 International Journal of Organic Chemistry, 2011, 1, 191-201 doi:10.4236/ijoc.2011.14028 Published Online December 2011 (http://www.SciRP.org/journal/ijoc) Copyright © 2011 SciRes. IJOC 191 Investigation of the Role of Electrogenerated N-Heterocyclic Carbene in the Staudinger Synthesis in Ionic Liquid Marta Feroci Department of Foundamental and Applied Sciences for Engineering (SBAI), Sapienza University of Rome, Rome, Italy E-mail: marta.feroci@uniroma1.it Received November 5, 2011; revised December 11, 2011; accepted December 24, 2011 Abstract Electrogenerated N-heterocyclic carbene (NHC), obtained by cathodic reduction of Bmim-BF4, behaves as organocatalyst and base in the Staudinger synthesis from an acyl chloride and a deactivated imine in ionic liquid to yield -lactams. The effect of many parameters (temperature, amount of electricity, substituents, presence of an external base) has been evaluated and a tentative mechanism for the Staudinger synthesis in a very polar medium like the ionic liquid reported. The yields of isolated -lactams are good, starting from non-electrophilic imines, and predominantly trans lactams are obtained with a good diastereomeric ratio. Keywords: Electrosynthesis, NHC, Staudinger Synthesis, -Lactams, Organocatalyst, Ionic Liquid 1. Introduction The use of ionic liquids (ILs) [1-3] as solvents in organic reactions has, during the last two decades, undergone a rapid acceleration as these salts can, in many cases, sub- stitute the classical organic solvents (VOCs), which can be considered air-pollutant due to their volatility. In fact, ionic liquids have a virtually null vapour pressure and this characteristic leads to their easy recycling. One of the most studied and used class of ILs in or- ganic chemistry is the imidazolium based one, due to its air and water-stability, low cost and wide temperature window of the liquid phase [4,5]. Nevertheless, these ILs are not mere solvents. In fact, recently the “non inno- cent” nature of imidazolium ILs has been described by many authors, [6,7] as, in particular experimental condi- tions, these cations can give rise to the formation of N- heterocyclic carbenes (NHC). NHC can be easily pre- pared by deprotonation of imidazolium cations [8] not substituted in the 2-position (Scheme 1) [9-17]. N-Heterocyclic carbenes have been frequently used in organic chemistry as ligands and, more recently, as orga- nocatalysts [18-21] in many reactions, such as the annu- lation of enals and sulfonylimines, [10,11] the Aza-Mo- rita-Baylis-Hillman reaction of cyclic enones and N-to- sylimines, [12] the benzoin condensation, [9,13] the Stet- ter reaction, [9,13] the Mannich Reaction [15] and the Staudinger reaction [16,17]. These stabilized singlet car- benes behave as nucleophilic organic catalysts, due to the presence of p-donor heteroatoms adjacent to the divalent carbon atom [22]. ILs can be conveniently used in electrochemistry as valid substituents of common solvent-supporting elec- trolyte systems, as they are molten salts (and so, electro- lytes) [23]. Electrochemistry can also be useful in the ge- neration of NHCs from the corresponding IL. In fact, the monoelectronic cathodic reduction of an imidazolium cation leads to the formation of the corresponding car- bene and molecular hydrogen (Scheme 2) [19,24-26]. Scheme 1. Chemical generation of NHCs. Scheme 2. Electrochemical generation of NHCs.  M. FEROCI 192 Many reactions have been successfully catalyzed by electrogenerated NHC, such as the Henry reaction, the N-functionalization of benzoxazolones or oxazolidinones, the benzoin condensation, the Stetter reaction and the cyc- lization of linear amides to -lactams [26,27]. -Lactams are well known molecules whose impor- tance spreads over many fields, from industrial and che- mical to pharmaceutical and biological [28,29]. There are many reactions to form the azetidin-2-one ring, but pro- bably the most important is the Staudinger synthesis. It is described as a [2 + 2] ketene-imine cycloaddition (Sche- me 3) [30]. Although reported for the first time in 1907, its me- chanism is still now uncertain and object of many studies. [31-34]. Both ketene and imine are species that can act as either nucleophiles or electrophiles, depending on their substituents, so the mechanism and outcome (cis or trans -lactams) depend on the structure of the reagents. How- ever, usually this is a reaction that needs catalysis. [35-37] Among the catalysts, NHCs have been recently used in the Staudinger synthesis [10,16,17,38-40] starting from highly electrophilic imines (e.g., N-Boc, N-Ts and N-pNs imines). These reactions are successfully carried out in classical VOCs, using a disubstituted ketene (pre-pre- pared) and an electrophilic imine, in the presence of an NHC as organocatalyst. As reported by Smith and coworkers, [17] the order of addition of reactants is critical to the successful genear- tion of -lactams, the right order being ketene+NHC and then the imine. In fact, it seems that in these conditions (disubstituted ketene and electrophilic imine in aprotic solvent) a NHC activation of ketene is probable, as con- firmed by Ye and coworkers, [16] while the reaction NHC-electrophilic imine leads to a stable adduct (in some cases isolated). On the other hand, Wilhelm and cowork- ers [38] add all reagents (N-pNs imine, ketene and NHC) in toluene and obtain the desired -lactam, proposing that both activation of ketene and of imine are, in principle, possible and their studies could not rule out one of them. To the best of my knowledge, only two papers have been published in which the Staudinger synthesis has been carried out in ionic liquids, the first using ytter- bium(III) triflate [41] as catalyst in N-butylpyridinium Scheme 3. The Staudinger synthe sis. tetrafluoroborate, while the second reports the use of an IL-supported imine, [42] an acyl chloride and triethyl- amine in 1-butyl-3-methylimidazolium hexafluorophos- phate; in no case an NHC was used as an organocatalyst in IL. In a previous short communication, [43] our first re- sults on a Staudinger synthesis in ionic liquids, from imi- ne and acyl chloride, catalyzed by an electrogenerated NHC were described. Here extension of the method and a hypothesis of mechanism is reported. In this case, 1- butyl-3-methylimidazolium tetrafluoroborate (Bmim-BF4) acts both as a solvent and as a precatalyst. 2. Experimental General Procedure Constant current electrolyses were carried out using a glass two-compartment home-made cell. Anolyte (ca. 0.5 ml) and catholyte (ca. 1.5 ml) were separated through a glass disk (porosity 4). The electrode apparent surface areas were 1.0 cm2 for the cathodic Pt spiral (99.9%) and 0.8 cm2 for the anodic Pt spiral (99.9%). The current density was 15 mA/cm2. Electrolyses were carried out at 60˚C, under nitrogen atmosphere, using BMIM-BF4 as anolyte and catholyte. After the consumption of the nu- mber of Faradays per mol of imine reported in Tables 1 and 2, the current was switched off and imine (1 mmol) was added to the catholyte under stirring; when the dis- solution was complete, phenylacetyl chloride (1 mmol) was added. The mixture was kept at 60˚C for 2 h. In the cases in which triethylamine was necessary (see Tables 1 and 2), NEt3 was added to the catholyte with the imine. The catholyte was extracted with diethyl ether, the sol- vent was removed under vacuum and the residue was an- alyzed by 1H-NMR and purified by flash-chromato- graphy, affording the corresponding pure -lactam. All -lactams are known compounds and gave spectral data in accordance with the ones reported in the literature. Recycling of the catholyte: after the ethereal extraction, the catholyte was kept under reduced pressure at 60˚C for 1 hr to eliminate completely diethyl ether traces, then it was reused for a new electrolysis. Trans 1,3,4-Triphenylazetidin-2-one 2a. [44] 1H NMR (200 MHz, CDCl3): 7.38 - 7.21 (m, 14H), 7.09 - 7.02 (m, 1H), 4.95 (d, J = 2.5 Hz, 1H), 4.27 (d, J = 2.5 Hz, 1H). 13C NMR (50 MHz, CDCl3): 165.6, 137.5, 137.4, 134.7, 129.3, 129.1, 129.0, 128.6, 127.9, 127.4, 125.9, 124.0, 117.2, 65.1, 63.7. C21H17NO: calcd. C 84.25, H 5.72, N 4.68; found C 83.84, H 6.03, N 4.52. Trans 1-(4-Methoxyphenyl)-3,4-diphenylazetidin-2- one 2b. [44] 1H NMR (200 MHz, CDCl3): 7.36 - 7.24 (m, 12H), 6.78 (d, J = 9.2 Hz, 2H), 4.89 (d, J = 2.2 Hz, Copyright © 2011 SciRes. IJOC  M. FEROCI 193 1H), 4.24 (d, J = 2.2 Hz, 1H), 3.73 (s, 3H). 13C NMR (50 MHz, CDCl3): 164.9, 156.1, 137.6, 134.8, 131.0, 129.2, 129.0, 128.6, 127.8, 127.4, 125.9, 118.5, 114.3, 65.1, 63.8, 55.4. C22H19NO2: calcd. C 80.22, H 5.81, N 4.25; found C 80.04, H 6.12, N 4.21. Trans 1,4-bis(4-methoxyphenyl)-3-phenylazetidin-2- one 2c. [44] 1H NMR (200 MHz, CDCl3): 7.36 - 7.27 (m, 9H), 6.93 (d, J = 8.8 Hz, 2H), 6.83 (d, J = 9.2 Hz, 2H), 4.88 (d, J = 2.4 Hz, 1H), 4.25 (d, J = 2.4 Hz, 1H), 3.83 (s, 3H), 3.77 (s, 3H). 13C NMR (50 MHz, CDCl3): 165.2, 159.9, 156.1, 135.0, 131.1, 129.4, 129.0, 127.8, 127.5, 127.3, 118.6, 114.7, 114.3, 65.2, 63.6, 55.4, 55.3. C23H21NO3: calcd. C 76.86, H 5.89, N 3.90; found C 76.67, H 6.11, N 3.77. Trans 1-(4-methoxyphenyl)-4-(4-nitrophenyl)-3-ph- enyl azetidin-2-one 2d. [45] 1H NMR (200 MHz, CDCl3): 8.28 (d, J = 8.7 Hz, 2H), 7.57 (d, J = 8.7 Hz, 2H), 7.41 - 7.24 (m, 7H), 6.84 (d, J = 9.0 Hz, 2H), 5.03 (d, J = 2.6 Hz, 1H), 4.26 (d, J = 2.6 Hz, 1H), 3.78 (s, 3H). 13C NMR (50 MHz, CDCl3): 164.3, 156.6, 148.1, 144.9, 134.0, 133.3, 129.4, 128.6, 127.5, 126.9, 124.6, 118.5, 116.3, 65.3, 62.9, 55.5. C22H18N2O4: calcd. C 70.58, H 4.85, N 7.48; found C 70.41, H 4.93, N 7.32. 3,3-dichloro-1-(4-methoxyphenyl)-4-phenylazetidin -2-one 2e. [46] 1H NMR (200 MHz, CDCl3): 7.46 - 7.43 (m, 3H), 7.35 - 7.26 (m, 4H), 6.86 - 6.82 (m, 2H), 5.48 (s, 1H), 3.78 (s, 3H). 13C NMR (50 MHz, CDCl3): 157.9, 157.3, 131.8, 129.9, 129.3, 129.2, 127.8, 119.5, 114.6, 84.2, 74.1, 55.5. C16H13Cl2NO2: calcd. C 59.65, H 4.07, N 4.35; found C 59.48, H 4.13, N 4.22. Trans 1-benzyl-3,4-diphenylazetidin-2-one 2f. [47] 1H NMR (200 MHz, CDCl3): 7.43 - 7.20 (m, 15H), 4.99 (d, J = 14.8 Hz, 1H), 4.37 (d, J = 2.2 Hz, 1H), 4.22 (d, J = 2.2 Hz, 1H), 3.85 (d, J = 14.8 Hz, 1H). 13C NMR (50 MHz, CDCl3): 168.3, 137.2, 135.6, 135.0, 129.1, 128.9, 128.8, 128.7, 128.6, 127.8, 127.6, 127.4, 126.5, 65.2, 63.1, 44.6. C22H19NO: calcd. C 84.31, H 6.11, N 4.47; found C 84.17, H 6.23, N 4.32. 1-benzyl-3,3-dichloro-4-phenylazetidin-2-one 2g. [46] 1H NMR (200 MHz, CDCl3): 7.47 - 7.44 (m, 3H), 7.36 - 7.33 (m, 3H), 7.28 - 7.23 (m, 2H), 7.18 - 7.13 (m, 2H), 4.98 (d, J = 14.8 Hz, 1H), 4.85 (s, 1H), 3.96 (d, J = 14.8 Hz, 1H). 13C NMR (50 MHz, CDCl3): 161.9, 133.5, 131.7, 129.9, 129.1, 128.8, 128.4, 128.1, 128.0, 84.9, 73.3, 45.0. C16H13Cl2NO: calcd. C 62.76, H 4.28, N 4.57; found C 62.68, H 4.32, N 4.51. Trans 3,4-diphenyl-1-p-tolylazetidin-2-one 2h. [48] 1H NMR (200 MHz, CDCl3): 7.41 - 7.36 (m, 10H), 7.28 - 7.24 (m, 2H), 7.10 - 7.06 (m, 2H), 4.94 (d, J = 2.6 Hz, 1H), 4.27 (d, J = 2.6 Hz, 1H), 2.29 (s, 3H). 13C NMR (50 MHz, CDCl3): 165.3, 137.7, 134.9, 134.8, 133.7, 129.6, 129.3, 129.0, 128.6, 127.9, 127.5, 125.9, 117.2, 65.1, 63.7, 20.9. C22H19NO: calcd. C 84.31, H 6.11, N 4.47; found C 84.02, H 6.13, N 4.32. Reaction of electrogenerated 1-butyl-3-methylimi- dazol-2-ylidene with imine 1b. Preparative electrolysis was carried out as previously described and the current was switched off after 97 C (1 mF). Then, 1 mmol of 4-benzylidene-4-methoxyaniline 1b was added and the catholyte was kept under stirring at 60˚C for two hours. Then the usual workup gave a crude that was purified by crystallization from hexane. The mother liquor gave a mixture of imine 1b and dimer 1- butyl-2-(1-butyl-2,3-dihydro-3-methyl-1H-imidazol-2-yl) -2,3-dihydro-3- methyl-1H-imidazole 5 (every attempt to purify 5 led to its decomposition) and column chromato- graphy of the precipitate giave N-(4-methoxy phenyl) benzamide 3 and N-((1-butyl-1H-imidazol-2-yl) (phenyl) methylene)-4-methoxybenzenamine 4. N-(4-methoxyphenyl)benzamide 3. [49] 1H NMR (200 MHz, CDCl3): 7.90 - 7.85 (m, 2H), 7.8 (bs, 1H), 7.59 - 7.49 (m, 5H), 6.92 (d, J = 9.0 Hz, 2H), 3.83 (s, 3H). 13C NMR (50 MHz, CDCl3): 165.6, 156.6, 135.0, 131.0, 129.1, 127.4, 126.6, 122.5, 114.5, 55.7. C14H13NO2: calcd. C 73.99, H 5.77, N 6.16; found C 73.78, H 5.85, N 6.02. N-((1-butyl-1H-imidazol-2-yl)(phenyl)met hylen e)-4- methoxybenzenamine 4. [50] 1H NMR (200 MHz, CD- Cl3): 7.91 - 7.87 (m, 2H), 7.69 - 7.36 (m, 7H), 7.21 - 7.17 (m, 2H), 3.67 (t, J = 7.0 Hz, 2H), 3.19 (s, 3H), 1.70 - 1.62 (m, 2H), 1.41 - 1.34 (m, 2H), .96 (t, J = 7.2 Hz, 3H). 13C NMR (50 MHz, CDCl3): 159.6, 158.9, 143.4, 139.0, 131.9, 129.1, 128.8, 127.0, 124.9, 124.6, 120.1, 60.3, 39.2, 30.0, 19.8, 13.4. C21H23N3O: calcd. C 75.65, H 6.95, N 12.60; found C 75.33, H 7.18, N 12.32. 1-butyl-2-(1-butyl-2,3-d ihydro-3-methyl-1H-imidaz ol-2-yl) -2,3-dihydro-3-methyl-1 H-imidazole 5 in mix- ture with starting imine 1b. 1H NMR (200 MHz, CDCl3): 6.10 - 6.08 (m, 4H), 3.75 (d, J = 5.9 Hz, 2H), 3.51 (app. t, J = 7.2 Hz, 4H), 3.16 (s, 3H), 1.59 - 1.52 (m, 4H), 1.32 - 1.20 (m, 4H), 0.85 (t, J = 7.2 Hz, 6H). 13C NMR (50 MHz, CDCl3): 114.3, 113.9, 111.1, 109.9, 43.2, 31.5, 30.3, 19.7, 13.6. Reaction of electrogenerated 1-butyl-3-methy-limi- dazol-2-ylidene with phenylacetyl chloride. Preparative electrolysis was carried out as previously described and the current was switched off after 97 C (1 mF). Then, 1 mmol of phenylacetyl chloride was added and the catholyte was kept under stirring at 60˚C for two hours. Then column chromatography of the catholyte gave 1-(1-methyl-1H-imidazol-2-yl)-2-phenylethenol 6. 1-(1-methyl-1H-imidazol-2-y l)-2-phenylethenol 6. [51] 1H NMR (200 MHz, CDCl3): 7.41 - 7.34 (m, 5H), 7.22 - 7.21 (m, 3H), 6.73 (s, 1H), 3.89 (s, 3H). 13C NMR (50 MHz, CDCl3): 172.1, 132.6, 130.9, 130.1, 129.5, 129.1, 127.9, 119.7, 114.6, 41.0. GC-MS (EI) m/z: M+. absent, 144 (1%), 118 (26%), 91 (100%), 77 (2%), 65 (18%), 51 (6%). Copyright © 2011 SciRes. IJOC 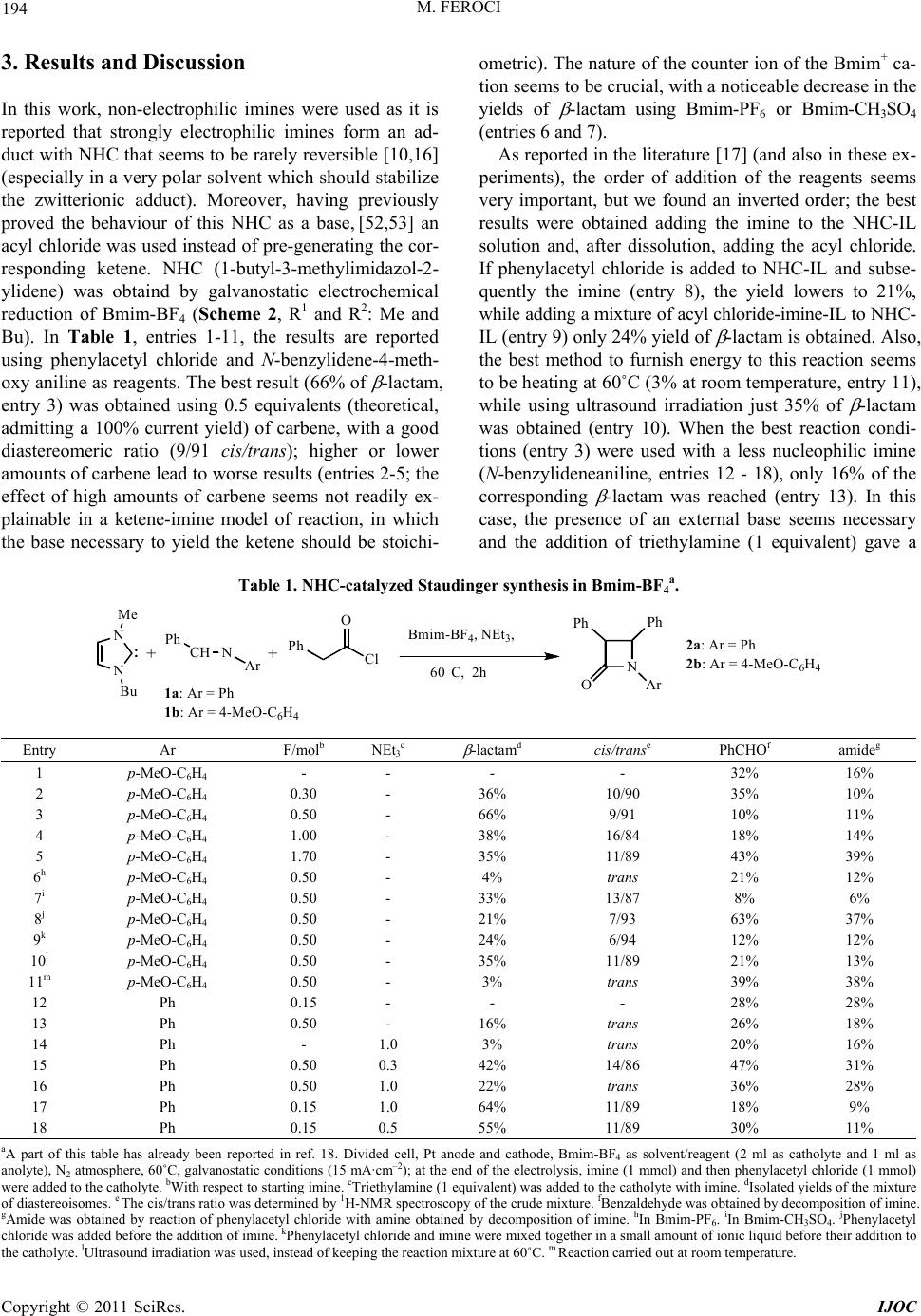 M. FEROCI Copyright © 2011 SciRes. IJOC 194 3. Results and Discussion In this work, non-electrophilic imines were used as it is reported that strongly electrophilic imines form an ad- duct with NHC that seems to be rarely reversible [10,16] (especially in a very polar solvent which should stabilize the zwitterionic adduct). Moreover, having previously proved the behaviour of this NHC as a base, [52,53] an acyl chloride was used instead of pre-generating the cor- responding ketene. NHC (1-butyl-3-methylimidazol-2- ylidene) was obtaind by galvanostatic electrochemical reduction of Bmim-BF4 (Scheme 2, R1 and R2: Me and Bu). In Table 1, entries 1-11, the results are reported using phenylacetyl chloride and N-benzylidene-4-meth- oxy aniline as reagents. The best result (66% of -lactam, entry 3) was obtained using 0.5 equivalents (theoretical, admitting a 100% current yield) of carbene, with a good diastereomeric ratio (9/91 cis/trans ); higher or lower amounts of carbene lead to worse results (entries 2-5; the effect of high amounts of carbene seems not readily ex- plainable in a ketene-imine model of reaction, in which the base necessary to yield the ketene should be stoichi- ometric). The nature of the counter ion of the Bmim+ ca- tion seems to be crucial, with a noticeable decrease in the yields of -lactam using Bmim-PF6 or Bmim-CH3SO4 (entries 6 and 7). As reported in the literature [17] (and also in these ex- periments), the order of addition of the reagents seems very important, but we found an inverted order; the best results were obtained adding the imine to the NHC-IL solution and, after dissolution, adding the acyl chloride. If phenylacetyl chloride is added to NHC-IL and subse- quently the imine (entry 8), the yield lowers to 21%, while adding a mixture of acyl chloride-imine-IL to NHC- IL (entry 9) only 24% yield of -lactam is obtained. Also, the best method to furnish energy to this reaction seems to be heating at 60˚C (3% at room temperature, entry 11), while using ultrasound irradiation just 35% of -lactam was obtained (entry 10). When the best reaction condi- tions (entry 3) were used with a less nucleophilic imine (N-benzylideneaniline, entries 12 - 18), only 16% of the corresponding -lactam was reached (entry 13). In this case, the presence of an external base seems necessary and the addition of triethylamine (1 equivalent) gave a Table 1. NHC-catalyzed Staudinger synthesis in Bmim-BF4a. Ph CH NAr Cl O Ph N N Me Bu Bmim-BF4, NEt3, 60 C, 2h ++N Ph Ph ArO 1a: Ar = Ph 1b: Ar = 4-MeO-C6H4 2a: Ar = Ph 2b: Ar = 4-MeO-C6H4 Entry Ar F/molb NEt3c -lactamd cis/transe PhCHOf amideg 1 p-MeO-C6H4 - - - - 32% 16% 2 p-MeO-C6H4 0.30 - 36% 10/90 35% 10% 3 p-MeO-C6H4 0.50 - 66% 9/91 10% 11% 4 p-MeO-C6H4 1.00 - 38% 16/84 18% 14% 5 p-MeO-C6H4 1.70 - 35% 11/89 43% 39% 6h p-MeO-C6H4 0.50 - 4% trans 21% 12% 7i p-MeO-C6H4 0.50 - 33% 13/87 8% 6% 8j p-MeO-C6H4 0.50 - 21% 7/93 63% 37% 9k p-MeO-C6H4 0.50 - 24% 6/94 12% 12% 10l p-MeO-C6H4 0.50 - 35% 11/89 21% 13% 11m p-MeO-C6H4 0.50 - 3% trans 39% 38% 12 Ph 0.15 - - - 28% 28% 13 Ph 0.50 - 16% trans 26% 18% 14 Ph - 1.0 3% trans 20% 16% 15 Ph 0.50 0.3 42% 14/86 47% 31% 16 Ph 0.50 1.0 22% trans 36% 28% 17 Ph 0.15 1.0 64% 11/89 18% 9% 18 Ph 0.15 0.5 55% 11/89 30% 11% aA part of this table has already been reported in ref. 18. Divided cell, Pt anode and cathode, Bmim-BF4 as solvent/reagent (2 ml as catholyte and 1 ml as anolyte), N2 atmosphere, 60˚C, galvanostatic conditions (15 mA·cm–2); at the end of the electrolysis, imine (1 mmol) and then phenylacetyl chloride (1 mmol) were added to the catholyte. bWith respect to starting imine. cTriethylamine (1 equivalent) was added to the catholyte with imine. dIsolated yields of the mixture of diastereoisomes. e The cis/trans ratio was determined by 1H-NMR spectroscopy of the crude mixture. fBenzaldehyde was obtained by decomposition of imine. gAmide was obtained by reaction of phenylacetyl chloride with amine obtained by decomposition of imine. hIn Bmim-PF6. iIn Bmim-CH3SO4. jPhenylacetyl chloride was added before the addition of imine. kPhenylacetyl chloride and imine were mixed together in a small amount of ionic liquid before their addition to the catholyte. lUltrasound irradiation was used, instead of keeping the reaction mixture at 60˚C. m Reaction carried out at room temperature.  M. FEROCI 195 good yield (entry 17, 64% with a cis/trans ratio of 11/89) only lowering the amount of carbene to 0.15 theoretical equivalents. Again, it is diffucult to understand the dif- ferent behaviour of NHC varying the imine in a ketene- imine model of reaction (NHC should deprotonate the same acyl chloride in both cases). The presence of NHC as an organocatalyst seems ne- cessary also using triethylamine; in fact, using solely NEt3 in Bmim-BF4 only 3% of -lactam was isolated (entry 14). It has to be underlined that Bmim-BF4 be- haves with imines not only as a solvent. In fact, when this reaction is carried out in the absence of both NHC and triethylamine (Table 1, entry 1), part of the starting imine decomposes into its constituents (aldehyde and amine) and the same behaviour is obtained in the pres- ence of NHC and base (Table 1, all other entries), al- though in these cases it is difficult to rule out a participa- tion of these two reagents. To better understand this re- action, which seems to be hardly explained with reported models, this methodology was extended to imines of dif- ferent nucleophilicity and to dichloroacetyl chloride us- ing two different sets of experimental conditions (depen- ding on the starting imine). These results are reported in Table 2. The yields of -lactams obtained in the absence of triethylamine are sometimes higher than the stoi- chiometric 50% value (Table 2, entries 3, 5, 9, 11 and 13), assuming that electrogenerated NHC acts as a base with acyl chloride to yield the corresponding ketene; in fact, hypothesizing a current yield of 100% in the elec- troreduction of the Bmim+ cation, with the generation of 0.5 equivalents of carbene in a monoelectronic process (0.5 F/mol of imine, odd entries of Table 2), the theo- retical maximum amount of ketene (obtained by NHC- deprotonation of the acyl chloride) is 0.5 equivalents, corresponding to a maximum yield of 50% of -lactams. These results are therefore not in line with a ketene- imine model of reaction. It should be considered, how- ever, that Bmim-BF4 is not a neutral solvent for imines; in fact (Table 1, entry 1), as previously stated, this IL is able to decompose this molecule into its constituents, aldehyde and amine (and the amine is isolated as amide, after reaction with the acyl chloride). It is therefore pos- sible that the amine, which derives from the decomposi- tion of the imine, takes part in the Staudinger synthesis, enhancing the yields. Table 2. NHC-catalyzed Staudinger reac tion in Bmim-BF4a. R1 CH NR2CH Cl O R3 R4 N N Me Bu Bmim-BF4, NEt3, 60 C, 2h ++N R3 R4 R1 R2 O 2a-h Entry R1 R 2 R 3 R 4 F/molb NEt3c -lactamd cis/transe 1 Ph Ph Ph H 0.50 - 2a, 16% trans 2 Ph Ph Ph H 0.15 1 eq. 2a, 64% 11/89 3 Ph 4-MeO-C6H4 Ph H 0.50 - 2b, 66% 9/91 4 Ph 4-MeO-C6H4 Ph H 0.15 1 eq. 2b, 32% trans 5 4-MeO-C6H4 4-MeO-C6H4 Ph H 0.50 - 2c, 65% 6/94 6 4-MeO-C6H4 4-MeO-C6H4 Ph H 0.15 1 eq. 2c, 22% trans 7 4-NO2-C6H4 4-MeO-C6H4 Ph H 0.50 - 2d, 23% 22/78 8 4-NO2-C6H4 4-MeO-C6H4 Ph H 0.15 1 eq. 2d, 33% trans 9 Ph 4-MeO-C6H4 Cl Cl 0.50 - 2e, 63% 10 Ph 4-MeO-C6H4 Cl Cl 0.15 1 eq. 2e, 14% 11 Ph Ph-CH2 Ph H 0.50 - 2f, 56% 21/79 12 Ph Ph-CH2 Ph H 0.15 1 eq. 2f, 22% 35/65 13 Ph Ph-CH2 Cl Cl 0.50 - 2g, 61% 14 Ph Ph-CH2 Cl Cl 0.15 1 eq. 2g, 30% 15 Ph 4-Me-C6H4 Ph H 0.50 - 2h, 9% trans 16 Ph 4-Me-C6H4 Ph H 0.15 1 eq. 2h, 68% 9/91 aDivided cell, Pt anode and cathode, Bmim-BF4 as solvent/reagent (2 ml as catholyte and 1 ml as anolyte), N2 atmosphere, 60˚C, galvanostatic conditions (15 mA·cm–2); at the end of the electrolysis, imine (1 mmol) and then acyl chloride (1 mmol) were added to the catholyte. bWith respect to starting imine. cTriethylamine (1 equivalent) was added to the catholyte with imine. dIsolated yields of the mixture of diastereoisomes. eThe cis/trans ratio was determined by 1H-NMR spectroscopy of the crude mixture. Copyright © 2011 SciRes. IJOC 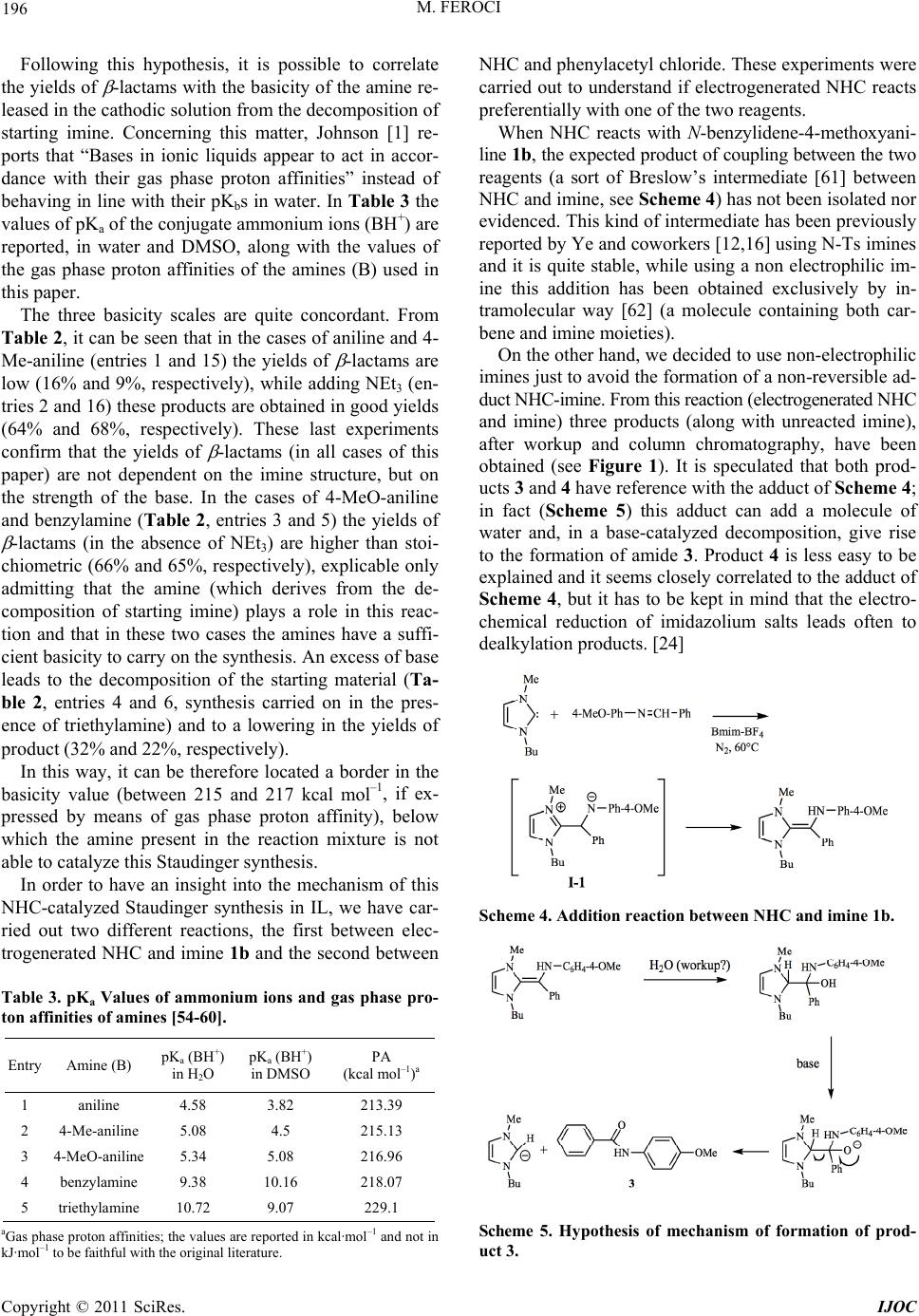 M. FEROCI 196 Following this hypothesis, it is possible to correlate the yields of -lactams with the basicity of the amine re- leased in the cathodic solution from the decomposition of starting imine. Concerning this matter, Johnson [1] re- ports that “Bases in ionic liquids appear to act in accor- dance with their gas phase proton affinities” instead of behaving in line with their pKbs in water. In Table 3 the values of pKa of the conjugate ammonium ions (BH+) are reported, in water and DMSO, along with the values of the gas phase proton affinities of the amines (B) used in this paper. The three basicity scales are quite concordant. From Table 2, it can be seen that in the cases of aniline and 4- Me-aniline (entries 1 and 15) the yields of -lactams are low (16% and 9%, respectively), while adding NEt3 (en- tries 2 and 16) these products are obtained in good yields (64% and 68%, respectively). These last experiments confirm that the yields of -lactams (in all cases of this paper) are not dependent on the imine structure, but on the strength of the base. In the cases of 4-MeO-aniline and benzylamine (Table 2, entries 3 and 5) the yields of -lactams (in the absence of NEt3) are higher than stoi- chiometric (66% and 65%, respectively), explicable only admitting that the amine (which derives from the de- composition of starting imine) plays a role in this reac- tion and that in these two cases the amines have a suffi- cient basicity to carry on the synthesis. An excess of base leads to the decomposition of the starting material (Ta- ble 2, entries 4 and 6, synthesis carried on in the pres- ence of triethylamine) and to a lowering in the yields of product (32% and 22%, respectively). In this way, it can be therefore located a border in the basicity value (between 215 and 217 kcal mol–1, if ex- pressed by means of gas phase proton affinity), below which the amine present in the reaction mixture is not able to catalyze this Staudinger synthesis. In order to have an insight into the mechanism of this NHC-catalyzed Staudinger synthesis in IL, we have car- ried out two different reactions, the first between elec- trogenerated NHC and imine 1b and the second between Table 3. pKa Values of ammonium ions and gas phase pro- ton affinities of amines [54-60]. Entry Amine (B) pKa (BH+) in H2O pKa (BH+) in DMSO PA (kcal mol–1)a 1 aniline 4.58 3.82 213.39 2 4-Me-aniline 5.08 4.5 215.13 3 4-MeO-aniline 5.34 5.08 216.96 4 benzylamine 9.38 10.16 218.07 5 triethylamine 10.72 9.07 229.1 aGas phase proton affinities; the values are reported in kcal·mol–1 and not in kJ·mol–1 to be faithful with the original literature. NHC and phenylacetyl chloride. These experiments were carried out to understand if electrogenerated NHC reacts preferentially with one of the two reagents. When NHC reacts with N-benzylidene-4-methoxyani- line 1b, the expected product of coupling between the two reagents (a sort of Breslow’s intermediate [61] between NHC and imine, see Scheme 4) has not been isolated nor evidenced. This kind of intermediate has been previously reported by Ye and coworkers [12,16] using N-Ts imines and it is quite stable, while using a non electrophilic im- ine this addition has been obtained exclusively by in- tramolecular way [62] (a molecule containing both car- bene and imine moieties). On the other hand, we decided to use non-electrophilic imines just to avoid the formation of a non-reversible ad- duct NHC-imine. From this reaction (electrogenerated NHC and imine) three products (along with unreacted imine), after workup and column chromatography, have been obtained (see Figure 1). It is speculated that both prod- ucts 3 and 4 have reference with the adduct of Scheme 4; in fact (Scheme 5) this adduct can add a molecule of water and, in a base-catalyzed decomposition, give rise to the formation of amide 3. Product 4 is less easy to be explained and it seems closely correlated to the adduct of Scheme 4, but it has to be kept in mind that the electro- chemical reduction of imidazolium salts leads often to dealkylation products. [24] Scheme 4. Addition reaction between NHC and imine 1b. Scheme 5. Hypothesis of mechanism of formation of prod- uct 3. Copyright © 2011 SciRes. IJOC 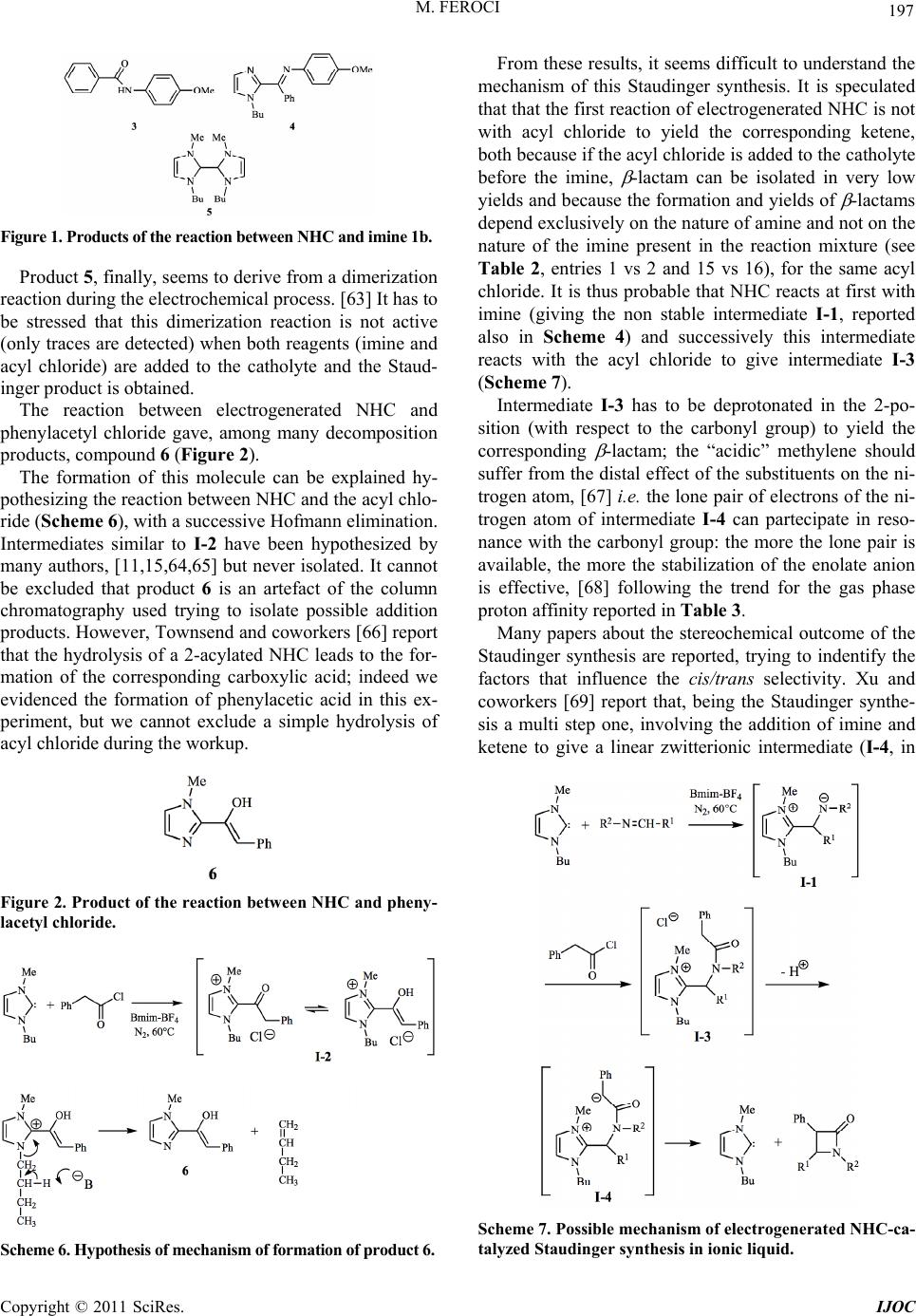 M. FEROCI 197 Figure 1. Products of the reaction between NHC and imine 1b. Product 5, finally, seems to derive from a dimerization reaction during the electrochemical process. [63] It has to be stressed that this dimerization reaction is not active (only traces are detected) when both reagents (imine and acyl chloride) are added to the catholyte and the Staud- inger product is obtained. The reaction between electrogenerated NHC and phenylacetyl chloride gave, among many decomposition products, compound 6 (Figure 2). The formation of this molecule can be explained hy- pothesizing the reaction between NHC and the acyl chlo- ride (Scheme 6), with a successive Hofmann elimination. Intermediates similar to I-2 have been hypothesized by many authors, [11,15,64,65] but never isolated. It cannot be excluded that product 6 is an artefact of the column chromatography used trying to isolate possible addition products. However, Townsend and coworkers [66] report that the hydrolysis of a 2-acylated NHC leads to the for- mation of the corresponding carboxylic acid; indeed we evidenced the formation of phenylacetic acid in this ex- periment, but we cannot exclude a simple hydrolysis of acyl chloride during the workup. Figure 2. Pr oduct of the reaction between NHC and phe ny- lacetyl chloride. Scheme 6. Hypothesis of mechan ism of formation of product 6. From these results, it seems difficult to understand the mechanism of this Staudinger synthesis. It is speculated that that the first reaction of electrogenerated NHC is not with acyl chloride to yield the corresponding ketene, both because if the acyl chloride is added to the catholyte before the imine, -lactam can be isolated in very low yields and because the formation and yields of -lactams depend exclusively on the nature of amine and not on the nature of the imine present in the reaction mixture (see Table 2, entries 1 vs 2 and 15 vs 16), for the same acyl chloride. It is thus probable that NHC reacts at first with imine (giving the non stable intermediate I-1, reported also in Scheme 4) and successively this intermediate reacts with the acyl chloride to give intermediate I-3 (Scheme 7). Intermediate I-3 has to be deprotonated in the 2-po- sition (with respect to the carbonyl group) to yield the corresponding -lactam; the “acidic” methylene should suffer from the distal effect of the substituents on the ni- trogen atom, [67] i.e. the lone pair of electrons of the ni- trogen atom of intermediate I-4 can partecipate in reso- nance with the carbonyl group: the more the lone pair is available, the more the stabilization of the enolate anion is effective, [68] following the trend for the gas phase proton affinity reported in Table 3. Many papers about the stereochemical outcome of the Staudinger synthesis are reported, trying to indentify the factors that influence the cis/trans selectivity. Xu and coworkers [69] report that, being the Staudinger synthe- sis a multi step one, involving the addition of imine and ketene to give a linear zwitterionic intermediate (I-4, in Scheme 7. Possible mechanism of electrogenerated NHC-ca- talyzed Staudinger synthesis in ionic liquid. Copyright © 2011 SciRes. IJOC  M. FEROCI 198 our hypothesis) and the subsequent ring closure of this intermediate (Scheme 3), “the product ratio (cis/trans) only depends on the rate constants of the direct ring clo- sure (k1) and the isomerization (k2)”. In fact, the zwit- terionic intermediate can isomerize by rotation along the N-C bond of the imine portion of the intermediate itself. It seems that when the ring closure of the zwitterion is fast, a cis -lactam is obtained, when it is slow (in com- parison with the isomerization) a trans product is gained. The competition between these two reactions relies on electronic effects of the substituents on imine and ketene, and on the steric hindrance of the same. The solvent can play a role in this reaction; in particu- lar, apolar solvents favour cis -lactams, while polar solvents favour tran s ones, probably because polar sol- vents stabilize the zwitterionic intermediate, permitting its isomerization. The solvent of this reaction is an ionic liquid, highly polar, and the main product is a trans - lactam, on line with this theory. As regards the possibility of recycling the cathodic io- nic liquid after the isolation of the products, the IL used in Table 2, entry 3, was kept under vacuum (to eliminate residual diethyl ether) and used for a new electrolysis. In this case, however, the yield in -lactam fell to 29%, and only traces of product were obtained during the third cycle. This is probaly due to a gradual degradation of the ionic liquid during the cathodic reduction, with the for- mation of by-products which could interfere with this synthesis. 4. Conclusions The first example of synthesis of -lactams via Staud- inger synthesis in ionic liquid catalyzed by an N-he- terocyclic carbene is described. This NHC is easily ob- tained by cathodic reduction of Bmim-BF4, under galva- nostatic conditions, and it behaves as base and/or nucleo- philic organocatalyst (so, under these experimental con- ditions, IL plays the double role of solvent and precata- lyst). Good yields of -lactams, in predominantly trans configuration have been obtained starting from non elec- trophilic imines and acyl chloride and a hypothesis of mechanism is given, not involving the formation of a ke- tene. 5. Acknowledgements This study was supported by MIUR and CNR (Rome). The author thanks Mr. Marco Di Pilato for his contribu- tion to the experimental part of this work. 6. References [1] K. E. Johnson, “What’s an Ionic Liquid?” Interface, Vol. 16, No. 1, 2007, pp. 38-41. [2] P. J. Dyson and T. J. Geldbach, “Applications of Ionic Liquids in Synthesis and Catalysis,” Interface, Vol. 16, No. 1, 2007, pp. 50-53. [3] M. J. Earle and K. R. Seddon, “Ionic Liquids. Green Sol- vents for the Future,” Pure and Applied Chemistry, Vol. 72, No. 7, 2000, pp. 1391-1398. doi:10.1351/pac200072071391 [4] J. Dupont, C. S. Consorti and J. Spencer, “Room Tem- perature Molten Salts: Neoteric ‘Green’ Solvents for Chemical Reactions and Processes,” Journal of the Bra- zilian Chemical Society, Vol. 11, No. 4, 2000, pp. 337- 344. doi:10.1590/S0103-50532000000400002 [5] J. G. Huddleston, A. E. Visser, W. M. Reichert, H. D. Willauer, G. A. Broker and R. D. Rogers, “Charact- erization and Comparison of Hydrophilic and Hydro- phobic Room Temperature Ionic Liquids Incorporating the Imidazolium Cation,” Green Chemistry, Vol. 3, No. 4, 2001, pp. 156-164. doi:10.1039/b103275p [6] J. Dupont and J. Spencer, “On the Noninnocent Nature of 1,3-Dialkylimidazolium Ionic Liquids,” Angewandte Che- mie International Edition, Vol. 43, No. 40, 2004, pp, 5296-5297. doi:10.1002/anie.200460431 [7] X. Cui, S. Zhang, F. Shi, Q. Zhang, X. Ma, L. Lu and Y. Deng, “The Influence of the Acidity of Ionic Liquids on Catalysis,” ChemSusChem, Vol. 3, No. 9, 2010, pp. 1043- 1047. doi:10.1002/cssc.201000075 [8] Y. Chu, H. Deng and J.-P. Cheng, “An Acidity Scale of 1,3-Dialkylimidazolium Salts in Dimethyl Sulfoxide So- lutions,” Journal of Organic Chemistry, Vol. 72, No. 20, 2007, pp. 7790-7793. doi:10.1021/jo070973i [9] A. P. Dove, R. C. Pratt, B. G. G. Lohmeijer, H. Li, E. C. Hagberg, R. M. Waymouth and J. L. Hedrick, “N-Hetero- cyclic Carbenes as Organic Catalysts,” In: S. P. Nolan, Ed., N-Heterocyclic Carbenes in Synthesis, Wiley-VCH, Weinheim, 2006, pp. 275-296. doi:10.1002/9783527609451.ch12 [10] M. He and J. W. Bode, “Catalytic Synthesis of -Lactams via Direct Annulations of Enals and N-Sulfonylimines,” Organic Letters, Vol. 7, No. 14, 2005, pp. 3131-3134. doi:10.1021/ol051234w [11] M. Heand and J. W. Bode, “Enantioselective, NHC- Catalyzed Bicyclo- -Lactam Formation via Direct An- nulations of Enals and Unsaturated N-Sulfonyl Ketimi- nes,” Journal of the American Chemical Society, Vol. 130, No. 2, 2008, pp. 418-419. doi:10.1021/ja0778592 [12] L. He, T.-Y. Jian and S. Ye, “N-Heterocyclic Carbene Catalyzed Aza-Morita-Baylis-Hillman Reaction of Cyclic Enones with N-Tosylarylimines,” Journal of Organic Chemistry, Vol. 72, No. 19, 2007, pp. 7466-7468. doi:10.1021/jo071247i [13] D. Enders and T. Balensiefer, “Nucleophilic Carbenes in Asymmetric Organocatalysis,” Accounts of Chemical Research, Vol. 37, No. 8, 2004, pp. 534-541. doi:10.1021/ar030050j [14] J. Pesch, K. Harms and T. Bach, “Preparation of Axially Copyright © 2011 SciRes. IJOC  M. FEROCI 199 Chiral N, N’-Diarylimidazolium and N-Arylthiazolium Salts and Evaluation of Their Catalytic Potential in the Benzoin and in the Intramolecular Stetter Reactions,” European Journal of Organic Chemistry, Vol. 2004, No. 9, 2004, pp. 2025-2035. doi:10.1002/ejoc.200300762 [15] Y. Kawanaka, E. M. Phillips and K. A. Scheidt, “N-Hete- rocyclic Carbene-Catalyzed Enantioselective Mannich Reactions with -Aryloxyacetaldehydes,” Journal of the American Chemical Society, Vol. 131, No. 50, 2009, pp. 18028-18029. doi:10.1021/ja9094044 [16] Y.-R. Zhang, L. He, X. Wu, P.-L. Shao and S. Ye, “Chiral N-Heterocyclic Carbene Catalyzed Staudinger Reaction of Ketenes with Imines: Highly Enantioselec- tive Synthesis of N-Boc -Lactams,” Organic Letters, Vol. 10, No. 2, 2008, pp. 277-280. doi:10.1021/ol702759b [17] N. Duguet, C. D. Campbell, A. M. Z. Slawin and A. D. Smith, “N-Heterocyclic Carbene Catalysed -lactam Syn- thesis,” Organic and Biomolecular Chemistry, Vol. 6, No. 6, 2008, pp. 1108-1113. doi:10.1039/b800857b [18] V. Nair, S. Bindu and V. Sreekumar, “N-Heterocyclic Carbenes: Reagents, Not Just Ligands!” Angewandte Chemie International Edition, Vol. 43, 2004, pp. 5130- 5135. doi:10.1002/anie.200301714 [19] J. P. Canal, T. Ramnial, D. A. Dickie and J. A. C. Cly- burne, “From the Reactivity of N-Heterocyclic Carbenes to New Chemistry in Ionic Liquids,” Chemical Commu- nications, Vol. 17, 2006, pp. 1809-1818. doi:10.1039/b512462j [20] N. Marion, S. Díez-González and S. P. Nolan, “N-Hete- rocyclic Carbenes as Organocatalysts,” Angewandte Chemie International Edition, Vol. 46, No. 17, 2007, pp. 2988-3000. doi:10.1002/anie.200603380 [21] D. Enders, O. Niemeier and A. Henseler, “Organocataly- sis by N-Heterocyclic Carbenes,” Chemical Reviews, Vol. 107, No. 12, 2007, pp. 5606-5655. doi:10.1021/cr068372z [22] D. Bourissou, O. Guerret, F. P. Gabbai and G. Bertrand, “Stable Carbenes,” Chemical Reviews, Vol. 100, No. 1, 2000, pp. 39-92. doi:10.1021/cr940472u [23] P. Hapiot and C. Lagrost, “Electrochemical Reactivivty in Room-Temperature Ionic Liquids,” Chemical Reviews, Vol. 108, No. 7, 2008, pp. 2238-2264. doi:10.1021/cr0680686 [24] L. Xiao and K. E. Johnson, “Electrochemistry of 1-Butyl- 3-methyl-1H-imidazolium Tetrafluoroborate Ionic Liq- uid,” Journal of the Electrochemical Society, Vol. 150, No. 6, 2003, pp. E307-E311. doi:10.1149/1.1568740 [25] B. Gorodetsky, T. Ramnial, N. R. Branda and J. A. C. Clyburne, “Electrochemical Reduction of an Imidazolium Cation: A Convenient Preparation of Imidazol-2-ylidenes and Their Observation in an Ionic Liquid,” Chemical Communications, Vol. 7, No. 17, 2004, pp. 1972-1973. doi:10.1039/b407386j [26] M. Feroci, I. Chiarotto, M. Orsini, G. Sotgiu and A. Inesi, “Reactivity of Electrogenerated N-Heterocyclic Carbene in Room-Temperature Ionic Liquids. Cyclization to 2-Azetidinone Ring via C3-C4 Bond Formation,” Ad- vanced Synthesis and Catalysis, Vol. 350, No. 9, 2008, pp. 1355-1359. doi:10.1002/adsc.200800049 [27] M. Feroci, M. N. Elinson, L. Rossi and A. Inesi, “The Double Role of Ionic Liquids in Organic Electrosynthesis: Precursors of N-Heterocyclic Carbenes and Green Sol- vents. Henry Reaction,” Electrochemistry Communica- tions, Vol. 11, No. 7, 2009, pp. 1523-1526. doi:10.1016/j.elecom.2009.05.045 [28] A. Brandi, S. Cicchi and F. M. Cordero, “Novel Synthe- ses of Azetidines and Azetidinones,” Chemical Reviews, Vol. 108, No. 9, 2008, pp. 3988-4035. doi:10.1021/cr800325e [29] D. Lednicer, “Strategies for Organic Drug Synthesis and Design,” 2nd Edition, John Wiley & Sons, Inc., Hoboken, 2009, pp. 545-575. [30] H. Staudinger, “Zur Kenntniss der Ketene. Diphenyl- keten,” Justus Liebigs Annalen der Chemie, Vol. 356, No. 1-2, 1907, pp. 51-123. doi:10.1002/jlac.19073560106 [31] A. Arrieta, B. Lecea and F. P. Cossió, “Origins of the Stereodivergent Outcome in the Staudinger Reaction between Acyl Chlorides and Imines,” Journal of Organic Chemistry, Vol. 63, No. 17, 1998, pp. 5869-5876. doi:10.1021/jo9804745 [32] Y. Wang, Y. Liang, L. Jiao, D.-M. Du and J. Xu, “Do Reaction Conditions Affect the Stereoselectivity in the Staudinger Reaction?” Journal of Organic Chemistry, Vol. 71, No. 18, 2006, pp. 6983-6990. doi:10.1021/jo0611521 [33] F. P. Cossió, A. Arrieta and M. A. Sierra, “The Mech- anism of the Ketene-Imine (Staudinger) Reaction in Its Centennial: Still an Unsolved Problem?” Accounts of Chemical Research, Vol. 41, No. 8, 2008, pp. 925-936. doi:10.1021/ar800033j [34] J. Xu, “Stereoselectivity in the Synthesis of 2-Aze- tidinones from Ketenes and Imines via the Staudinger Reaction,” ARKIVOC, Vol. ix, 2009, pp. 21-44. [35] S. France, A. Weatherwax, A. E. Taggi and T. Lectka, “Advances in the Catalytic, Asymmetric Synthesis of -Lactams,” Accounts of Chemical Research, Vol. 37, No. 8, 2004, pp. 592-600. doi:10.1021/ar030055g [36] E. C. Lee, B. L. Hodous, E. Bergin, C. Shih and G. C. Fu, “Catalytic Asymmetric Staudinger Reactions to Form -Lactams: An Unanticipated Dependence of Diastereo- selectivity on the Choice of the Nitrogen Substituent,” Journal of the American Chemical Society, Vol. 127, No. 33, 2005, pp. 11586-11587. doi:10.1021/ja052058p [37] A. Weatherwax, C. J. Abraham and T. Lectka, “An Ani- onic Nucleophilic Catalyst System for the Diastereoselec- tive Synthesis of trans- -Lactams,” Organic Letters, Vol. 7, No. 16, 2005, pp. 3461-3463. doi:10.1021/ol0511070 [38] O. Sereda, A. Blanrue and R. Wilhelm, “Enantiopure Imi- dazolium-Dithiocarboxylates as Highly Selective Novel Organocatalysts,” Chemical Communications, Vol. 9, 2009, pp. 1040-1042. doi:10.1039/b817991c Copyright © 2011 SciRes. IJOC  M. FEROCI 200 [39] N. Duguet, A. Donaldson, S. M. Leckie, J. Douglas, P. Shapland, T. B. Brown, G. Churchill, A. M. Z. Slawin and A. D. Smith, “Chiral Relay in NHC-Mediated Asy- mmetric -Lactam Synthesis I; Substituent Effects in NHCs Derived from (1R,2R)-Cyclohexane-1,2-diamine,” Tetrahedron: Asymmetry, Vol. 21, No. 5, 2010, pp. 582- 600. doi:10.1016/j.tetasy.2010.03.001 [40] N. Duguet, A. Donaldson, S. M. Leckie, E. A. Kallström, C. D. Campbell, P. Shapland, T. B. Brown, A. M. Z. Slawin and A. D. Smith, “Chiral Relay in NHC-Mediated Asymmetric -Lactam Synthesis II; Asymmetry from NHCs Derived from Acyclic 1,2-Diamines,” Tetrahedron: Asymmetry, Vol. 21, No. 5, 2010, pp. 601-616. doi:10.1016/j.tetasy.2010.03.002 [41] R. Chen, B.Yang and W. Su, “Ytterbium(III) Triflate- Catalyzed Stereoselective Synthesis of β-Lactams via [2+2] Cyclocondensation in Ionic Liquid,” Synthetic Communications, Vol. 36, No. 21, 2006, pp. 3167-3174. doi:10.1080/00397910600908843 [42] X.-L. Tao, M. Lei and Y.-G. Wang, “Ionic Liquid Sup- ported Synthesis of -Lactam Library in Ionic Liquid Batch,” Tetrahedron Letters, Vol. 48, No. 28, 2007, pp. 5143-5146. doi:10.1016/j.tetlet.2007.05.053 [43] M. Feroci, I. Chiarotto, M. Orsini and A. Inesi, “Elec- trogenerated NHC as an Organocatalyst in the Staudinger Reaction,” Chemical Communications, Vol. 46, No. 23, 2010, pp. 4121-4123. doi:10.1039/c002325f [44] M. M.-C. Lo and G. C. Fu, “Cu(I)/Bis(azaferrocene)-Cata lyzed Enantioselective Synthesis of -Lactams via Cou- plings of Alkynes with Nitrones,” Journal of the Ameri- can Chemical Society, Vol. 124, No. 17, 2002, pp. 4572- 4573. doi:10.1021/ja025833z [45] S. Kikuchi and Y. Hashimoto, “Novel Method for the Synthesis of β-Lactams by the Reaction of α-Bromo- carboxylic Acids with Imines Mediated by Tripheny- lphosphine,” Heterocycles, Vol. 68, No. 3, 2006, pp. 453- 457. doi:10.3987/COM-05-10661 [46] M. A. Casadei, A. Inesi, F. Micheletti Moracci and D. Occhialini, “Electrochemical Studies on -Lactams. Part 4. Electroacetylation of -Lactams,” Tetrahedron, Vol. 45, No. 21, 1989, pp. 6885-6890. doi:10.1016/S0040-4020(01)89156-0 [47] L. Zhao and C.-J. Li, “Highly Efficient Three-Component Synthesis of β-Lactams from N-methylhydroxylamine, Aldehydes, and Phenylacetylene,” Chemistry—An Asian Journal, Vol. 1, No. 1-2, 2006, pp. 203-209. doi:10.1002/asia.200600097 [48] M.-C. Ye, J. Zhou and Y. Tang, “Triazoline/Cu(II)-Pro- moted Kinugasa Reaction. Enantioselective Synthesis of -Lactams,” Journal of Organic Chemistry, Vol. 71, No. 9, 2006, pp. 3576-3582. doi:10.1021/jo0602874 [49] M. A. Mohamed, K.-J. Yamada and K. Tomioka, “Ac- cessing the Amide Functionality by the Mild and Low- Cost Oxidation of Imine,” Tetrahedron Letters, Vol. 50, No. 26, 2009, pp. 3436-3438. doi:10.1016/j.tetlet.2009.02.174 [50] G. Steiner, A. Krajete, H. Kopacka, K.-H. Ongania, K. Wurst, P. Preishuber-Pflügl and B. Bildstein, “[1,2]-Rear- rangement of Imino-N-heterocyclic Carbenes—Synth- esis and Structures of Chelating Iminoimidazole Pd and Ni Complexes,” European Journal of Inorganic Chem- istry, Vol. 2004, No. 14, 2004, pp. 2827-2836. doi:10.1002/ejic.200400070 [51] G. A. Taylor, “Keten. Part XIV. Adducts of Diphenylke- ten with Aza-Arenes,” Journal of the Chemical Society Perkin Transactions I, Vol. 11, 1975, pp. 1001-1009. doi:10.1039/p19750001001 [52] I. Chiarotto, M. M. M. Feeney, M. Feroci and A. Inesi, “Electrogenerated N-Heterocyclic Carbene. N-Acylation of Chiral Oxazolidin-2-Ones in Ionic Liquids,” Electro- chimica Acta, Vol. 54, No. 5, 2009, pp. 1638-1644. doi:10.1016/j.electacta.2008.09.057 [53] I. Chiarotto, M. Feroci, M. Orsini, G. Sotgiu and A. Inesi, “Electrogenerated N-Heterocyclic Carbene. N-Function- alization of Benzoxazolones,” Tetrahedron, Vol. 65, No. 18, 2009, pp. 3704-3710. doi:10.1016/j.tet.2009.02.057 [54] M. R. Crampton and I. A.Robotham, “Acidities of Some Substituted Ammonium Ions in Dimethyl Sulfoxide,” Journal of Chemical Research, Vol. 1, 1997, pp. 22-23. doi:10.1039/a606020j [55] http://ifs.massey.ac.nz/outreach/resources/chem/orgbases. php [56] J. Courtot-Coupez and M. Le Démézet, “Electrochimie dans le Diméthylsulfoxyde. II. Fonctionnement de l’électrode à Hydrogéne,” Bulletin Societe Chimique de France, Vol. 3, 1969, pp. 1033-1040. [57] B. H. M. Asghar and M. R. Crampton, “Rate-Limiting Proton-Transfer in the σ-Adduct Forming Reactions of 1,3,5-Trinitrobenzene and 4-Nitrobenzofuroxan with Sub- stituted Anilines in Dimethyl Sulfoxide,” Organic and Biomolecular Chemistry, Vol. 3, No. 21, 2005, pp. 3971-3978. doi:10.1039/b511644a [58] A. N. Pankratov, I. M. Uchaeva, S. Y. Doronin and R. K. Chernova, “Correlations between the Basicity and Proton Affinity of Substituted Anilines,” Journal of Structural Chemistry, Vol. 42, No. 5, 2001, pp. 739-746. doi:10.1023/A:1017909131054 [59] J. Cao, C. Aubry and J. L. Holmes, “Proton Affinities of Simple Amines; Entropies and Enthalpies of Activation and Their Effect on the Kinetic Method for Evaluating Proton Affinities,” Journal of Physical Chemistry A, Vol. 104, No. 44, 2000, pp. 10045-10052. doi:10.1021/jp0021244 [60] D. H. Aue, H. M. Webb and M. T. Bowers, “A Complete Thermodynamic Analysis of the ‘Anomalous Order’ of Amine Basicity in Solution,” Journal of the American Chemical Society, Vol. 94, No. 13, 1972, pp. 4726-4728. doi:10.1021/ja00768a049 [61] R. Breslow, “On the Mechanism of Thiamine Action. IV. Evidence from Studies on Model Systems,” Journal of the American Chemical Society, Vol. 80, No. 14, 1958, pp. 3719-3726. doi:10.1021/ja01547a064 [62] S. Simonovic, J.-C. Frison, H. Koyuncu, A. C. Whitwood and R. E. Douthwaite, “Addition of N-Heterocyclic Car- Copyright © 2011 SciRes. IJOC  M. FEROCI Copyright © 2011 SciRes. IJOC 201 benes to Imines: Phenoxide Assisted Deprotonation of an Imidazolium Moiety and Generation of Breslow Inter- mediates Derived from Imines,” Organic Letters, Vol. 11, No. 1, 2009, pp. 245-247. doi:10.1021/ol802572n [63] M. C. Kroon, W. Buijs, C. J. Peters and G.-J. Witkamp, “Decomposition of Ionic Liquids in Electrochemical Pro- cessing,” Green Chemistry, Vol. 8, No. 3, 2006, pp. 241- 245. doi:10.1039/b512724f [64] Y. Suzuki, K. Yamauchi, K. Muramatsu and M. Sato, “First Example of Chiral N-Heterocyclic Carbenes as Catalysts for Kinetic Resolution,” Chemical Communica- tions, Vol. 23, 2004, pp. 2770-2771. doi:10.1039/b411855c [65] N. T. Reynolds, J. Read de Alaniz, J and T. Rovis, “Conversion of α-Haloaldehydes into Acylating Agents by an Internal Redox Reaction Catalyzed by Nucleophilic Carbenes,” Journal of the American Chemical Society, Vol. 126, No. 31, 2004, pp. 9518-9519. doi:10.1021/ja046991o [66] N. Khaleeli, R. Li and C. A. Townsend, “Origin of the β-Lactam Carbons in Clavulanic Acid from an Unusual Thiamine Pyrophosphate-Mediated Reaction,” Journal of the American Chemical Society, Vol. 121, No. 39, 1999, pp. 9223-9224. doi:10.1021/ja9923134 [67] J. Ho, C. J. Easton and M. L. Coote, “The Distal Effect of Electron-Withdrawing Groups and Hydrogen Bonding on the Stability of Peptide Enolates,” Journal of the Amer- ican Chemical Society, Vol. 132, No. 15, 2010, pp. 5515- 5521. doi:10.1021/ja100996z [68] A. Fersner, J. M. Karty and Y. Mo, “Why Are Esters and Amides Weaker Carbon Acids than Ketones and Acid Fluorides? Contributions by Resonance and Inductive Effects,” Journal of Organic Chemistry, Vol. 74, No. 19, 2009, pp. 7245-7253. doi:10.1021/jo901225t [69] L. Jiao, Y. Liang and J. Xu, “Origin of the Relative Stereochemistry of the -Lactam Formation in the Sta- udinger Reaction,” Journal of the American Chemical Society, Vol. 128, No. 18, 2006, pp. 6060-6069. doi:10.1021/ja056711k
|