A. KEBEDE ET AL.
Copyright © 2011 SciRes. WJNSE
91
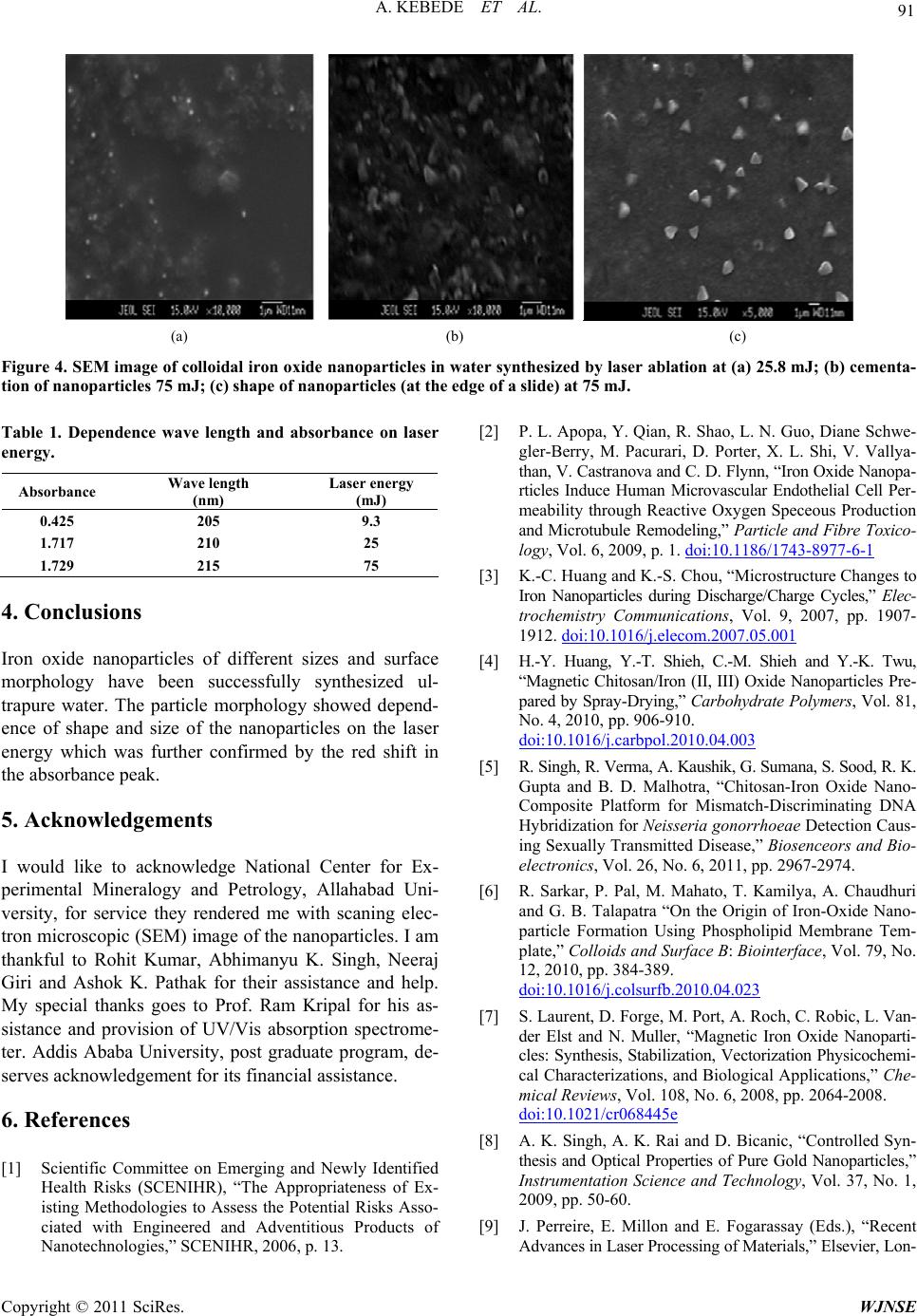
(a) (b) (c)
Figure 4. SEM image of colloidal iron oxide nanoparticles in water synthesized by laser ablation at (a) 25.8 mJ; (b) cementa-
tion of nanoparticles 75 mJ; (c) shape of nanoparticles (at the edge of a slide) at 75 mJ.
Table 1. Dependence wave length and absorbance on laser
energy.
Absorbance Wave length
(nm) Laser energy
(mJ)
0.425 205 9.3
1.717 210 25
1.729 215 75
[2] P. L. Apopa, Y. Qian, R. Shao, L. N. Guo, Diane Schwe-
gler-Berry, M. Pacurari, D. Porter, X. L. Shi, V. Vallya-
than, V. Castranova and C. D. Flynn, “Iron Oxide Nanopa-
rticles Induce Human Microvascular Endothelial Cell Per-
meability through Reactive Oxygen Speceous Production
and Microtubule Remodeling,” Particle and Fibre Toxico-
logy, Vol. 6, 2009, p. 1. doi:10.1186/1743-8977-6-1
[3] K.-C. Huang and K.-S. Chou, “Microstructure Changes to
Iron Nanoparticles during Discharge/Charge Cycles,” Elec-
trochemistry Communications, Vol. 9, 2007, pp. 1907-
1912. doi:10.1016/j.elecom.2007.05.001
4. Conclusions
Iron oxide nanoparticles of different sizes and surface
morphology have been successfully synthesized ul-
trapure water. The particle morphology showed depend-
ence of shape and size of the nanoparticles on the laser
energy which was further confirmed by the red shift in
the absorbance peak.
[4] H.-Y. Huang, Y.-T. Shieh, C.-M. Shieh and Y.-K. Twu,
“Magnetic Chitosan/Iron (II, III) Oxide Nanoparticles Pre-
pared by Spray-Dry ing,” Carbohydrate Polymers, Vol. 81,
No. 4, 2010, pp. 906-910.
doi:10.1016/j.carbpol.2010.04.003
[5] R. Singh, R. Verma, A. Kaushik, G. Sumana, S. Sood, R. K.
Gupta and B. D. Malhotra, “Chitosan-Iron Oxide Nano-
Composite Platform for Mismatch-Discriminating DNA
Hybridization for Neisseria gonorrhoeae Detection Caus-
ing Sexually Transmitted Disease,” Biosenceors and Bio-
electronics, Vol. 26, No. 6, 2011, pp. 2967-2974.
5. Acknowledgements
I would like to acknowledge National Center for Ex-
perimental Mineralogy and Petrology, Allahabad Uni-
versity, for service they rendered me with scaning elec-
tron microscopic (SEM) image of the nanoparticles. I am
thankful to Rohit Kumar, Abhimanyu K. Singh, Neeraj
Giri and Ashok K. Pathak for their assistance and help.
My special thanks goes to Prof. Ram Kripal for his as-
sistance and provision of UV/Vis absorption spectrome-
ter. Addis Ababa University, post graduate program, de-
serves acknowledgement for its financial assistance.
[6] R. Sarkar, P. Pal, M. Mahato, T. Kamilya, A. Chaudhuri
and G. B. Talapatra “On the Origin of Iron-Oxide Nano-
particle Formation Using Phospholipid Membrane Tem-
plate,” Colloids and Surface B: Biointerface, Vol. 79, No.
12, 2010, pp. 384-389.
doi:10.1016/j.colsurfb.2010.04.023
[7] S. Laurent, D. Forge, M. Port, A. Roch, C. Robic, L. Van-
der Elst and N. Muller, “Magnetic Iron Oxide Nanoparti-
cles: Synthesis, Stabilization, Vectorization Physicochemi-
cal Characterizations, and Biological Applications,” Che-
mical Reviews, Vol. 108, No. 6, 2008, pp. 2064-2008.
doi:10.1021/cr068445e
6. References [8] A. K. Singh, A. K. Rai and D. Bicanic, “Controlled Syn-
thesis and Optical Properties of Pure Gold Nanoparticles,”
Instrumentation Science and Technology, Vol. 37, No. 1,
2009, pp. 50-60.
[1] Scientific Committee on Emerging and Newly Identified
Health Risks (SCENIHR), “The Appropriateness of Ex-
isting Methodologies to Assess the Potential Risks Asso-
ciated with Engineered and Adventitious Products of
Nanotechnologies,” SCENIHR, 2006, p. 13. [9] J. Perreire, E. Millon and E. Fogarassay (Eds.), “Recent
Advances in Laser Processing of Materials,” Elsevier, Lon-