K. D. KLIKA
216
oriented b onds, r espectively, are r eplaced analogously by
a hollow wedge bond and a hashed wedge bond with a
line through it, respectively3. For example, if the relative
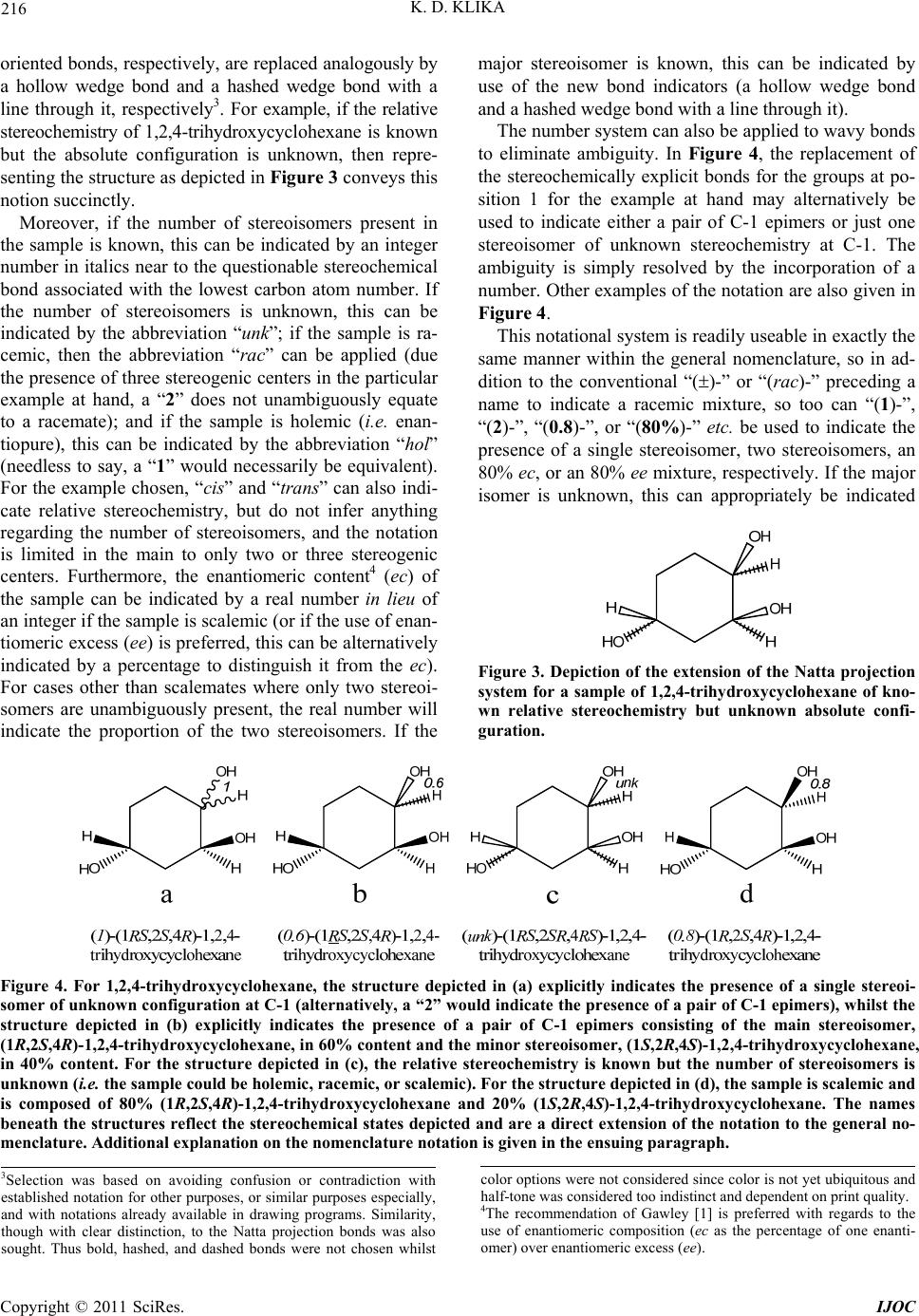
stereochemistry of 1,2,4-trihydroxycyc lohexane is known
but the absolute configuration is unknown, then repre-
senting the structure as depicted in Figure 3 conveys this
notion succinctly.
Moreover, if the number of stereoisomers present in
the sample is known, this can be indicated by an integer
number in italics near to the questionable stereochemical
bond associated with the lowest carbon atom number. If
the number of stereoisomers is unknown, this can be
indicated by the abbreviation “unk”; if the sample is ra-
cemic, then the abbreviation “rac” can be applied (due
the presence of three stereogenic centers in the particular
example at hand, a “2” does not unambiguously equate
to a racemate); and if the sample is holemic (i.e. enan-
tiopure), this can be indicated by the abbreviation “hol”
(needless to say, a “1” would necessarily be equivalent).
For the example chosen, “cis” and “trans” can also indi-
cate relative stereochemistry, but do not infer anything
regarding the number of stereoisomers, and the notation
is limited in the main to only two or three stereogenic
centers. Furthermore, the enantiomeric content4 (ec) of
the sample can be indicated by a real number in lieu of
an integer if the sample is scalemic (or if the use of enan-
tiomeric excess (ee) is preferred, this can be alternatively
indicated by a percentage to distinguish it from the ec).
For cases other than scalemates where only two stereoi-
somers are unambiguously present, the real number will
indicate the proportion of the two stereoisomers. If the
major stereoisomer is known, this can be indicated by
use of the new bond indicators (a hollow wedge bond
and a hashed wedge bond with a line through it).
The number system can also be applied to wavy bonds
to eliminate ambiguity. In Figure 4, the replacement of
the stereochemically explicit bonds for the groups at po-
sition 1 for the example at hand may alternatively be
used to indicate either a pair of C-1 epimers or just one
stereoisomer of unknown stereochemistry at C-1. The
ambiguity is simply resolved by the incorporation of a
number. Other examples of the notation are also given in
Figure 4.
This notational system is readily useab le in exactly the
same manner within the general nomenclature, so in ad-
dition to the conventional “()-” or “(ra c)-” preceding a
name to indicate a racemic mixture, so too can “(1)-”,
“(2)-”, “(0.8)-”, or “(80%)-” etc. be used to indicate the
presence of a single stereoisomer, two stereoisomers, an
80% ec, or an 80% ee mixture, respectively. If the major
isomer is unknown, this can appropriately be indicated
Figure 3. Depiction of the extension of the Natta projection
system for a sample of 1,2,4-trihydroxycyclohexane of kno-
wn relative stereochemistry but unknown absolute confi-
guration.
Figure 4. For 1,2,4-trihydroxycyclohexane, the structure depicted in (a) explicitly indicates the presence of a single stereoi-
somer of unknown configuration at C-1 (alter natively, a “2” would indicate the presence of a pair of C-1 epimers), whilst the
structure depicted in (b) explicitly indicates the presence of a pair of C-1 epimers consisting of the main stereoisomer,
(1R,2S,4R)-1,2,4-trihydroxycyclohexane, in 60% content and the minor stereoisomer, (1S,2R,4S)-1,2,4-trihydroxycyclohexane,
in 40% content. For the structure depicted in (c), the relative stereochemistry is known but the number of stereoisomers is
unknown (i.e. the sample could be holemic, racemic, or scalemic). For the structure depicted in (d), the sample is scalemic and
is composed of 80% (1R,2S,4R)-1,2,4-trihydroxycyclohexane and 20% (1S,2R,4S)-1,2,4-trihydroxycyclohexane. The names
beneath the structures reflect the stereochemical states depicted and are a direct extension of the notation to the general no-
menclature. Additional explanation on the nomenclature notation is given in the ensuing paragraph.
color options were not considered since color is not yet ubiquitous and
half-tone was considered too in d i stinct and dependent on print quality.
4The recommendation of Gawley [1] is preferred with regards to the
use of enantiomeric composition (ec as the percentage of one enanti-
omer) over enantiomeric excess (ee).
3Selection was based on avoiding confusion or contradiction with
established notation for other purposes, or similar purposes especially,
and with notations already available in drawing programs. Similarity,
though with clear distinction, to the Natta projection bonds was also
sought. Thus bold, hashed, and dashed bonds were not chosen whils
Copyright © 2011 SciRes. IJOC