 International Journal of Organic Chemistry, 2011, 1, 176-182 doi:10.4236/ijoc.2011.14026 Published Online December 2011 (http://www.SciRP.org/journal/ijoc) Copyright © 2011 SciRes. IJOC BF3·OEt2-Mediated Benzylation of Arenes and Heteroarenes with Benzyl Ether Derivatives Ling Dang, Qiang Li, Tongmei Ma*, Sheng Sheng, Wei Zeng* School of Chemistry and Chemical Engineering, South China University of Technology , Guangzhou, China E-mail: {*zengwei, *tongmei}@scut.edu.cn Received October 18, 2011; revised November 26, 2011; accepted December 3, 2011 Abstract An efficient BF3·Et2O-promoted benzylation of arenes and heteroarenes with various benzyl ether derivatives has been developed. This method provided alternative access to valuable diarylmethane in good yields under mild conditions via an easy work-up procedure. Keywords: Friedel-Crafts Alkylation, BF3·Et2O, Diarylmethane, Benzyl Ether Derivatives, Arenes 1. Introduction Diarylmethane motifs are an important class of aromatic building blocks which are found in various biologically active compounds such as anastrozole, papaverine, piri- trexim, avrainvilleol, and beclobrate [1-5]. They are also used as key precursors in the synthesis of electroactive and photoactive oligomers and polymers [6,7]. Consequ- ently, various methodologies for the alkylation of arenes and heteroarenes have been developed [8-10]. Among the various functionalization reactions of arenes, Friedel- Crafts alkylation (FCA) provides an efficient access to building a diarylmethane framwork via the electrophilic addition of cationic species to aromatic compounds. Previous work about FCA has mainly centred on using alcohols [11-14], benzyl halides [15-18], aldehyde [19- 21], carboxylic acid ester [22,23] or alkenes [24-26] as the alkylating agents in the presence of kinds of Brønsted acids, Lewis acids and transition metal salts [11-26], but there have not been many trials for the development of FCA with ethers as electrophilics possibly due to the stable ether bond. Nevertheless, recently Peris and Shiina group, etc. demonstrated that Ir-NHC complexes, SiCl4/ AgOTf and Hf(OTf)4 et al. could efficiently catalyze alk- yaltion of arenes using ethers as alkyalting agents succes- sively [24,27-30]. Considering the wide application of dia- rylmethane derivatives in the synthesis of biological mo- lecules, the exploration of more economic catalysts for al- kylation of arenes with ethers under mild conditions is de- sirable. Recently, we have focused our attention on Lewis acid- catalyzed N-alkylation of sulfonamides using unactive ethers as alkyalting agents. During the period that we investigated the effect of various Lewis acids on the N- alkylation of 4-toluene sulfonamide 5 in toluene solvent, we found BF3·Et2O could enhance the formation of by- product diarylmethane 3a and 4a (around 10% yield) from the alkyaltion of toluene 2a and benzyl ether 1a (see Scheme 1), and further studies indicated that bypro- duct diarylmethane 3a and 4a did not form in the pres- ence of other Lewis acids including AlCl3, TiCl4, Cu- (OAc)2·2H2O, CdCl2·2.5H2O, ZrCl4, MgO, etc. Herein, we reported our further studies about BF3·Et2O-promoted benzylation of arenes with benzyl ether derivatives. 2. Results and Discussions Initially, the alkylation of toluene with dibenzyl ether was employed as a model reaction, optimization studies were carried out under different conditions, and the correspon- ding results were summarized in Table 1. As shown in Table 1, when the FCA of toulene (6.0 equiv.) with di- benzyl ether (0.5 mmol) was carried out using BF3·Et2O (3.0 equiv.) as promoter under refluxing condition in Scheme 1. Lewis acid-poromoted N-alkylation of sulfonami- de and alkylation of toluene. 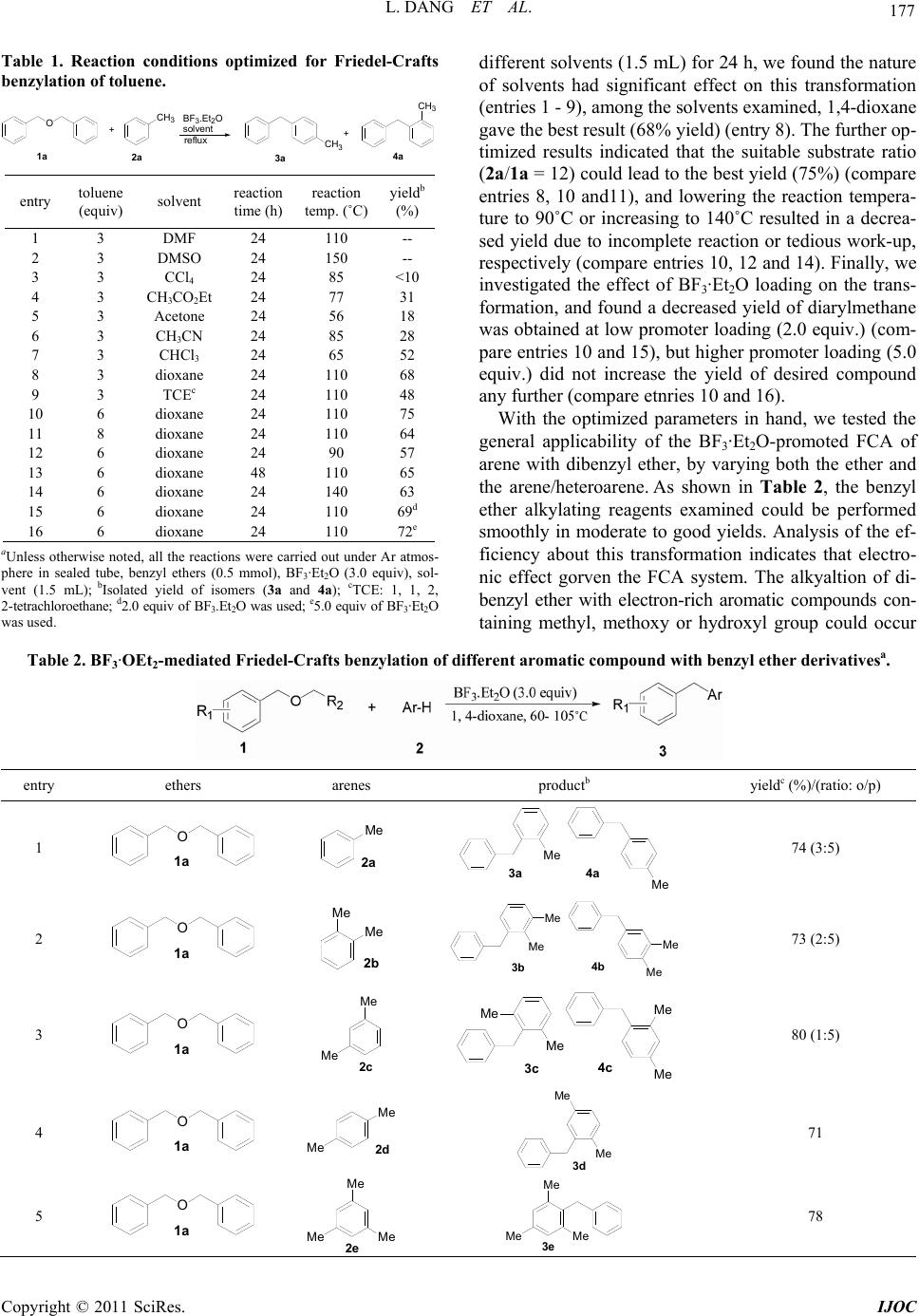 177 L. DANG ET AL. Table 1. Reaction conditions optimized for Friedel-Crafts benzylation of toluene. O+CH 3 BF 3 .Et 2 O solvent CH 3 CH 3 reflux + 1a 2a 3a 4a entry toluene (equiv) solvent reaction time (h) reaction temp. (˚C) yieldb (%) 1 3 DMF 24 110 -- 2 3 DMSO 24 150 -- 3 3 CCl4 24 85 <10 4 3 CH3CO2Et 24 77 31 5 3 Acetone 24 56 18 6 3 CH3CN 24 85 28 7 3 CHCl3 24 65 52 8 3 dioxane 24 110 68 9 3 TCEc 24 110 48 10 6 dioxane 24 110 75 11 8 dioxane 24 110 64 12 6 dioxane 24 90 57 13 6 dioxane 48 110 65 14 6 dioxane 24 140 63 15 6 dioxane 24 110 69d 16 6 dioxane 24 110 72e aUnless otherwise noted, all the reactions were carried out under Ar atmos- phere in sealed tube, benzyl ethers (0.5 mmol), BF3·Et2O (3.0 equiv), sol- vent (1.5 mL); b Isolated yield of isomers (3a and 4a); cTCE: 1, 1, 2, 2-tetrachloroethane; d2.0 equiv of BF3.Et2O was used; e5.0 equiv of BF3·Et2O was used. different solvents (1.5 mL) for 24 h, we found the nature of solvents had significant effect on this transformation (entries 1 - 9), among the solvents examined, 1,4-dioxane gave the best result (68% yield) (entry 8). The further op- timized results indicated that the suitable substrate ratio (2a/1a = 12) could lead to the best yield (75%) (compare entries 8, 10 and11), and lowering the reaction tempera- ture to 90˚C or increasing to 140˚C resulted in a decrea- sed yield due to incomplete reaction or tedious work-up, respectively (compare entries 10, 12 and 14). Finally, we investigated the effect of BF3·Et2O loading on the trans- formation, and found a decreased yield of diarylmethane was obtained at low promoter loading (2.0 equiv.) (com- pare entries 10 and 15), but higher promoter loading (5.0 equiv.) did not increase the yield of desired compound any further (compare etnries 10 and 16). With the optimized parameters in hand, we tested the general applicability of the BF3·Et2O-promoted FCA of arene with dibenzyl ether, by varying both the ether and the arene/heteroarene. As shown in Table 2, the benzyl ether alkylating reagents examined could be performed smoothly in moderate to good yields. Analysis of the ef- ficiency about this transformation indicates that electro- nic effect gorven the FCA system. The alkyaltion of di- benzyl ether with electron-rich aromatic compounds con- taining methyl, methoxy or hydroxyl group could occur Table 2. BF3·OEt2-mediated Friedel-Crafts benzylation of different aromatic compound with benzyl ether derivativesa. entry ethers arenes productb yieldc (%)/(ratio: o/p) 1 O 1a Me 2a M 3a Me 4a 74 (3:5) 2 O 1a Me Me 2b Me 3b Me 4b Me Me 73 (2:5) 3 O 1a Me 2c Me M 3c Me 4c Me Me 80 (1:5) 4 O 1a 2d Me Me Me 3d Me 71 5 O 1a 2e Me Me Me 3e Me Me Me 78 Copyright © 2011 SciRes. IJOC 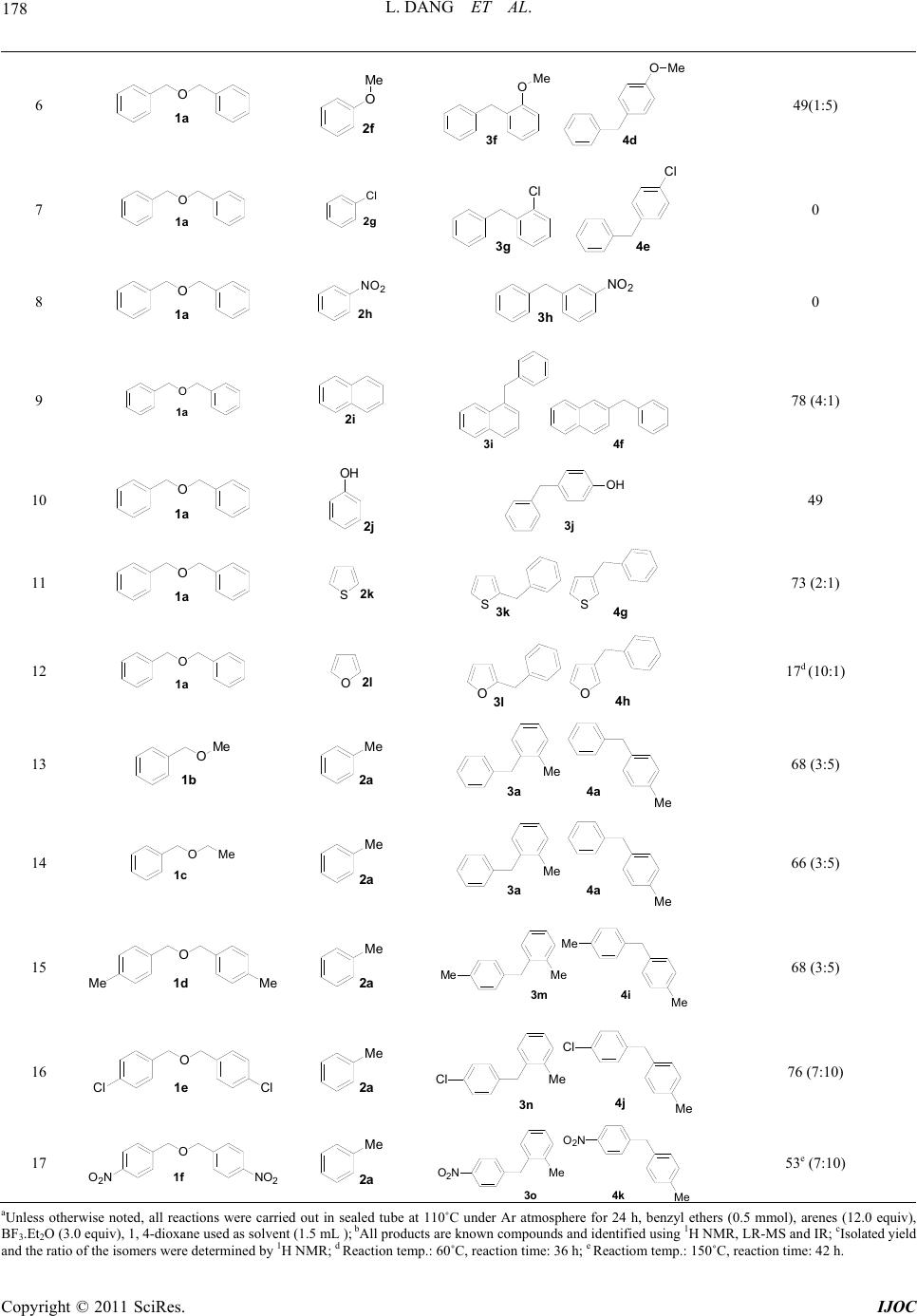 L. DANG ET AL. 178 6 O 1a O Me 2f 3f O 4d OMe Me 49(1:5) 7 O 1a Cl 2g 3g Cl 4e Cl 0 8 O 1a NO 2 2h 3h NO 2 0 9 O 1a 2i 3i 4 78 (4:1) 10 O 1a OH 2 OH 3 49 11 O 1a S2k SS 3k 4 73 (2:1) 12 O 1a O2l OO 3l 4h 17d (10:1) 13 OMe 1b Me 2a M 3a Me 4a 68 (3:5) 14 O 1c Me Me 2a M 3a Me 4a 66 (3:5) 15 O 1d Me Me Me 2a M 3m Me 4i Me Me 68 (3:5) 16 O 1e Cl Cl Me 2a M 3n Me 4j Cl Cl 76 (7:10) 17 O 1f O NNO Me 2a Me 3o Me 4k O 2 N O 2 N 53e (7:10) aUnless otherwise noted, all reactions were carried out in sealed tube at 110˚C under Ar atmosphere for 24 h, benzyl ethers (0.5 mmol), arenes (12.0 equiv), BF3.Et2O (3.0 equiv), 1, 4-dioxane used as solvent (1.5 mL ); bAll products are known compounds and identified using 1H NMR, LR-MS and IR; cIsolated yield and the ratio of the isomers were determined by 1H NMR; d Reaction temp.: 60˚C, reaction time: 36 h; e Reactiom temp.: 150˚C, reaction time: 42 h. Copyright © 2011 SciRes. IJOC  L. DANG ET AL. Copyright © 2011 SciRes. IJOC 179 efficiently to afford diarylmethan in moderate to good yield (entries 1 - 6). On the contray, benzyl ether deri- vates with an electon-withdrawing group such as nitro gave lower yield of the corresponding alkyalted products (compare entries 1 and 17). Even worse, if arenes had a chloride or nitro group, the FCA did not occur at all (en- tries 7 and 8). It is worth to note that BF3.Et2O could also efficiently promote the benzylation of heteroarenes such as thiophene and furan to give corresponding dihet- eroaryl-methane (entries 11 and 12), but we found that the arene substrates containing oxygen hetero-atom gave low yield (17% - 49%) of alkyalted product (entries 6, 10 and 12), this is possibly due to that the coordination of the ether oxygen or phenolic oxygen with B(III) decreased the electron density in the arene ring, and resulted in poor reactivity. It is more interesting that oxygen heteroatom- containing substrates such as 2f, 2j and 2l gave poor yields of benzylated products, but better regioselectivity was observed (compare entries 1 and 6, 10 and 12), espe- cially for substrate 2j, 100% regioselectivity was achi- eved (entry 10). 3. Conclusions In conclusion, we have demonstrated a BF3·Et2O-medi- ated benzylation of arenes and heteroarenes using benzyl ethers as the alkylating agents. Various ethers, arenes and heteroarenes were systematically studied, and low reac- tivity of oxygen-hetero atom-containging arenes was also observed. This method used inexpensive and comer- cially available BF3·Et2O as promoter, and provided com- plementary access to valuable diarylmethane under mild conditions. 4. General Experimental Informatio n Unless otherwise noted, all experiments were performed under Ar atmosphere in a sealed tube. All reagents were purchased from TCI, Acros or strem. Solvents were treat- ed with 4Å molecular sieves or sodium and distilled prior to use. Purifications of reaction products were car- ried out by flash chromatography using silica gel (40 - 63 mm) from Qingdao Haiyang Chemical Co. Ltd. Infrared spec- tra (IR) were recorded on a Brucker TENSOR 27 FTIR spectrophotometer and are reported as wavelength num- bers (cm–1). 1H NMR spectra were recorded on a Bruker Avance 400 MHz spectrometer. Chemical shifts are re- ported in parts per million (ppm) and coupling con- stants are reported as Hertz (Hz). Splitting patterns are designated as singlet (s), broad singlet (bs), doublet (d), triplet (t). Splitting patterns that could not be interpreted or easily visualized are designated as multiplet (m). Low resolution mass spectra were recorded using GC-MS. General Procedure Benzyl ether derivatives (0.5 mmol), arenes or heteroa- renes (6.0 mmol), BF3.OEt2 (3.0 equiv, 1.5 mmol) and 1, 4-dioxane (1.5 mL) were combined in a pressure tube equipped with a stir bar, The mixture was heated to given temperature and stirred for the given time under Ar at- mosphere. When the starting material has disappeared (monitored by TLC), the reaction mixture was treated with 10.0 mL of H2O, filtrated and the corresponding filtrate was extracted with ethyl acetate (3 × 15 mL), the combined organic layers were concentrated, and the residue was purified by flash column chromatography (si- lica gel) to furnish target product. All products are kno- wn compounds and identified using 1H NMR, LRMS and IR by comparison with previously reported data. 1-Benzyl-4-methylbenzene (3a) [31] and 1-benzyl-2- methylbenzene (4a) [32] (3a/4a = 5:3), liquid. 3a: 1H NMR (400 MHz, CDCl3) δ: 7.24 (d, J = 6.1 Hz, 2 H), 7.04 (m, 7 H), 3.92 (s, 2H), 2.29 (s, 3 H); 4a: 1H NMR (400 MHz, CDCl3) δ: 7.24 (d, J = 6.1 Hz, 2 H), 7.04 (m, 7 H), 3.97 (s, 2 H), 2.22 (s, 3 H); MS (ESI): m/z = 182.26 [M]+; IR (KBr): 3294, 1720, 1597, 1443, 1332, 1210, 1167, 1027 cm–1. 4-Benzyl-1,2-dimethylbenzene (3b) [33] and 1-ben- zyl-2,3-dimethylbenzene (4b) [34] ( 3b/4b = 5:2), liquid. 3b 1H NMR (400 MHz, CDCl3) δ: 7.28 - 7.22 (m, 2 H), 7.17 (d, J = 7.3 Hz, 2 H), 7.10 (d, J = 7.4 Hz, 1H), 7.08 - 7.00 (m, 1H), 6.99 - 6.94 (m, 1H), 6.91 (d, J = 7.6 Hz, 1 H), 3.90 (s, 2 H), 2.21 (s, 6H); 4b: 1H NMR (400 MHz, CDCl3) δ: 7.28 - 7.22 (m, 2 H), 7.17 (d, J = 7.3 Hz, 2 H), 7.10 (d, J = 7.4 Hz, 1 H), 7.08 - 7.00 (m, 1 H), 6.99 - 6.94 (m, 1 H), 6.91 (d, J = 7.6 Hz, 1 H), 4.00 (s, 2 H), 2.27 (s, 3 H), 2.12 (s, 3 H); MS (ESI): m/z = 196.28 [M]+; IR (KBr): 2923, 2854, 1817, 1764, 1727, 1588, 1481, 1095, 1026, 827, 741, 666, 567 cm–1. 1-Benzyl-2,4-dimethylbenzene (3c) [35] and 2-benzyl- 1,3-dimethylbenzene (4c) [33] (3c/4c = 5:1), liquid. 3c 1H NMR (400 MHz , CDCl3) δ: 7.29 - 6.90 (m, 7 H), 3.93 (s, 2 H), 2.29 (s, 3 H),2.19 (s, 3 H); 4c: 1H NMR (400 MHz, CDCl3 ) δ: 7.29 - 6.90 (m, 7 H), 4.04 (s, 2 H), 2.23 (s, 6 H); MS (ESI): m/z = 196.34 [M]+, IR (KBr): 3119, 2921, 2853, 1742, 1696, 1516, 1462, 1355, 1229, 853, 634, 499 cm–1. 2-Benzyl-1,4-dimethylbenzene (3d) [33], liquid. 1H NMR (400 MHz, CDCl3) δ: 7.25 (t, J = 7.4 Hz, 2 H), 7.14 (dd, J = 25.9, 8.2 Hz, 3 H), 7.04 (d, J = 7.9 Hz, 1 H), 6.97 - 6.90 (m, 2 H), 3.94 (s, 2 H), 2.28 (s, 3 H), 2.18 (s, 3 H); MS (ESI): m/z = 196.28 [M]+, IR (KBr): 2924, 2856, 1734, 1504, 1458, 1377, 811, 735, 628 cm–1. 2-Benzyl-1,3,5-trimethylbenzene (3e) [33], liquid. 1H NMR (400 MHz , CDCl3) δ: 7.20 (d, J = 7.8 Hz, 2 H), 7.14 (d, J = 7.1 Hz, 1 H), 7.00 (d, J = 7.5 Hz, 2 H), 6.88  L. DANG ET AL. 180 (s, 2 H), 4.01 (s, 2 H), 2.28 (s, 3 H), 2.20 (s, 6 H); MS (ESI): m/z = 210.31 [M]+, IR (KBr): 2923, 2856, 1640, 1515, 1457, 1385, 1319, 1257, 1097, 852, 691, 591 cm–1. 1-Benzyl-4-methoxybenzene (3f) [36] and 1-benzyl-2- methoxybenzene (4d) [15] (3f/4d = 5:1), liquid. 3f: 1H NMR (400 MHz, CDCl3 ) δ: 7.30 - 7.14 (m, 6 H), 7.08 (dd, J = 16.4, 7.5 Hz, 1 H), 6.88 – 6.80 (m, 2 H), 3.92 (s, 2 H), 3.77 (s, 3 H); 4d: 1H NMR (400 MHz, CDCl3 ) δ: 7.30 - 7.14 (m, 6 H), 7.08 (dd, J = 16.4, 7.5 Hz, 1 H), 6.88 - 6.80 (m, 2 H), 3.97 (s, 2 H), 3.80 (s, 3 H); MS (ESI): m/z= 198.26 [M]+, IR (KBr): 3459, 2924, 2856, 1726, 1599, 1459, 1384, 1248, 1101, 1033, 740, 547 cm–1. 2-Benzylnaphthalene (3i) [37] and 1-benzylnaphtha- lene (4f)14 (3i/4f =3:1), liquid. 3i: 1H NMR (400 MHz, CDCl3) δ: 7.76 (dd, J = 13.3, 7.8 Hz, 3 H), 7.28 - 7.13 (m, 9 H), 4.12 (s, 2 H); 4f: 1H NMR (400 MHz, CDCl3 ) δ: 7.99 - 7.96 (m, 2 H), 7.85 - 7.84 (m, 2 H), 7.44 - 7.37 (m, 8 H), 4.43 (s, 2 H); MS (ESI): m/z = 218.29 [M]+; IR (KBr): 3054, 2922, 2855, 1812, 1724, 1596, 1496, 1446, 1388, 1263, 1076, 856, 786, 738, 706, 590, 513 cm–1. 4-Benzylphenol (3j) [38], liquid. 3j: 1H NMR (400 MHz, CDCl3) δ: 7.31 - 7.25 (m, 2 H), 7.24 - 7.17 (m, 3 H), 7.12 (dd, J = 11.5, 4.5 Hz, 2 H), 6.91 - 6.85 (m, 1 H), 6.75 (d, J = 8.2 Hz, 1 H), 4.75 (s, 1 H), 3.98 (s, 2 H); MS (ESI): m/z = 184.23 [M]+, IR (KBr): 3678, 3029, 2890, 2824, 1746, 1700, 1496, 1333, 844, 746 cm–1. 2-Benzylthiophene (3k) [39] and 3-benzylthiophene (4g) 18 (3k/4g = 2:1), liquid. 3k: 1H NMR (400 MHz , CDCl3) δ: 7.34 - 7.10 (m, 6 H), 6.90 (d, J = 0.8 Hz, 2 H), 4.14 (s, 2 H); 4g: 1H NMR (400 MHz, CDCl3) δ: 7.34 - 7.10 (m, 6 H), 6.90 (d, J = 0.8 Hz, 2 H), 3.97 (s, 2 H); MS (ESI): m/z = 174.29 [M]+; IR (KBr): 2923, 2855, 1739, 1639, 1458, 1384, 1264, 1203, 1264, 1203, 1096, 1030, 932, 741, 704, 558 cm–1. 2-Benzylfuran (3l) [18] and 3-benzylfuran (4h) [39] (3l/4h = 10:1), liquid. 3l: 1H NMR (400 MHz, CDCl3) δ: 7.30 (dd, J = 12.0, 3.7 Hz, 3 H), 7.23 (d, J = 7.0 Hz, 3 H), 6.28 (dd, J = 2.9, 1.9 Hz, 1 H), 6.00 (dd, J = 3.1, 0.7 Hz, 1 H), 3.96 (s, 2 H); 4h: 1H NMR (400 MHz, CDCl3) δ: 7.30 (dd, J = 12.0, 3.7 Hz, 3 H), 7.23 (d, J = 7.0 Hz, 3 H), 6.28 (dd, J = 2.9, 1.9 Hz, 1 H), 6.00 (dd, J = 3.1, 0.7 Hz, 1 H), 3.77 (s, 2 H); MS (ESI): m/z = 158.20 [M]+; IR (KBr): 2924, 2855, 1884, 1724, 1598, 1456, 1385, 1263, 1096, 1027 ,932, 740, 556 cm–1. di-p-Tolylmethane (3m) [40] and o-tolyl(p-tolyl)meth- ane (4i) [19] (3m/4i = 5:3), liquid. 3m: 1H NMR (400 MHz, CDCl3 ) δ: 7.17 - 6.95 (m, 8 H), 3.88 (s, 2 H), 2.29 (s, 6 H); 4i: 1H NMR (400 MHz, CDCl3) δ: 7.17 - 6.95 (m, 8 H), 3.93 (s, 2 H), 2.29 (s, 3 H), 2.23 (s, 3 H); MS (ESI): m/z = 196.29 [M]+, IR (KBr): 2924, 2857, 1732, 1597, 1456, 1382, 1263, 1104, 1034, 799, 739, 607 cm–1. 1-(4-Chlorobenzyl)-4-methylbenzene (3n) [41] and 1 -(4-chlorobenzyl)-2-methylbenzene (4j) [42], (3n/4j = 10:7), liquid. 3n: 1H NMR (400 MHz, CDCl3 ) δ: 7.26 - 7.20 (m, 2 H), 7.15 (d, J = 9.1 Hz, 2 H), 7.13 - 7.00 (m, 4 H), 6.95 (d, J = 8.6 Hz, 1 H), 3.89 (s, 2 H), 2.31 (s, 3 H); 4j: 1H NMR (400 MHz,, CDCl3 ) δ: 7.26 - 7.20 (m, 2 H), 7.15 (d, J = 9.1 Hz, 2 H), 7.13 - 7.00 (m, 4 H), 3.93 (s, 2 H), 2.21 (s, 3 H); MS (ESI): m/z = 216.70[M]; IR (KBr): 2923, 2856, 1885, 1811, 1720, 1660, 1593, 1454, 1384, 1261, 1096, 798, 739, 698, 541 cm–1. 1-(4-Nitrobenzyl)-4-methylbenzene (3o) [43] and 1- (4-nitrobenzyl)-2-methylbenzene (4k) [43] (3o/4k = 10: 7), liquid. 3o: 1H NMR (400 MHz, CDCl3 ) δ: 8.13 (dd, J = 8.2, 3.8 Hz, 2 H), 7.33 (dd, J = 8.5, 3.6 Hz, 2 H), 7.26 (d, J = 9.3 Hz, 2 H), 7.23 - 7.16 (m, 2 H), 4.03 (s, 2 H), 2.33 (s, 3 H); 4k: 1H NMR (400 MHz, CDCl3) δ: 8.13 (dd, J = 8.2, 3.8 Hz, 2 H), 7.33 (dd, J = 8.5, 3.6 Hz, 2 H), 7.26 (d, J = 9.3 Hz, 2 H), 7.23 - 7.16 (m, 2 H), 4.08 (s, 2 H), 2.21 (s, 3 H); MS (ESI): m/z = 227.25 [M]+; IR (KBr): 3021, 2922, 2858, 1924, 1729, 1599, 1515, 1342, 1179, 1106, 1026, 854, 736, 540 cm–1. 5. Acknowledgements The financial supports for this work from the Program for New Century Excellent Talents in University by Mi- nistry of Education (Grant No. NCET-10-0371) and the Fundamental Research Funds for the Central Universities (Grant No. 2009ZM0262, 2009ZM0126) are gratefully acknowledged. 6. References [1] M. G. Nordberg, K. Kolmodin, J. Aquist, S. F. Queemer and A. J. Hallberg, “Design, Synthiesis, Computational Prediction, and Biological Evaluation of Ester Soft Drug as Inhibitors of Dihydrofolate Reductase from Pneumo- cystis Carinii,” Journal of Medicinal Chemistry, Vol. 44, No. 15, 2001, pp. 2391-2402. doi:10.1021/jm010856u [2] H. H. Sun, V. J. Paul and W. Fenical, “Avrainvilleol, a Brominated Diphenylmethane Derivative with Feeding Deterrent Properties from the Tropical Green Alga Avrainvillea Longicaulis,” Phytochemistry, Vol. 22, No. 3, 1983, pp. 743-745. doi:10.1016/S0031-9422(00)86974-5 [3] H. Hoshina, K. Maekawa, K. Taie, T. Igarashi and T. Salurai, “A New Route to Papaverine Analogs via Photocyclization of Substituted N-Acyl-α-dehydrophen- ylalaninamides,” Heterocycles, Vol. 60, 2003, pp. 1779- 1786. [4] C. Manzoni, M. R. Lovati, A. Bonelli, G. Galli and C. R. Sirtori, “Differential Effects of Beclobrate on Lipid/ Lipoprotein Distribution in Normal and Hypercholes- terolemic Rats,” European Journal of Pharmacology, Vol. 190, No. 1-2, 1990, pp. 39-49. doi:10.1016/0014-2999(90)94110-J Copyright © 2011 SciRes. IJOC  181 L. DANG ET AL. [5] C. Rose, O. Vtoraya, A. Pluzanska, N. Davidson, M. Gershanovich, R. Thomas, S. Johnson, J. Caicedo, H. Gervasio, G. Manikhas, F. B. Ayed, S. B. Radoux, H. A. C. Ross and R. Lang, “An Open Randomised Trial of Second-Line Endocrine Therapy in Advanced Breast Cancer: Comparison of the Aromatase Inhibitors Letro- zole and Anastrozole,” European Journal of Cancer, Vol. 39, No. 16, 2003, pp. 2318-2327. doi:10.1016/S0959-8049(03)00630-0 [6] V. B. Andela, “Functional Antagonism between NF-kB and Nuclear Receptors: Implications in Carcinogenesis and Strategies for Optimal Cancer Chemopreventive Interventions,” Current Cancer Drug Targets, Vol. 4, No. 4, 2004, pp. 337-344. doi:10.2174/1568009043332952 [7] M. S. Khan, M. R. A. Al-Mandhary, M. K. Al-Suti, B. Ahrens, M. F. Mahon, L. Male, P. R. Raithby, C. E. Boothby and A. Kohler, “Synthesis, Characterisation and Optical Spectroscopy of Diynes and Poly-Ynes Con- taining Derivatised Fluorenes in the Backbone,” Dalton Transaction, Vol. 1, 2003, pp. 74-84. doi:10.1039/b208963g [8] S. L. You, Q. Cai and M. Zeng, “Chiral Brønsted Acid Catalyzed Friedel-Crafts Alkylation Reactions,” Chemi- cal Society Reviews, Vol. 38, No. 8, 2009, pp. 2190-2201. doi:10.1039/b817310a [9] H. M. Peng, L. X. Dai and S. L. You, “Enantioselective Palladium-Catalyzed Direct Alkylation and Olefination Reaction of Simple Arenes,” Angewandte Chemie Inter- national Edition, Vol. 49, No. 34, 2010, pp. 5826-5828. doi:10.1002/anie.201000799 [10] S. Messaoudi, J. D. Brion and M. Alami, “Transition- Metal-Catalyzed Direct C-H Alkenylation, Alkynylation, Benzylation, and Alkylation of (Hetero) Arenes,” Euro- pean Journal of Organic Chemistry, Vol. 2010, No. 34, 2010, pp. 6495-6516. doi:10.1002/ejoc.201000928 [11] C. Zhang, X. Gao, J. Zhang and X. Peng, “Fe/CuBr2- Catalyzed Benzylation of Arenes and Thiophenes with Benzyl Alcohols,” Synlett, Vol. 2, 2010, pp. 261-265. doi:10.1055/s-0029-1218571 [12] W. Yao, P. makowski, C. Giordano and F. Goettmann, “Synthesis of Early-Transition-Metal Carbide and Nitride Nanoparticles through the Urea Route and Their Use as Alkylation Catalysts,” Chemistry—A European Journal, Vol. 15, No. 44, 2009, pp. 11999-12004. doi:10.1002/chem.200901496 [13] P. Makowski, R. Rothe, A. Thomas, M. Niederberger and F. Moettmann, “Chlorine Borrowing: An Efficient Method for an Easier Use of Alcohols as Alkylation Agents,” Green Chemistry, Vo1. 11, 2009, pp. 34-37. [14] F. Wang and W. Ueda, “High Catalytic Efficiency of Nanostructured Molybdenum Trioxide in the Benzylation of Arenes and an Investigation of the Reaction Mech- anism,” Chemistry—A European Journal, Vol. 15, No. 3, 2009, pp. 742-753. doi:10.1002/chem.200801153 [15] M. Kitano, K. Nakajima, J. N. Kondo, S. Hayashi and M. Hara, “Protonated Titanate Nonotubes as Solid Acid Catalyst,” Journal of the American Chemical Society, Vol. 132, No. 19, 2010, pp. 6622-6623. doi:10.1021/ja100435w [16] P. K. Thallapally, C. A. Fernandez, R. K. Motkuri, S. K. Nune, J. Liu and C. H. F. Peden, “Micro and Mesoporous Metal-Organic Frameworks for Catalysis Applications,” Dalton Transactions, Vol. 39, No. 7, 2010, pp. 1692- 1694. doi:10.1039/b921118g [17] V. L. Budarin, J. H. Clark, R. Luque and D. Macquarrie, “Versatile Mesoporous Carbonaceous Materials for Acid Catalysis,” Chemical Communications, Vol. 6, 2007, pp. 634-636. doi:10.1039/b614537j [18] G. A. Sereda, “Alkylation on Graphite in the Absence of Lewis Acids,” Tetrahedron Letters, Vol. 45, No. 39, 2004, pp. 7265-2667. doi:10.1016/j.tetlet.2004.08.026 [19] Y. Hashimoto, K. Hirata, H. Kagoshima, N. Kihara, M. Hasegawa and K. Saigo, “Gallium Dichloride-Mediated Reductive Friedel-Crafts Reaction,” Tetrahedron, Vol. 49, No. 27, 1993, pp. 5969-5978. doi:10.1016/S0040-4020(01)87183-0 [20] J. M. Aizpurua, B. Lecea and C. Palomo, “Reduction of Carbonyl Compounds Promoted by Silicon Hydrides un- der the Influence of Trimethylsilyl-Based Reagents,” Canadian Journal of Chemistry, Vol. 64, No. 12, 1986, pp. 2342-2347. doi:10.1139/v86-386 [21] T. Miyai, Y. Onishi and A. Baba, “Indium Trichloride Catalyzed Reductive Friedel-Crafts Alkylation of Aro- matics Using Carbonyl Compounds,” Tetrahedron Le- tters, Vol. 39, No. 35, 1998, pp. 6291-6294. doi:10.1016/S0040-4039(98)01333-1 [22] K. Mertins, I. Iovel, J. Kischel, A. Zapf and M. Beller, “Transition-Metal-Catalyzed Benzylation of Arenes and Heteroarenes,” Angewandte Chemie International Edition, Vol. 44, 2005, pp. 238-242. doi:10.1002/anie.200460666 [23] M. Rueping, B. J. Nachtsheim and W. Ieawsuwan, “An Effective Bismuth-Catalyzed Benzylation of Arenes and Heteroarenes,” Advanced Synthesis & Catalysis, Vol. 348, No. 9, 2006, pp. 1033-1037. doi:10.1002/adsc.200606068 [24] A. Prades, R. Corberan, M. Poyatos and E. Peris, “A Simple Catalyst for the Efficient Benzylation of Arenes by Using Alcohols, Ethers, Styrenes, Aldehydes, or Ke- tones,” Chemistry—A European Journal, Vol. 15, No. 18, 2009, pp. 4610-4613. doi:10.1002/chem.200802740 [25] Y. P. Xiao, X. Y. Liu and C. M. Che, “Highly Efficient Gold(III)-Catalyzed Intermolecular Hydroarylation of Unactivated Alkenes with Arenes under Mild Condi- tions,” Journal of Organometallic Chemistry, Vol. 694, No. 4, 2009, pp. 494-501. doi:10.1016/j.jorganchem.2008.07.035 [26] K. M. Reddy, N. S. Babu, P. S. S. Prasad and N. Lingaiah, “Aluminium-Exchanged Tungstophosphoric Acid: An Ef- ficient Catalyst for Intermolecular Hydroarylation of Vi- nyl Arenes,” Catalysis Communications, Vol. 9, No. 15, 2008, pp. 2525-2531. doi:10.1016/j.catcom.2008.07.007 [27] I. Shiina and M. Suzuki, “The Catalytic Friedel-Crafts Alkylation Reaction of Aromatic Compounds with Ben- zyl or Allyl Silyl Ethers Using Cl2Si(OTf)2 or Hf(OTf)4,” Tetrahedron Letters, Vol. 43, No. 36, 2002, pp. 6391- Copyright © 2011 SciRes. IJOC  L. DANG ET AL. Copyright © 2011 SciRes. IJOC 182 6394. doi:10.1016/S0040-4039(02)01376-X [28] S. A. Ardizzone, P. Beltrame and G. Zuretti, “Kinetics of the Reaction of Toluene with Benzyl Alcohol over Sul- fated Zirconia,” Applied Catalysis A: General, Vol. 314, No. 2, 2006, pp. 240-247. doi:10.1016/j.apcata.2006.08.026 [29] J. R. Satam and R. V. Jayaram, “Liquid Phase Friedel- Crafts Benzylation of Aromatics on a Polymer-Supported 12-Tungstophosphoric Acid Catalyst,” Catalysis Com- munications, Vol. 9, No. 9, 2008, pp. 1937-1940. doi:10.1016/j.catcom.2008.03.018 [30] B. Q. Wang, S. K. Xiang, Z. P. Sun, B. T. Guan, P. Hu, K. Q. Zhao and Z. J. Shi, “Benzylation of Arenes through FeCl3-Catalyzed Friedel-Crafts Reaction via C-O Activa- tion of Benzyl Ether,” Tetrahedron Letters, Vol. 49, No. 27, 2008, pp. 4310-4312. doi:10.1016/j.tetlet.2008.04.117 [31] N. Christoph and M. Herbert, “Kinetics of the Solvolyses of Fluoro-Substituted Benzhydryl Derivatives: Reference Electrofuges for the Development of a Comprehensive Nucleofugality Scale,” European Journal of Organic Ch- emistry, Vol. 2010, No. 8, 2010, pp. 1435-1439. doi:10.1002/ejoc.200901400 [32] K. Tobias, V. Katja and L. Torsten, “Regioselective Arene Functionalization: Simple Substitution of Carboxylate by Alkyl Groups,” Chemistry—A European Journal, Vol. 15, No. 44, 2009, pp. 12082-12091. doi:10.1002/chem.200901774 [33] H. B. Sun, B. Li, S. J. Chen, J. Li and R. M. Hua, “An Efficient Synthesis of Unsymmetrical Diarylmethanes from the Dehydration of Arenes with Benzyl Alcohols Using InCl3·4H2O/Acetylacetone Catalyst System,” Tetr- ahedron, Vol. 63, No. 41, 2007, pp. 10185-10188. doi:10.1016/j.tet.2007.07.093 [34] M. Kristin, L. Irina, K. Jette and Z. Alexander, “Gold- Catalyzed Benzylation of Arenes and Heteroarenes,” Ad- vanced Synthesis & Catalysis, Vol. 348, No. 6, 2006, pp. 691-695. doi:10.1002/adsc.200505433 [35] F. Alice, T. Amy and B. William, “Palladium-Catalyzed Cross-Coupling of B-Benzyl-9-Borabicyclo[3.3.1]nonane to Furnish Methylene-Linked Biaryls,” Organic Letters, Vol. 7, No. 22, 2005, pp. 4975-4978. doi:10.1021/ol051929x [36] A. Muriel and G. Corinne, “Synthesis of Functionalised Diarylmethanes via a Cobalt-Catalysed Cross-Coupling of Arylzinc Species with Benzyl Chlorides,” Chemical Communications, Vol. 40, 2008, pp. 5019-5021. doi:10.1039/b809626k [37] M. Kenichi, K. Takeo, S. Norio, K. Kozo, G. Takahiro and N. J. Yukinori, “Synthesis of Novel Silyl Enol Ethers from Chlorodimethyl(Naphthylphenylmethyl)silanes Hav- ing a Chiral Centre and a Ketone and Their Chirality Transfer Effects in Crossed-Aldol Reactions,” Journal of Chemical Research, Vol. 2009, No. 1, 2009, pp. 46-51. doi:10.3184/030823409X393709 [38] C. R. Chen, S. L. Zhou and H. M. Gau, “Extremely Efficient Cross-Coupling of Benzylic Halides with Ary- ltitanium Tris(isopropoxide) Catalyzed by Low Load- ings of a Simple Palladium(II) Acetate/Tris(p-tolyl) pho- sphine System,” Advanced Synthesis & Catalysis, Vol. 352, No. 10, 2010, pp. 1718-1727. doi:10.1002/adsc.201000311 [39] H. Nicolas, “One-Pot Dual Substitutions of Bromobenzyl Chloride, 2-Chloromethyl-6-halogenoimidazo[1,2-a] py- ridine and -[1,2-b]pyridazine by Suzuki-Miyaura Cross- Coupling Reactions,” European Journal of Organic Ch- emistry, Vol. 2008, No. 28, 2008, pp. 4824-4827. doi:10.1002/adsc.201000311 [40] Z. K. Yu, “Synthesis and Structural Characterization of Complexes Derived from Treatment of Gallium Trichlo- ride with 3,5-Diphenylpyrazole,” Polyhedron, Vol. 21, No. 11, 2002, pp. 1117-1123. doi:10.1016/S0277-5387(02)00931-2 [41] T. Lloyd Fletcher and J. Blackwell, “Experiments in the Colchicine Field. VI. A Method for the Synthesis of Ring B1,” Journal of the Chemical Society, 1961, pp. 1400- 1420. [42] J. R. Schmink and N. E. Leadbeater, “Palladium-Cata- lyzed Synthesis of Diarylmethanes: Exploitation of Car- banionic Leaving Groups,” Organic Letters, Vol. 11, No. 12, 2009, pp. 2575-2578. doi:10.1021/ol900874z [43] T. Z. Nichele and A. L. Monteiro, “Synthesis of Diaryl- methane Derivatives from Stille Cross-Coupling Reac- tions of Benzylic Halides,” Tetrahedron Letters, Vol. 48, 2007, pp. 7472-7475.
|