 Applied Mathematics, 2011, 2, 1507-1514 doi:10.4236/am.2011.212213 Published Online December 2011 (http://www.SciRP.org/journal/am) Copyright © 2011 SciRes. AM Pattern Formation in Tri-Trophic Ratio-Dependent Food Chain Model Dawit Melese, Sunita Gakkhar Department of Mat hematics, Indian Institute of Technology Roorkee, Roorkee, India E-mail: mahifikir@gmail.com, sungkfma@iitr.ernet.in Received October 27, 2011; revised November 26, 2011 ; accepted December 5, 2011 Abstract In this paper, a spatial tri-trophic food chain model with ratio-dependent Michaelis-Menten type functional response under homogeneous Neumann boundary conditions is studied. Conditions for Hopf and Turing bi- furcation are derived. Sufficient conditions for the emergence of spatial patterns are obtained. The results of numerical simulations reveal the formation of labyrinth patterns and the coexistence of spotted and stripe- like patterns. Keywords: Reaction-Diffusion Equations, Hopf Bifurcation, Turing Instability, Turing Pattern, Food Chain 1. Introduction Food chains in the environment are very important sys- tems in ecological science, applied mathematics, eco- nomic and engineering science [1]. They have been ap- plied to management in Aquatic ecosystem for problems like water quality and lake management [2]. Modeling of food chain dynamics has challenges in the fields of both theoretical ecology and applied mathematics. Tri-trophic food chain models have been studied in both spatially homogeneous [1-4] and spatially inhomogeneous envi- ronments [5-10] for the last two decades. Those models exhibit rich and complex dynamics and nonlinear mathe- matical behavior, including varying numbers and stabili- ty of equilibrium states, limit cycles, patterns and chaos. It is well known that in real life, the resources are not uniformly distributed in the habitat. The biological spe- cies move (diffuse) from place to place in search of food in their habitat and hence interact with each other and with the environment. This movement (diffusiveness) of species has an impact on their trophic interactions. Con- sequently, spatial pattern s evolve. One can easily observe patterns in both aquatic and terrestrial populations [10]. A number of recent contribu- tions have begun to suggest that it is critical to begin to fully consider the implications of spatial flows on the dynamics of ecological communities [7]. In the litera- tures [11,12], the spatial component of ecological inter- actions (trophic-interactions) is identified as an important factor in shaping ecological communities. However, there is lack of recognition about the role of spatial considera- tions in food chain dynamics [7]. In addition to this, ig- norance of spatial scales of fo od chains limits our ability to predict how trophic interactions will vary in different contexts, across space and time [2]. The dynamics and stability of the interacting species in relation to spatial phenomena such as pattern formation has recently be- come a focus of intensive research in theoretical ecology [13], chemical and biological systems [14]. The spatial patterns modify the temporal dynamics at a range of spa- tial scales, whose effects must be incorporated in tempo- ral ecological models that do not represent space explic- itly. In recent decades, stationary patterns induced by diffusion have been studied extensively, and lots of im- portant phen o mena have been obs erved. Spatial patterns of interacting species are ubiquitous in nature and may occur due to stochastic process, environ- mental fluctuations or variability, or deterministic proc- ess, growth and movement of interacting species. The deterministic process is intrinsic to the interacting spe- cies and results in population-driven and self-organized spatial patterns. The formation of population-driven spa- tial patterns was first pointed out by Alan Turing. Turing, one of the key scientist of the 20th century, mathemati- cally showed that a system of coupled reaction-diffusion equations can give rise to spatial concentration patterns of a fixed characteristic length from an arbitrary initial configuration due to diffusion—driven instability or Tur- ing instability [15]. Reaction-Diffusion systems are capable of qualitati- vely imitating many biological patterns such as the stri- pes of a zebra, tiger and snakes or spots of a cheetah and  D. MELESE ET AL. 1508 even more irregular patterns such as those on leopards and giraffes, the patterns on exotic fish, butterflies or beetles through Turing instab ility. Pattern formation for two species model [11-14,16-19] based on coupled reaction diffusion equations has been intensively investigated. The necessary and sufficient condition for Turing instability, which leads to the for- mation of spatial patterns, has been derived [9,16,17,20] and very interesting patterns are also obtained from the numerical simulation results [9,21,22]. For three species case, the authors [2,23] used a three species interacting discrete model to study the formation of pattern. In the literatures [9,10,24], the authors have considered a food chain model with diffusion and inves- tigate the persistence of the system, the stability of the positive steady state solution of the system. In this paper, the formation of patterns in a tri-trophic food chain model with ratio-dependent Michaelis-Men- ten type functional response and diffusion has been in- vestigated. The organization of the paper is as follows: In Section 2 the mathematical model is given. Section 3 is devoted to the stability and bifurcation analysis of the system. Section 4 presents the results of numerical simulations. Section 5 is devoted to some conclusions. 2. The Mathematical Model Let and denote the densities of the prey, intermediate predator and top predator respectively at time T and position (,,), (,,)U XYTV XYT(,,)WXYT (,) Y in the habitat . The prey is assumed to grow logis- tically. The intermediate predator, V, and the top predator, W, follow the ratio-dependent Michaelis-Menten type functional response. Thus the mathematical model gov- erning the spatiotemporal dynamics of the three interact- ing species prey-predator community can be described by the following system of reaction-diffusion equations. 2 22 1 122 1 22 3 2 24 B V 22 12 22 5 36 22 2 0 1, ,, , ,, ,, , 0,, , ,,0, 0, BUV UU UU DrU TKUV XY XY BVW BUV VVV D TUVVW XY XY BVW WWW DBWXY TVW XY UVW XY UXYU XYV 0 0 ,,0, 0, ,,0, 0,,. XYV XY W XYWXYXY (1) The reaction parameters are assumed to be posi co tive nstants and have the usual biological meaning. The positive constants 12 ,DD and 3 D are the diffusion coefficients of ,UV Wrespectively. and is the outward unit norm smooth bounry al vector to theda . The initial population densities 0,,UXY 0 VX and ,Y 0,WXY are assumed to be ous f Introduce the f positive and continu- unctions. ollowing non-dimensional variables and parameters so as to reduce the number of parameters of the system (2.1): 112 3 1 13 222 ,, ,, ,,, VW 2 , uv wtrT KK K D D rr xXyY dd DDDD U 3 1 13 12 ,, 2,4,5 i i BB B cc ci rr r ,6. The spatio-temporal system (2.1) is transformed to the following system of equations in non-dimensional vari- ables: 22 1 122 22 3 24 22 22 5 36 22 00 1,, ,, , ,, , 0,, , ,,0, 0,,,0, 0, , cuv , uu duuxy tuv xy cvw cuv vvv cv xy tuvvw xy cvw www dcwxy tvw xy uvwxy uxyu xyvxyv xy wxy u 0 ,0, 0,,.wxy xy (2) . Stability and Bifurcation Analysis he spatio-temporal system (2) has at most three spati- 3 T ally homogeneous non-negative steady states: 1) predators free steady state: 01, 0, 0E 2) top predator free steady state: ,,Eu 10v 42 cc 124 11 ,1,uc vu cc 3) coexistence of the three species: ,,Euvw ; 11 c1 1,1uv A ,Au 525 63564 c 1,A= cc wv ccccc 5 . c The equilibrium point lies in the first octant if and E Copyright © 2011 SciRes. AM  D. MELESE ET AL.1509 only if 56 1 ,1,0 1 ccA c (3) From biological point of view the stability of the non- tri ity analysis, the spatio-tem- po vial steady stateEwhich ensures th e coexistence of the three species is of interest. To perform linear stabil ral system (2) is linearized at the spatially homogene- ous steady state E for small space and time dependent fluctuations. For this, set ,,,, ;,,, ,,,, ;,,, ,,,, ;,,. uxytuu xytuxytu vxytvv xytvxytv wxytw wxytwxytw Let us assume solutions of the form 0 1 2 ,, cos cos, ,, txy vx ytekx ky wxyt ,,uxyt where 0,1 is the growth rate of perturbation in time t, ,2 ii represent the amplitudes, k and k umber of the solutions. The cos pondi linearized system has the characteristic equation are theve n warre ng 20.JkD I (4) Here 13 ,1,Ddiagdd, 222 y kk k and 33 () ij x Ja e elementsis the community matrix of the system (2). Th are obtained as 1 11 11213 22 2 2 26 2 212243 225 2 2 366 6 233132 5336 25 5 1 11,,0, 1,1 ,0,1 ,1 c aca a AA Ac c c aacc c AA ccc c aaacac c c5 , . c The characteristic equation corresponding to is E 3222 2 (5 21 0 0,bkbk bk ) with 3 , 22 21122331 ((1 )),bka aakdd 2 111331122223332232112 213223 11133 413 13 1(1) () bkaa aaaaaaaa kddada da kd ddd 2 0113322113223122133 221 12112232233322311133 46 133223111313 (). bkaaaaaa aaa kaaaadaaaadaa kdaaddadkdd The reaction-diffusion systems have led to the charac- terization of two basic types of symmetry-breaking bi- furcations—Hopf and Turing bifurcation, responsible for the emergence of spatio-temporal patterns. See, for de tails, references [15,16,23,24]. - According to Routh-Hurwitz criteria Re 0k if and only if 22222 02120 0, 0,0bk bk bkbkbk (6) Contradiction of any one of the above c- plies the existence of an eigenvalue withl part, hence, in onditions im positive rea stability. Turing instability (or diffusion driven instability) occurs if the homogeneous steady is stable in the absence of diffusion ) state E 2 (0k but driven unstable by diffusion 2 (0)k. Thus we need two conditions which must hold simultaneously. First, the spatially uniform steady state must be stable to small perturbation, that is, all 2 k in Equation (5) have 20 0k Re , and second, only patte cer- tain spatial extent, that is, patterns within a definite range of wave length k, can begin to grow, with rns of a 2 Re0 0k . It is clear that the homous steady state E is lly stable if and only if ogene locally asymptotica 000,b 200b and 12 0 00 00bb b. But it will be driven unstable by diffusion if any of the Equation (3.4) fail to it ca conditions in easily hold. However,n be seen that diffusion driven instability cannot occur by contradicting 2 20bk . Hence, we have tor which reverse the other two condi- tions in Equation (3.4). The expressions for look fo conditions sign of the 2 0 bk and 22 2 12 0 bk bkbk are both cubic functions of 2 k of the form: 2642 321030 ;0,0.FkFkFkFk FFF (7) The coefficients 0, 1,2,3Fi are given i n Table 1. i 2 () kFor to be negative for some positive real number 0 2 k , the minimum must be negative. This minimum occurs at 2 2 13 23. 2 2 3 3 c FFF k (8) kF Now 2 c k is real and positive if 2 12 21 0or0and3 3 FFF F (9) Hence, Copyright © 2011 SciRes. AM 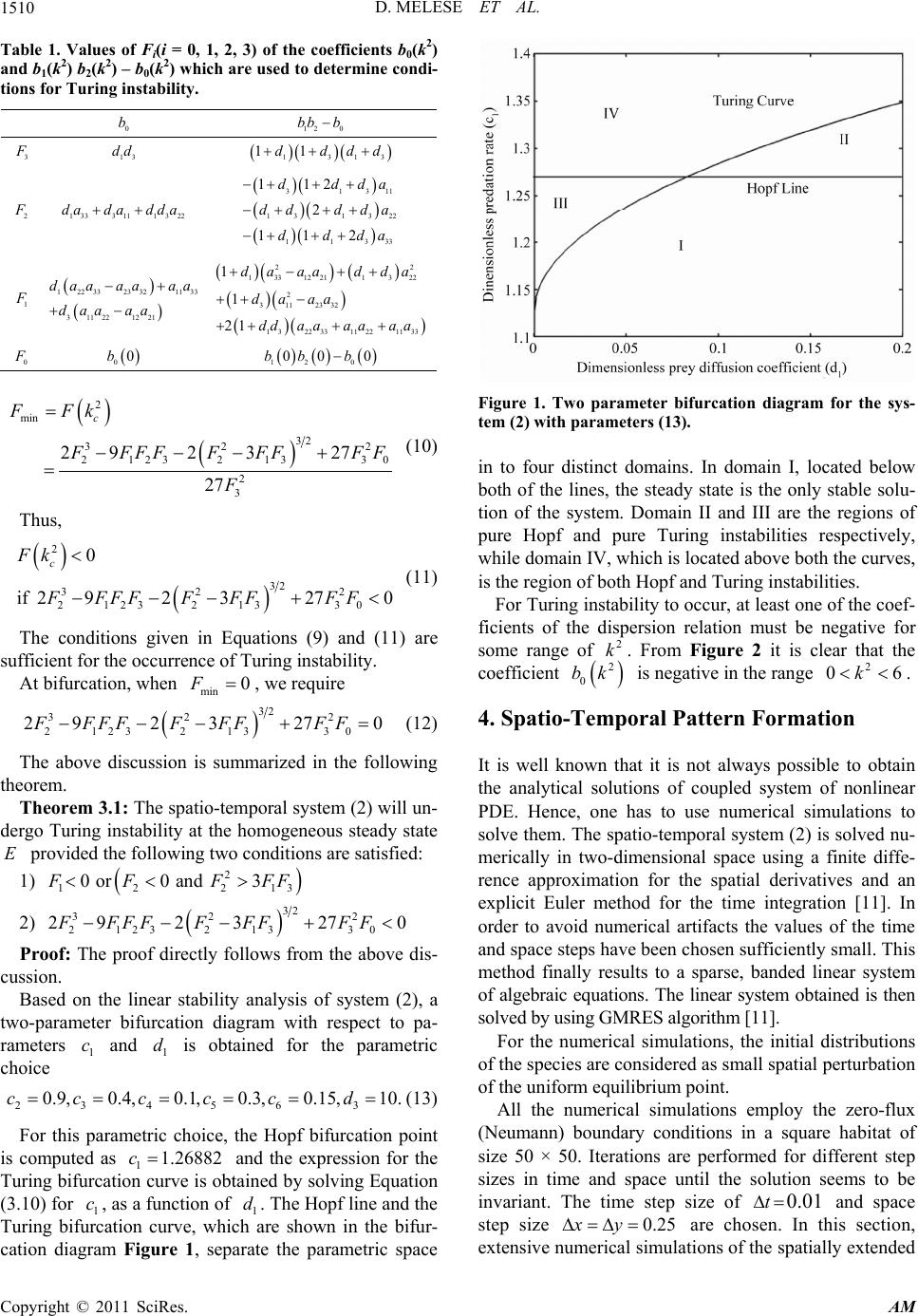 D. MELESE ET AL. 1510 Ta Va the coefficients b0(k2) and 1) b2k0d to determine co ndi- tions for Turing instability. ble 1. lues of Fi(i = 0, 1, 2, 3) of b(k2(2) – b(k2) which are use 0 b 12 0 bb b 3 13 dd 1313 11dddd 2 1 3331113 22 dada dda 112ddda 313 13 1322 1133 2 11 dd dda ddda 11 3 2 1 12233 23321133 31122 1221 daa aaaa daaaa 22 13312211322 2 3112332 1 3223311221133 1 1 21 da aadda daaa dd aaaaaa 0 00b 12 0 00 0bb b 2 min 32 32 21 2 3 2133 2 3 292 327 27 c FFk 2 0 FFFFFFF F F (10) Thus, 2 32 32 212321330 0 if 2923270 c Fk FFFF FFFFF 2 (11) ns given in Equations (9) and (11) are sufficient for the occurrence of Turing instability. At bifurcation, when The conditio min 0F , we require 32 32 2 292 3270FFFF FFFFF (12) lowing th Theorem 3.1: The spatio-temporal system (2) will un- dergo Turing instability at the homogeneous stead 3 21 23 21330 The above discussion is summarized in the fol eorem. y state E provided the following two conditions are satisfied: 1) 2 12 21 0or0and3 FFFF 2) 32 32 2 2123 21330 292 3270FFFF FFFFF Proof: The proof directly follows from the above dis- cussion. on the linear stability analysis of system (2), a twarameter bifurcation diagram with respect to ra rcattained by solving Equation (3.10) fo r . The Hopf line an Turi ca Based o-p pa- meters 1 c and 1 d is obtained for the parametric choice 234563 0.9,0.4, 0.1, 0.3,0.15, 10.cccc d(13) For this parametric choice, the Hopf bifurcation point is computed as 1.26882c and the expression for the Turing bifu c 1 ion curve is ob , as a function of 1 c1 dd the ng bifurcation curve, which are shown in the bifur- tion diagram Figure 1, separate the parametric space Figure 1. Two parameter bifurcation diagram for the sys- tem (2) with parameters (13). in to four distinct domains. In domain I, located below both of the lines, the steady state is the only stable solu- g instabilities respectively, hile domain IV, which is located above both the curves, tion of the system. Domain II and III are the regions of pure Hopf and pure Turin w is the region of both Hopf and Turing instabilities. For Turing instability to occur, at least one of the coef- ficients of the dispersion relation must be negative for some range of 2 k. From Figure 2 it is clear that the coefficient 2 0 bk is negative in the range 2 06k . 4 f nonlineto ed nu- erically in two-dimensional space using a finite diffe- re habitat of siz and space ste . Spatio-Temporal Pattern Formation It is well known that it is not always possible to obtain the analytical solutions of coupled system oar PDE. Hence, one has to use numerical simulations solve them. The spatio-temporal system (2) is solv m rence approximation for the spatial derivatives and an explicit Euler method for the time integration [11]. In order to avoid numerical artifacts the values of the time and space steps have been chosen sufficiently small. This method finally results to a sparse, banded linear system of algebraic equations. The linear system obtained is then solved by using GMRES algorithm [11]. For the numerical simulations, the initial distributions of the species are considered as small spatial perturbation of the uniform equilibrium point. All the numerical simulations employ the zero-flux (Neumann) boundary conditions in a squa e 50 × 50. Iterations are performed for different step sizes in time and space until the solution seems to be invariant. The time step size of 0.01t p size 0.25xy are chosen. In this section, extensive numerical simulations of the spatially extended Copyright © 2011 SciRes. AM 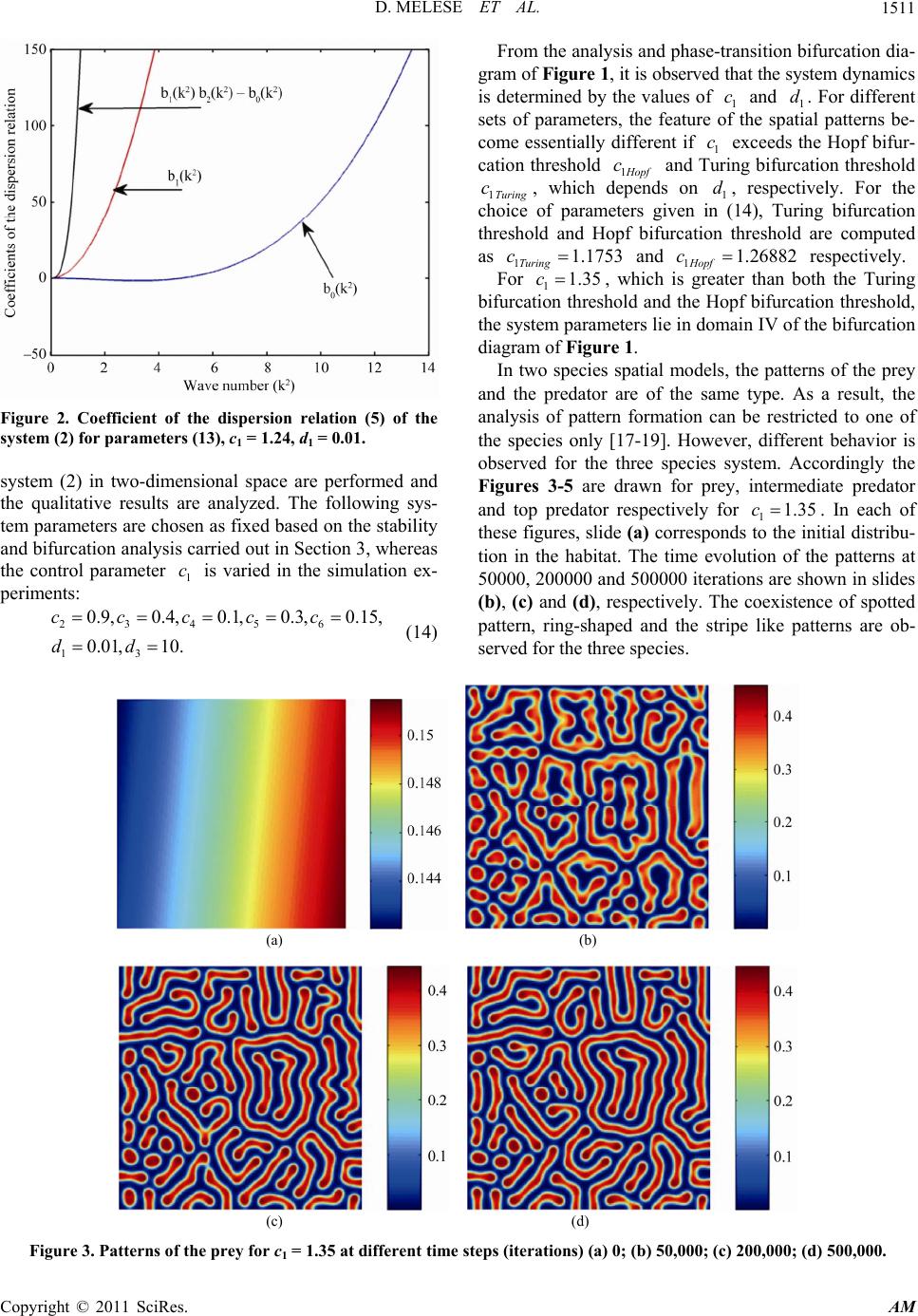 D. MELESE ET AL. Copyright © 2011 SciRes. AM 1511 From the analysis and phase-transition bifurcation dia- gram of Figure 1, it is observed tha t the syste m dy na mic s is determined by the valu es of 1 and 1. For different sets of parameters, the feature of the spatial patterns be- come essentially different if 1 exceeds the Hopf bifur- cation threshold 1 c d c opf and Turing bifu rcation threshold 1Turing , which depends on 1 d, respectively. For the choice of parameters given in (14), Turing bifurcation threshold and Hopf bifurcation threshold are computed as c 3 c c 1.175 and respectively. 1Turing 1Hopf For 1 1.268c82 1.35c , which is greater than both the Turing bifurcation threshold and the Hopf bifurcation threshold, the system parameters lie in domain IV of the bifurcation diagram of Figure 1. In two species spatial models, the patterns of the prey and the predator are of the same type. As a result, the analysis of pattern formation can be restricted to one of the species only [17-19]. However, different behavior is observed for the three species system. Accordingly the Figures 3-5 are drawn for prey, intermediate predator and top predator respectively for 1. In each of these figures, slide (a) corresponds to the initial distribu- tion in the habitat. The time evolution of the patterns at 50000, 200000 and 500000 iteration s are shown in slides (b), (c) and (d), respectively. The coexistence of spotted pattern, ring-shaped and the stripe like patterns are ob- served for the three species. 1.35c Figure 2. Coefficient of the dispersion relation (5) of the system (2) for parameters (13), c1 = 1.24, d1 = 0.01. system (2) in two-dimensional space are performed and the qualitative results are analyzed. The following sys e control parameter is varied in the simulation ex- - tem parameters are chosen as fixed based on the stability and bifurcation analysis carried out in Section 3, whereas th 1 periments: 23456 13 0.9, 0.4,0.1,0.3, 0.15, 0.01, 10. ccccc dd (14) c (a) (b) (c) (d) Figure 3. Patterns of the prey for c1 = 1.35 at different time steps (iterations) (a) 0; (b) 50,000; (c) 200,000; (d) 500,000. 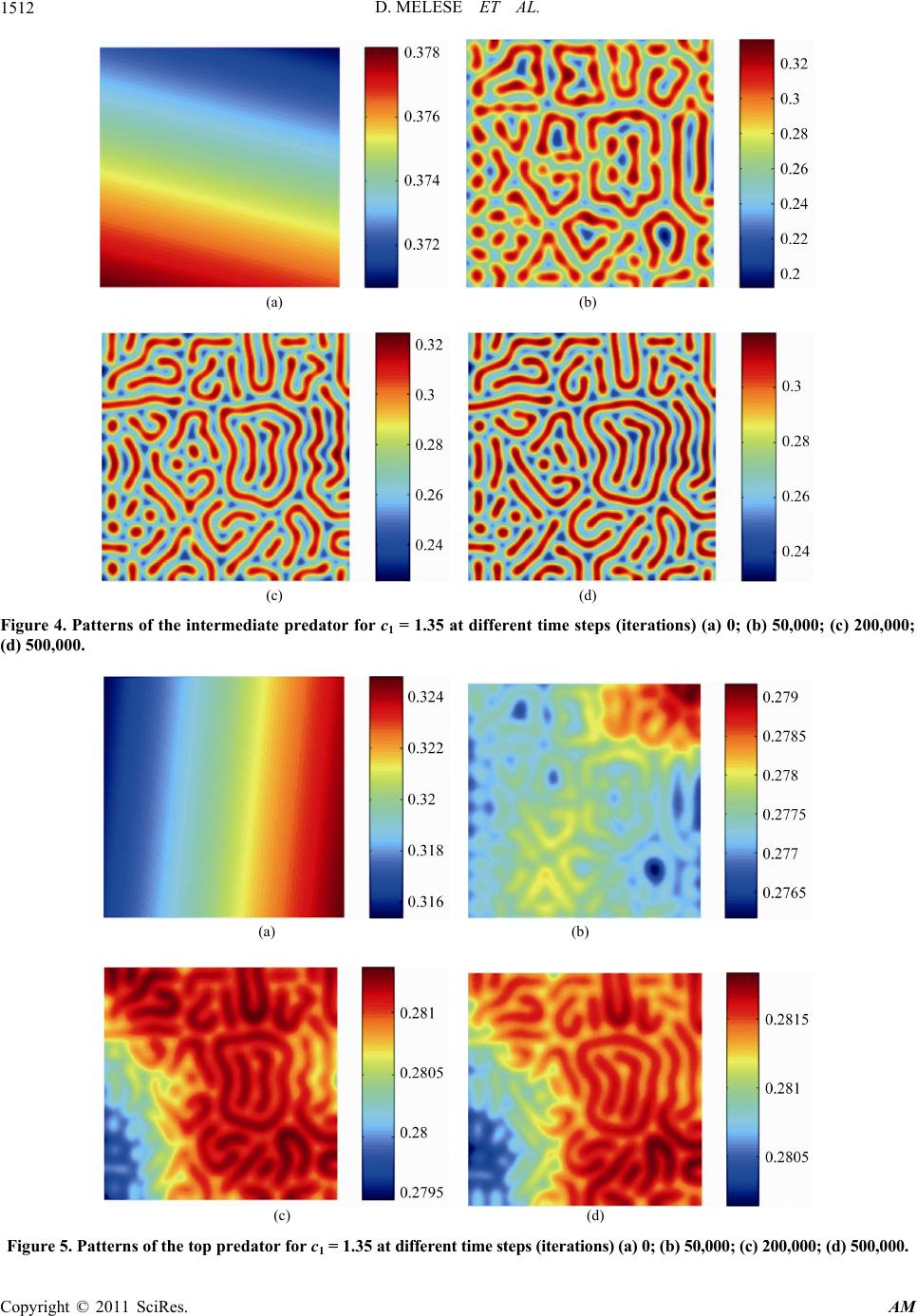 D. MELESE ET AL. 1512 (a) (b) (c) (d) Figure 4. Patterns of the intermediate preor for c1 = 1.35 at different time steps (iterations) (a) 0; (b) 50,000; (c) 200,000; (d) 500,000. dat (a) (b) (c) (d) Figure 5. Patterns of the top predator for c 1.35 at different time steps (iterations) (a) 0; (b) 50,000; (c) 200,000; (d) 500,000. 1 = Copyright © 2011 SciRes. AM  D. MELESE ET AL. Copyright © 2011 SciRes. AM 1513 In Figure 3, one can see that the small spatial perturb- bations to the homogeneous steady state of the spatio- temporal system (2) leads to the formation of spots and stripes of high prey density on a blue background of low prey density (c.f. Figure 3(b)). However, at later time some of the spots merge together to form stripes. This results in an increase in the number of stripes and a de- crease in the number of spots. Ring-shaped pattern is also formed (c.f. Figures 3(c) and (d)). The patterns of the intermediate predator shown in Figure 4 is structurally similar wi o ic ontrol parameter th that of the patterns f the prey shown in Figure 3. But, the background colo r s not spatially uniform and varying temporally. In par- i tular, the background density is decreasing with time. The basic skeleton of the pattern of the top predator (c.f. Figure 5) is similar with that of the patterns of the prey and intermediate predator. However the background density and the density in the patterns keep changing spatially as well as temporally. Patches of different den- sities are also visible. Figure 6 shows the steady state patterns of the three species for 11.24c. In this case, the c 1 c is greater than the Turing bifurcation threshold 1Turing c but less than the Hopf bifurcation threshold 1 opf c. That is, the parametric space is domain III of the bifurcation diagram of Figure 1. This means that the system has pure Turing instability and Turing patterns ar results show that the small spatial perturbations given to the homogeneous steady state lead to the formation of the stationary labyrinth like patterns. The density gradi- ent of the prey is greater than that of the intermediate predator and of the top predator. There is a sharp contrast between two consecutive ribbons in the case of prey. The contrast becomes progressively lower for intermediate predator to top predator. 5. Conclusions in region IV, both Turing and opf instability occur and ring, stripe-like and spotted In this study, the results show that the ratio-dependent In this paper, we have presented a theoretical analysis of evolutionary processes that involves organisms’ distribu- tion and trophic interaction in spatially extended envi- ronment with self diffusion. The numerical simulations were consistent with the theoretical findings that there are a range of parameters in 11 cd plane where the different spatial patterns emerge. When the parameters are located in region III of Figure 1, pure Turing insta- bility occurs and labyrinth like patterns emerge. Whereas when the parameters are H patterns coexist. tri-trophic model with Michaelis–Menten type functional response and diffusion represents rich spatial dynamics, such as labyrinth pattern, coexistence of ring-shaped pattern, spotted pattern and stripe-like pattern, which will e expected for this choice. The numerical simulation (b) (a) (c) Figure 6. Steady state patternop predator (c) for c1 = 1.24. s of the prey (a), intermediate predator (b) and t  D. MELESE ET AL. 1514 be useful for studying the dynamic complexity of eco- systems or physical systems. 6. References [1] A. Al-Khedhairi, “The Chaos and Control of Food Chain Model Using Nonlinear Feedback,” Applied Mathemati- cal Sciences, Vol. 3, No. 12, 2009, pp. 591-604. [2] N. J. Gotelli and A. M. Ellison, “Food-Web Models Pre- dict Species Abundances in Response to Habitat Change,” PLoS Biology, Vol. 4, No. 10, 2006, pp. 1869-1873. doi:10.1371/journal.pbio.0040324 [3] S. Gakkhar and R. K. Naji, “Chaos in Three Species Ra- tio Dependent Food Chain,” Chaos, Solitons and Fractals, Vol. 14, No. 5, 2002, pp. 771-778. doi:10.1016/S0960-0779(02)00038-3 4] S. B. Hsu, T. W. Hwang[ and Y. Kuang, “A R od Chain Model and Its Applications to trol,” Mathematical Biosciences, Vol. 181, No. 1, 2003, pp. 55-83. atio- Dependent Fo Biological Con doi:10.1016/S0025-5564(02)00127-X [5] K. A. J. White and C. A. Gilligan, “Spatial Heterogeneity in Three-Species, Plant-Parasite-Hyperparasite, Systems,” Philosophical Transactions of Royal Society B, Vol. 353, No. 1368, 1998, pp. 543-557. doi:10.1098/rstb.1998.0226 [6] C. Neuhauser and S. W. Pacala, “An Explicitly Spatial Version of Lotka-Voltera Model with Inter-Specific Competition,” The Annals of Applied Probability, Vol. 9, No. 4, 1999, pp. 1226-1259. doi:10.1214/aoap/1029962871 [7] K. S. McCann, J. B. Rasmussen and J. Umbanhowar, “The Dynamics of Spatially Coupled Food Webs,” Ecol- ogy Letters, Vol. 8, No. 5, 2005, pp. 513-523. doi:10.1111/j.1461-0248.2005.00742.x Chon and T. Ma-[8] S. H. Lee, H. K. Pak, H. S. Wi, T. S. tsumoto, “Growth Dynamics of Domain Pattern in a Three-Trophic Population Model,” Physica A, Vol. 334, No. 1-2, 2004, pp. 233-242. doi:10.1016/j.physa.2003.11.017 eis and M. A. M. de Aguiar, [9] D. O. Maionchi, S. F. dos R “Chaos and Pattern Formation in a Spatial Tritrophic Food Chain,” Ecological Modelling, Vol. 191, No. 2, 2006, pp. 291-303. doi:10.1016/j.ecolmodel.2005.04.028 [10] M. Wang, “Stationary Patterns f with Prey-Dependent and Rator a Prey-Predator Model io-Dependent Functional Responses and Diffusion,” Physica D, Vol. 196, No. 1-2, 2004, pp. 172-192. doi:10.1016/j.physd.2004.05.007 [11] M. R. Garvie, “Finite-Difference Schemes for Diffusion Equations Modeling Predator- Reac Prey Interactions tion- in MATLAB,” Bulletin of Mathematical Biology, Vol. 69, No. 3, 2007, pp. 931-956. doi:10.1007/s11538-006-9062-3 [12] A. B. Medvinsky, S. V. Petrovskii, I. A. Malchow and B. L. Li, “Spatiote Tikhonov, H. mporal Complexity of Plankton and Fish Dynamics,” SIAM Review, Vol. 44, No. 3, 2002, pp. 311-370. doi:10.1137/S0036144502404442 [13] S. V. Petrovskii, B. L. Li and H. Malchow, “Transition to Spatiotemporal Chaos Can Resolve the Paradox of En- richment,” Ecological Complexity, Vol. 1, No. 1, 2004, pp. 37-47. doi:10.1016/j.ecocom.2003.10.001 [14] H. Shen and Z. Jin, “Two Dimensional Pattern Formation of Prey-Predator System,” Eighth ACIS International Conference on Software Engineering, Artificial Intelli- gence, Networking, and Parallel/Distributed Computing, Qingdao, 30 July - 1 August 2007, pp. 343-346. doi:10.1109/SNPD.2007.215 genesis,” ol. 237, [15] A. M. Turing, “The Chemical Basis of Morpho Philosophical Transactions of Royal Society B, V No. 641, 1952, pp. 37-72. doi:10.1098/rstb.1952.0012 [16] M. Banerjee and S.V. Petrovskii, “Self-Organized Spatial Patterns and Chaos in a Ratio-Dependent Predator Prey System,” Theoretical Ecology, Vol. 4, No. 1, 2011, pp. 37-53. doi:10.1007/s12080-010-0073-1 [17] W. Wang, L. Zhang, Y. Xue and Z. Jin, “Spatiotemporal oral Com- with Constant Harvest Pattern Formation of Beddington-DeAngelis-Type Preda- tor-Prey Model,” arXiv: 0801.0797v1 [q-bio.PE], January 2008. [18] L. Zhang, W. Wang and Y. Xue, “Spatiotemp plexity of a Predator-Prey System Rate,” Chaos Solitons Fractals, Vol. 41, No. 1, 2009, pp. 38-46. doi:10.1016/j.chaos.2007.11.009 [19] W. Wang, Q. X. Liu and Z. Jin, “Spatiotemporal Com- plexity of a Ratio-Dependent P Physical Review E, Vol. 75, No. redator-Prey System,” 5, 2007, Article ID 051913. doi:10.1103/PhysRevE.75.051913 [20] J. D. Murray, “Mathematical Biology II: Spatial Models and Biomedical Applications,” Springer, Berlin, 2003. G. Sun, Z. Jin, Q. X. Liu and L. Li, “Pattern Fo[21] rmation in a Spatial S-I model with Nonlinear Incidence Rates,” Journal of Statistical Mechanics: Theory and Experiment, Vol. 2007, 2007, P11011. doi:10.1088/1742-5468/2007/11/P11011 [22] H. Malchow, “Spatio-Temporal Pattern Formation in Coupled Models of Plankton Dynamics and Fish School Motion,” Nonlinear Analysis: Real World Applications, Vol. 1, No. 1, 2000, pp. 53-67. doi:10.1016/S0362-546X(99)00393-4 [23] S. B. L. Araújo and M. A. M. de Aguiar, “Pattern For- mation, Outbreaks, and Synchronization in Food Chains with Two and Three Species,” Physical Review E, Vol. 75, No. 6, 2007, Article ID 061908. doi:10.1103/PhysRevE.75.061908 [24] W. Ko and I. Ahn, “Analysis of Ratio-Dependent Food Chain Model,” Journal of Mathematical Analysis Appli- cations, Vol. 335, No. 1, 2007, pp. 498-523. doi:10.1016/j.jmaa.2007.01.089 Copyright © 2011 SciRes. AM
|