10.1080/00397911003797809 [9] F. M. Abdelrazek, A. W. Erian and K. M. H. Hilmy, “Ni- triles in Heterocyclic Synthesis. A Novel Synthesis of 4-Phenacylpyrazole and Pyrrolo[2,3-c]pyrazole Deriva- tives,” Synthesis, Vol. 1986, No. 1, 1986, pp. 74-75. doi:10.1055/s-1986-31484 [10] F. M. Abdelrazek and A. A. Fadda, “Nitriles in Hete Cy- clic Synthesis: A Novel Synthesis of Polyfunctionally Substituted Pyrrole Derivatives,” Zeitschrift für Natur- forschung, Vol. 41B, 1986, pp. 499-501. [11] F. M. Abdelrazek, A. W. Erian and A. M. Torgoman, “Some Reactions with ω-Bromoacetophenone: Novel Synthesis of Poly-Substituted Pyrrole, Furan, Pyridine and Pyridazine Derivatives,” Chemistry & Industry, Vol. 1, 1988, pp. 30-32. [12] F. M. Abdelrazek, Z. E. Kandeel and A. M. Salah, “Some Reactions with ω-Bromoacetophenone: Synthesis of Δαβ- Butenolide and Its Transformation into Pyrrole Deriva- tives,” Heteroatom Chemistry, Vol. 6, No. 1, 1995, pp. 77-80. doi:10.1002/hc.520060116 [13] F. M. Abdelrazek and M. S. Bahbouh, “Heterocyclic Synthesis with Nitriles: Synthesis of Some Novel Pyrrole, Copyright © 2011 SciRes. IJOC  F. M. ABDELRAZEK ET AL. Copyright © 2011 SciRes. IJOC 223 pyrrolo[1,2-a]-quinazoline and Pyrrolo[1,2-a]triazine De- rivatives,” Phosphorous, Sulphur & Silicon, Vol. 116, 1996, pp. 235-241 [14] N. H. Metwally and F. M. Abdelrazek, “Heterocyclic Synthesis with Nitriles: Synthesis of Some Novel Pyr- rolo[2,1-b]thiadiazoline, Pyrrolo[2,1-b]thiadiazolo[3,2-a] pyrimidine and Pyridine Derivatives,” Journal für Prak- tische Chemie/Chemiker-Zeitung, Vol. 340, No. 7, 1998, pp. 676-678. doi:10.1002/prac.19983400713 [15] F. M. Abdelrazek, “Heterocyclic Synthesis with Nitriles: Synthesis of Some New Thiophene, Pyridazine, Oxazine, Thiopyran, Pyrrole and Pyrrolo[1,2-b]pyridazine Deriva- tives,” Synthetic Communications, Vol. 35, No. 17, 2005, pp. 2251-2258. doi:10.1080/00397910500184727 [16] F. M. Abdelrazek and N. H. Metwally, “Synthesis of Some New N-Substituted-pyrroles, pyrrolo[1,2-a]-quina- zoline and Diaza-as-Indacene Derivatives,” Synthetic Com- munications, Vol. 36, No. 1, 2006, pp. 83-89. doi:10.1080/00397910500330213 [17] K. M. H. Hilmy, “Synthesis of New Pyrrolo-[2,3-b]pyr- idines as a Potent Inhibitor of Tumor Necrosis Factor Alpha,” Archiv der Pharmazie, Vol. 337, No. 1, 2004, pp. 15-19. doi:10.1002/ardp.200300773 [18] F. M. Abdelrazek and N. H. Metwally, “Novel Syn The- sis of N-Arylpyrrole, Pyrrolo[1,2-a]quinazoline and Pyr- rolo[3,4-d]pyridazine Derivatives,” Synthetic Communi- cations, Vol. 39, 2009, pp. 4088-4099 [19] E. C. Taylor and A. McKillop, “The Chemistry of Cy Clic Enaminonitriles and o-Aminonitriles,” John Wiley & Sons, New York, 1970.
|