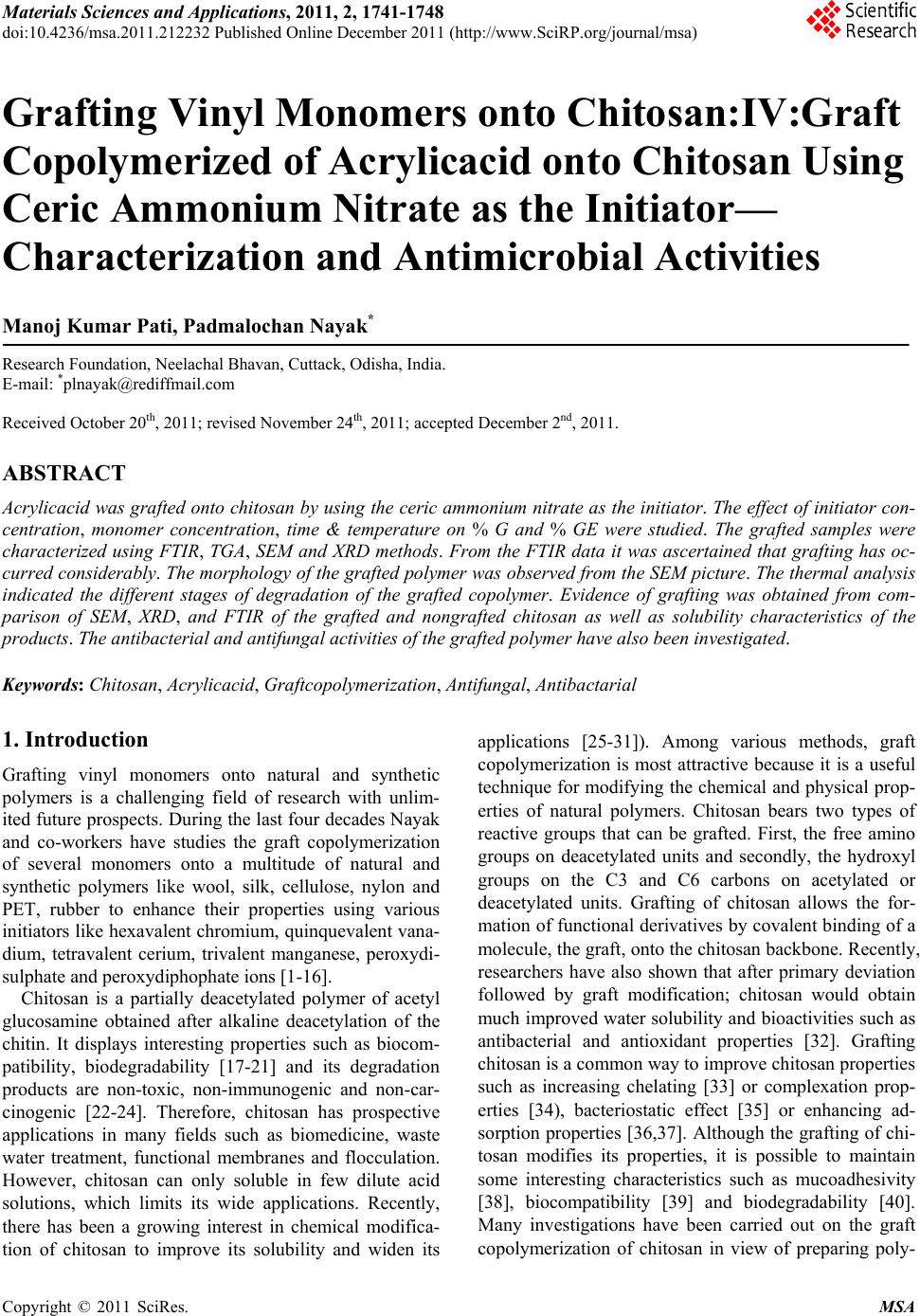
 Materials Sciences and Applicatio ns, 2011, 2, 1741-1748 doi:10.4236/msa.2011.212232 Published Online December 2011 (http://www.SciRP.org/journal/msa) Copyright © 2011 SciRes. MSA 1741 Grafting Vinyl Monomers onto Chitosan:IV:Graft Copolymerized of Acrylicacid onto Chitosan Using Ceric Ammonium Nitrate as the Initiator— Characterization and Antimicrobial Activities Manoj Kumar Pati, Padmalochan Nayak* Research Foundation, Neelachal Bhavan, Cuttack, Odisha, India. E-mail: *plnayak@rediffmail.com Received October 20th, 2011; revised November 24th, 2011; accepted December 2nd, 2011. ABSTRACT Acrylicacid was grafted onto chitosan by using the ceric ammonium nitrate as the initiator. The effect of initiator con- centration, monomer concentration, time & temperature on % G and % GE were studied. The grafted samples were characterized using FTIR, TGA, SEM and XRD methods. From the FTIR data it was ascertained that grafting has oc- curred considerably. The morphology of the grafted polymer was observed from the SEM picture. The thermal analysis indicated the different stages of degradation of the grafted copolymer. Evidence of grafting was obtained from com- parison of SEM, XRD, and FTIR of the grafted and nongrafted chitosan as well as solubility characteristics of the products. The antibacterial and antifungal activities of the grafted polymer have also been investigated. Keywords: Chitosan, Acrylicacid, Graftcopolymerization, Antifungal, Antibactarial 1. Introduction Grafting vinyl monomers onto natural and synthetic polymers is a challenging field of research with unlim- ited future prospects. During the last four decades Nayak and co-workers have studies the graft copolymerization of several monomers onto a multitude of natural and synthetic polymers like wool, silk, cellulose, nylon and PET, rubber to enhance their properties using various initiators like hexavalent chromium, quinquevalent vana- dium, tetravalent cerium, trivalent manganese, peroxydi- sulphate and peroxydiphophate ions [1-16]. Chitosan is a partially deacetylated polymer of acetyl glucosamine obtained after alkaline deacetylation of the chitin. It displays interesting properties such as biocom- patibility, biodegradability [17-21] and its degradation products are non-toxic, non-immunogenic and non-car- cinogenic [22-24]. Therefore, chitosan has prospective applications in many fields such as biomedicine, waste water treatment, functional membranes and flocculation. However, chitosan can only soluble in few dilute acid solutions, which limits its wide applications. Recently, there has been a growing interest in chemical modifica- tion of chitosan to improve its solubility and widen its applications [25-31]). Among various methods, graft copolymerization is most attractive because it is a useful technique for modifying the chemical and physical prop- erties of natural polymers. Chitosan bears two types of reactive groups that can be grafted. First, the free amino groups on deacetylated units and secondly, the hydroxyl groups on the C3 and C6 carbons on acetylated or deacetylated units. Grafting of chitosan allows the for- mation of functional derivatives by covalent binding of a molecule, the graft, onto the chitosan backbone. Recently, researchers have also shown that after primary deviation followed by graft modification; chitosan would obtain much improved water solubility and bioactivities such as antibacterial and antioxidant properties [32]. Grafting chitosan is a common way to improve chitosan properties such as increasing chelating [33] or complexation prop- erties [34), bacteriostatic effect [35] or enhancing ad- sorption properties [36,37]. Although the grafting of chi- tosan modifies its properties, it is possible to maintain some interesting characteristics such as mucoadhesivity [38], biocompatibility [39] and biodegradability [40]. Many investigations have been carried out on the graft copolymerization of chitosan in view of preparing poly-  Grafting Vinyl Monomers onto Chitosan:IV:Graft Copolymerized of Acrylicacid onto Chitosan 1742 Using Ceric Ammonium Nitrate as the Initiator—Characterization and Antimicrobial Activities saccharide-based advanced materials with unique bioac- tivities and thus widening their applications in biomedi- cine and environmental fields. A few review articles on the potential applications of chitosan for pharmaceutical, veterinary medicine, biomedical and environmental field have already been reported [41-49]. However, there is no review available on the graft copolymerization of chito- san and its applications, despites the considerable amount of works have been published in this field. Moreover, the potential applications of grafted chitosan in the various fields such as drug delivery, biomedical, tissue engineer- ing and environmental is also discussed. In the present research program, we wish to report the graft copolymerization of Acrylicacid onto chitosan us- ing ceric ammonium nitrate as the initiator. The graft co- polymrization was studied by varying the initiator, time, temperature and concentration of monomer. The grafted polymers were characterized by SEM, XRD and FTIR studies. The antibacterial and antifungal activity of the grafted samples have been reported. 2. Material and Method Chitosan with more than 95% deacylated was obtained as Gift sample from India Sea food, Kerala, India. Acrylicacid (Riedel) was extracted with an aqueous solution of 5% NaOH/20% NaCl to remove the hydro- quinone stabilizer. The monomer was distilled and the middle fraction were used Inhibitor free Acrylicacid (Fluka puriss) was also used. Reagent-grade ceric ammonium supplied by Merck was used to prepare the initiator solution, and was used without further purification. Other reagents were of ana- lytical grade and were used without further treatment. 2.1. Graft Copolymerization The chitosan powder sample was dispersed in a definite volume was dissolved in 2% acetic acid, in a thermo- stated reaction flask for 60 min. The ceric ammonium nitrate in 0.5 M nitric acid solution was then loaded into the reactor under continuous stirring. Then a known weight of Acrylicacid was also injected into the reactor. The reaction was assumed to have started at the moment the monomer was injected. Graft copolymerization was carried out at room tem- perature under constant stirring in nitrogen atmosphere for a particular time period. At the end of grafting co- polymerization the reaction mixture was neutralized with a 1 M NaOH solution, and the reaction products (graft copolymer and homopolymer) were filtered and thor- oughly washed with distilled water, and then dried to constant weight. The homopolymer was subsequently removed by extraction with N,N-dimethethyl formamide for 6 hrs. The remaining product, after drying to a con- stant weight, was considered to be a graft copolymer. Grafting percentage (%G) which designates the amount of polymer grafted on the substract backbone (chitosan) and grafting efficiency (%E), which indicates the effi- ciency of conversion of the initial Acrylicacid to the grafted PAN, were calculated from the increase in weight of the chitosan after graft copolymerization in following manner. 21 1 21 3 %1 %1 ww Gw ww Gw 00 00 2.2. Evidence of Grafting It has been seen that the amidecarbonyl absorption band from grafted chains appears at 1649 cm–1. The band is located at 1670 cm–1 for acrylamide homopolymer. On the other hand, the most typical absorption bands of chi- tosan situated at 1558 cm–1 and 1661 cm–1 corresponding to amide-I and amide-II bands respectively, are not clearly visible since they are hidden by strong carbonyl absorption band of PAA in this spectrum region. How- ever the CHI amide-I absorption can be observed as a shoulder at 1540 cm–1. The shift of the carbonyl absorp- tion band of PAA amide-I band of chitosan to lower fre- quencies could be due to inter—and/or intramolecular interacting though hydrogen bonding. Naturally, the in- solubility of PAA grafted CHI samples, inspite of con- taining a large number of amide groups, is produced by the cross linking (Figure 1). 2.3. X-Ray Diffraction Studies The formation and quality of compounds were checked by X-ray diffraction (XRD) spectrum. The XRD pattern was measured by drop coated grafting compound on glass plate and employed with X-ray diffractometer (INEL X-ray diffractometer) of characteristic Co-kα1 radiation (λ = 1.78 Å) in the range of 20˚ to 90˚ at a scan rate of 0.05˚/min with the time constant of 2 s. 2.4. SEM Analysis of Grafting Compound Scanning Electron Microscopic (SEM) analysis was done using Hitachi S-4500 SEM machine. The sample were prepared on a carbon coated copper grid by just dropping a very small amount of the sample on the grid, extra so- lution was removed using a blotting paper and then the sample on the SEM grid were allowed to dry by putting it under a mercury lamp for 5 min. Copyright © 2011 SciRes. MSA 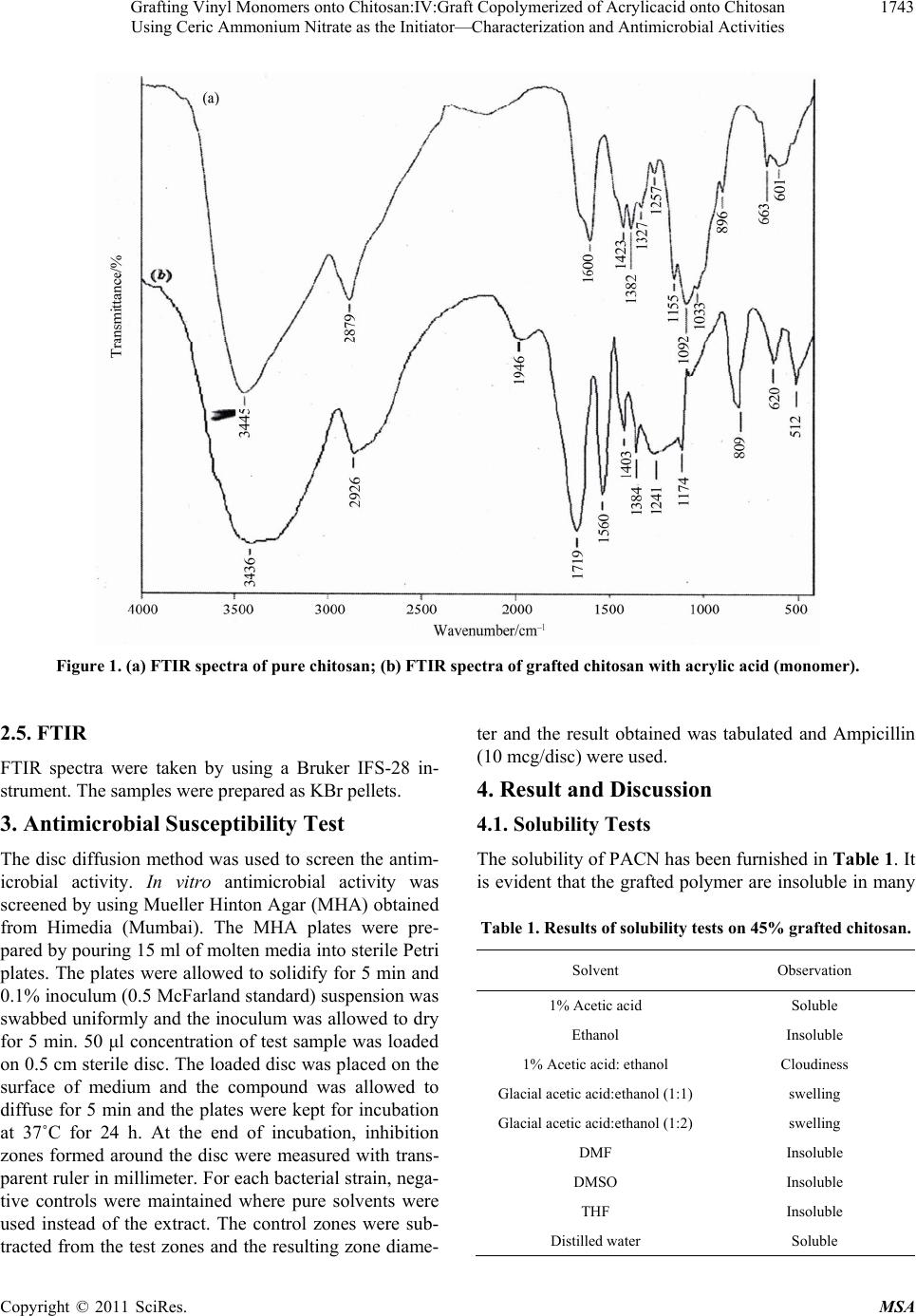 Grafting Vinyl Monomers onto Chitosan:IV:Graft Copolymerized of Acrylicacid onto Chitosan Using Ceric Ammonium Nitrate as the Initiator—Characterization and Antimicrobial Activities Copyright © 2011 SciRes. MSA 1743 Figure 1. (a) FTIR spectra of pure chitosan; (b) FTIR spectra of grafted chitosan with acrylic acid (monomer). 2.5. FTIR FTIR spectra were taken by using a Bruker IFS-28 in- strument. The samples were prepared as KBr pellets. 3. Antimicrobial Susceptibility Test The disc diffusion method was used to screen the antim- icrobial activity. In vitro antimicrobial activity was screened by using Mueller Hinton Agar (MHA) obtained from Himedia (Mumbai). The MHA plates were pre- pared by pouring 15 ml of molten media into sterile Petri plates. The plates were allowed to solidify for 5 min and 0.1% inoculum (0.5 McFarland standard) suspension was swabbed uniformly and the inoculum was allowed to dry for 5 min. 50 μl concentration of test sample was loaded on 0.5 cm sterile disc. The loaded disc was placed on the surface of medium and the compound was allowed to diffuse for 5 min and the plates were kept for incubation at 37˚C for 24 h. At the end of incubation, inhibition zones formed around the disc were measured with trans- parent ruler in millimeter. For each bacterial strain, nega- tive controls were maintained where pure solvents were used instead of the extract. The control zones were sub- tracted from the test zones and the resulting zone diame- ter and the result obtained was tabulated and Ampicillin (10 mcg/disc) were used. 4. Result and Discussion 4.1. Solubility Tests The solubility of PACN has been furnished in Ta bl e 1. It is evident that the grafted polymer are insoluble in many Table 1. Results of solubility tests on 45% grafted chitosan. Solvent Observation 1% Acetic acid Soluble Ethanol Insoluble 1% Acetic acid: ethanol Cloudiness Glacial acetic acid:ethanol (1:1) swelling Glacial acetic acid:ethanol (1:2) swelling DMF Insoluble DMSO Insoluble THF Insoluble Distilled water Soluble 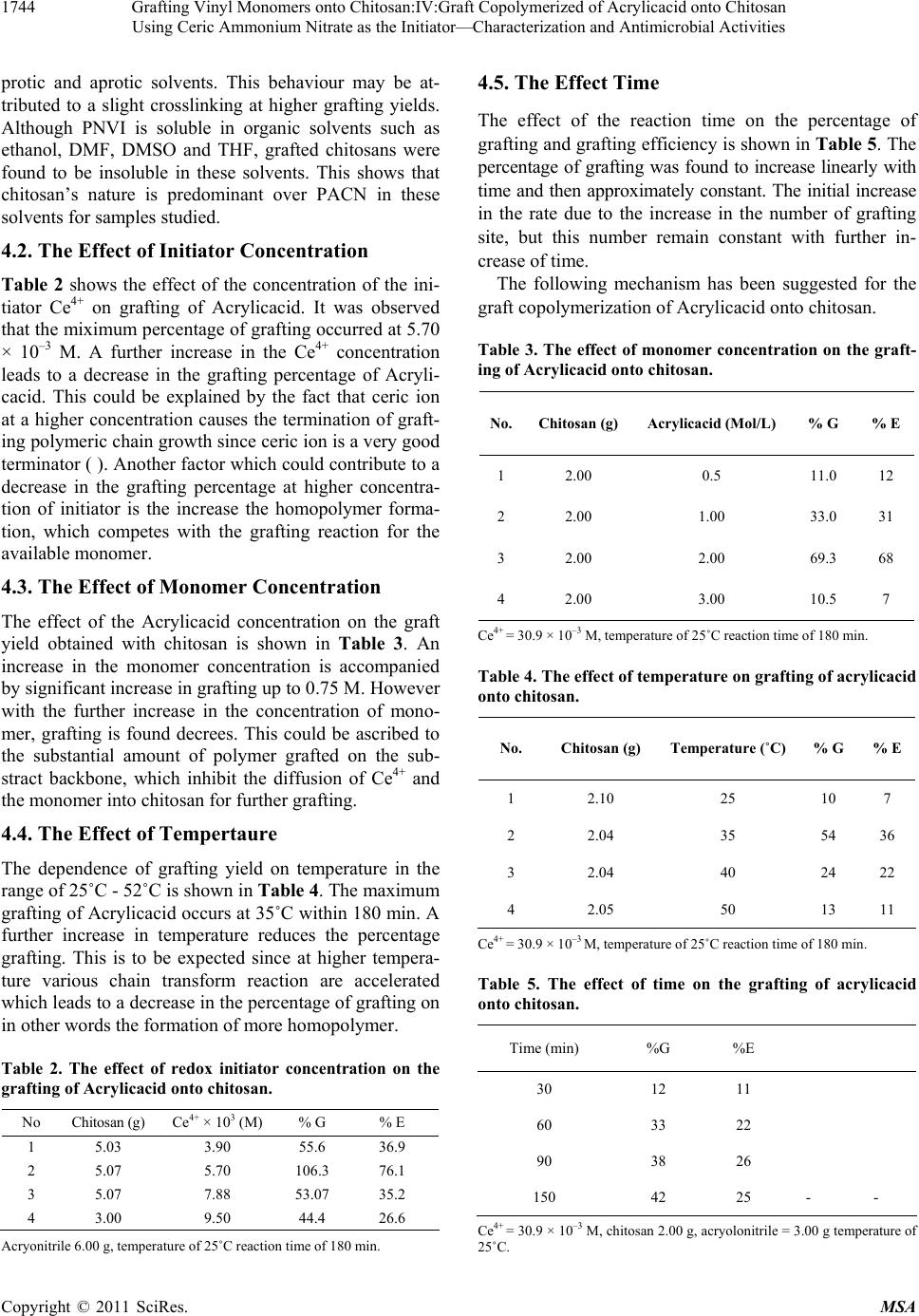 Grafting Vinyl Monomers onto Chitosan:IV:Graft Copolymerized of Acrylicacid onto Chitosan 1744 Using Ceric Ammonium Nitrate as the Initiator—Characterization and Antimicrobial Activities protic and aprotic solvents. This behaviour may be at- tributed to a slight crosslinking at higher grafting yields. Although PNVI is soluble in organic solvents such as ethanol, DMF, DMSO and THF, grafted chitosans were found to be insoluble in these solvents. This shows that chitosan’s nature is predominant over PACN in these solvents for samples studied. 4.2. The Effect of Initiator Concentration Table 2 shows the effect of the concentration of the ini- tiator Ce4+ on grafting of Acrylicacid. It was observed that the miximum percentage of grafting occurred at 5.70 × 10–3 M. A further increase in the Ce4+ concentration leads to a decrease in the grafting percentage of Acryli- cacid. This could be explained by the fact that ceric ion at a higher concentration causes the termination of graft- ing polymeric chain growth since ceric ion is a very good terminator ( ). Another factor which could contribute to a decrease in the grafting percentage at higher concentra- tion of initiator is the increase the homopolymer forma- tion, which competes with the grafting reaction for the available monomer. 4.3. The Effect of Monomer Concentration The effect of the Acrylicacid concentration on the graft yield obtained with chitosan is shown in Table 3. An increase in the monomer concentration is accompanied by significant increase in grafting up to 0.75 M. However with the further increase in the concentration of mono- mer, grafting is found decrees. This could be ascribed to the substantial amount of polymer grafted on the sub- stract backbone, which inhibit the diffusion of Ce4+ and the monomer into chitosan for further grafting. 4.4. The Effect of Tempertaure The dependence of grafting yield on temperature in the range of 25˚C - 52˚C is shown in Table 4 . The maximum grafting of Acrylicacid occurs at 35˚C within 180 min. A further increase in temperature reduces the percentage grafting. This is to be expected since at higher tempera- ture various chain transform reaction are accelerated which leads to a decrease in the percentage of grafting on in other words the formation of more homopolymer. Table 2. The effect of redox initiator concentration on the grafting of Acrylicacid onto chitosan. No Chitosan (g) Ce4+ × 103 (M) % G % E 1 5.03 3.90 55.6 36.9 2 5.07 5.70 106.3 76.1 3 5.07 7.88 53.07 35.2 4 3.00 9.50 44.4 26.6 Acryonitrile 6.00 g, temperature of 25˚C reaction time of 180 min. 4.5. The Effect Time The effect of the reaction time on the percentage of grafting and grafting efficiency is shown in Table 5. The percentage of grafting was found to increase linearly with time and then approximately constant. The initial increase in the rate due to the increase in the number of grafting site, but this number remain constant with further in- crease of time. The following mechanism has been suggested for the graft copolymerization of Acrylicacid onto chitosan. Table 3. The effect of monomer concentration on the graft- ing of Acrylicacid onto chitosan. No.Chitosan (g) Acrylicacid (Mol/L) % G % E 1 2.00 0.5 11.0 12 2 2.00 1.00 33.0 31 3 2.00 2.00 69.3 68 4 2.00 3.00 10.5 7 Ce4+ = 30.9 × 10–3 M, temperature of 25˚C reaction time of 180 min. Table 4. The effect of temperature on grafting of acrylicacid onto chitosan. No. Chitosan (g) Temper a ture (˚C) % G% E 1 2.10 25 10 7 2 2.04 35 54 36 3 2.04 40 24 22 4 2.05 50 13 11 Ce4+ = 30.9 × 10–3 M, temperature of 25˚C reaction time of 180 min. Table 5. The effect of time on the grafting of acrylicacid onto chitosan. Time (min) %G %E 30 12 11 60 33 22 90 38 26 150 42 25 - - Ce4+ = 30.9 × 10–3 M, chitosan 2.00 g, acryolonitrile = 3.00 g temperature of 25˚C. Copyright © 2011 SciRes. MSA 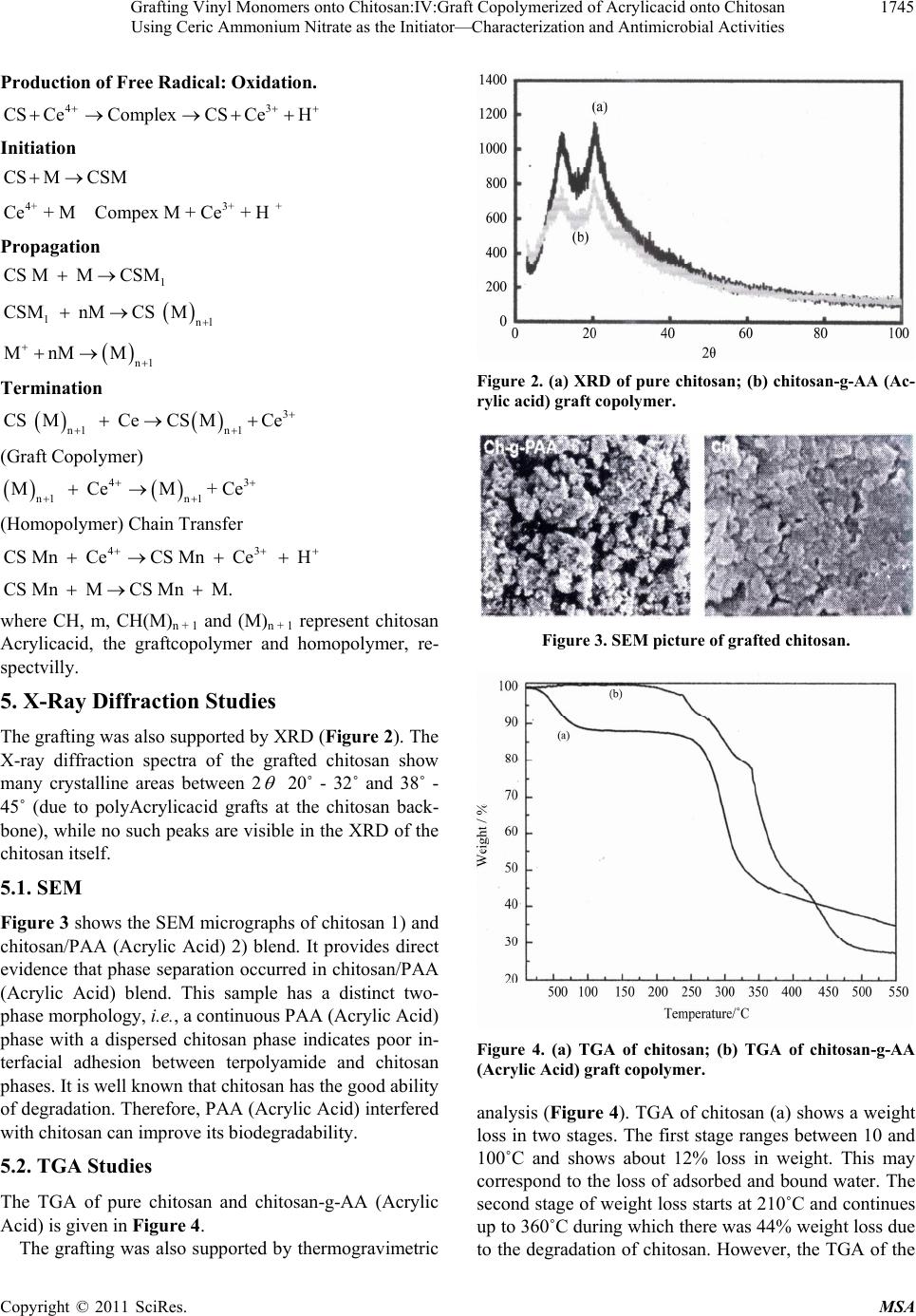 Grafting Vinyl Monomers onto Chitosan:IV:Graft Copolymerized of Acrylicacid onto Chitosan 1745 Using Ceric Ammonium Nitrate as the Initiator—Characterization and Antimicrobial Activities Production of Free Radical: Oxidation. 43 CS CeComplexCSCeH Initiation CS MCSM 4+ 3++ Ce+ M Compex M + Ce+ H Propagation 1 CS M MCSM 1nl CSM nMCS M n1 MnM M Termination 3 n1 n1 CS M CeCSMCe (Graft Copolymer) 43 n1 n1 M CeM+ Ce (Homopolymer) Chain Transfer 43 CS Mn CeCS Mn Ce H CS Mn MCS Mn M. where CH, m, CH(M)n + 1 and (M)n + 1 represent chitosan Acrylicacid, the graftcopolymer and homopolymer, re- spectvilly. 5. X-Ray Diffraction Studies The grafting was also supported by XRD (Figure 2). The X-ray diffraction spectra of the grafted chitosan show many crystalline areas between 2 20˚ - 32˚ and 38˚ - 45˚ (due to polyAcrylicacid grafts at the chitosan back- bone), while no such peaks are visible in the XRD of the chitosan itself. 5.1. SEM Figure 3 shows the SEM micrographs of chitosan 1) and chitosan/PAA (Acrylic Acid) 2) blend. It provides direct evidence that phase separation occurred in chitosan/PAA (Acrylic Acid) blend. This sample has a distinct two- phase morphology, i.e., a continuous PAA (Acrylic Acid) phase with a dispersed chitosan phase indicates poor in- terfacial adhesion between terpolyamide and chitosan phases. It is well known that chitosan has the good ability of degradation. Therefore, PAA (Acrylic Acid) interfered with chitosan can improve its biodegradability. 5.2. TGA Studies The TGA of pure chitosan and chitosan-g-AA (Acrylic Acid) is given in Figure 4. The grafting was also supported by thermogravimetric Figure 2. (a) XRD of pure chitosan; (b) chitosan-g-AA (Ac- rylic acid) graft copolymer. Figure 3. SEM picture of grafted chitosan. Figure 4. (a) TGA of chitosan; (b) TGA of chitosan-g-AA (Acrylic Acid) graft copolymer. analysis (Figure 4). TGA of chitosan (a) shows a weight loss in two stages. The first stage ranges between 10 and 100˚C and shows about 12% loss in weight. This may correspond to the loss of adsorbed and bound water. The second stage of weight loss starts at 210˚C and continues up to 360˚C during which there was 44% weight loss due to the degradation of chitosan. However, the TGA of the Copyright © 2011 SciRes. MSA  Grafting Vinyl Monomers onto Chitosan:IV:Graft Copolymerized of Acrylicacid onto Chitosan 1746 Using Ceric Ammonium Nitrate as the Initiator—Characterization and Antimicrobial Activities grafted product is different. The latter has three stage of weight loss between 10˚C and 550˚C. The first stage of weight loss starts at 180˚C and continues up to 340˚C, during which there was 22% weight loss due to the deg- radation of chitosan. The second stage from 340˚C to 420˚C and the third stage from 420˚C to 500˚C may con- tribute to the decomposition of different structure of the graft co-polymer. Below 430˚C the copolymer had lower weight loss than chitosan. These mean that the grafting of chitosan increases the thermal stability of chitosan in some extent. 6. Antibacterial Activity of Graft Copolymerized of Acrylicacid on Chitosan A preliminary study has been carried out to compare the antibacterial activity of grafted chitosan hydrochloride film samples with that of chitosan. The study was carried out against P. aeruginosa, E. coli, B. subtilis and S. aureus using the inhibition zone method. The results are shown in Table 3. It was observed that grafting of NVI improved the antibacterial activity of chitosans. While the inhibition zone diameter for chitosan film ranged between 9 and 11 mm against indicated bacteria, the in- hibition zone increased up to 17 mm (against B. subtilis) by grafting. Although the difference is not significant, activity of gram-positive bacteria seems to be more pro- nounced; increase in the inhibition zone diameter is 4 - 5 mm in gram-negative ones whereas it is 5 - 7 mm in gram-positive ones. Grafted samples showed an increas- ing antibacterial activity as the degree of grafting in- creased for all of gram-negative and gram positive bacte- rias; a minimum of 2 mm increase was observed consis- tently when the grafting percentage increased from 82.5% to 145%. Average film weight (thickness) also effected the degree of antimicrobial activity of both chi- tosan and grafted chitosan samples. And 3 - 4 mm in- crease was observed when the average film weight was increased from 134 to 296. Table 6. Antifungal activities of GRAFTING compounds minimal inhibitory concentration (MIC) mg/ml. Fungus MIC (mg/ml) C. albicans 15.22 ± 0.04 C. tropicalis 17.32 ± 0.07 C. krusei 20.28 ± 0.00 C. Kefyr 11.33 ± 0.44 A. flavus 18.63 ± 0.64 7. Antifungal Activity Further the grafting compound were found to be highly toxic against clinically isolated fungal species. At a con- centration of 50 μl grafting compound revealed a higher antifungal activity against C. albicans, Candida kefyr, Aspergillus niger whereas intermediated activity were showed against C. tropicalis, C. krusei, A. flavus, A. fu- migatus. The inhibitory activities of all the grafting compound are reported in Table 6. The data results were compared with the standard antimicrobics of Ketocona- zole (30 mg) and Itraconazole (30 mg). 8. Conclusions The grafting of acrylicacid onto chitosan, in the absence of N,N-methylenebisacrylamide as crosslinking agent, leads to slightly soluble products. When N,Nmethylene- bisacrylamide was used as crosslinker insoluble grafted chitosan was obtained which could be easily separated from the reaction medium and purified. Moreover, the insoluble grafted chitosan showed characteristics of SEM, XRD and FTIR. This study has demonstrated that graft- ing compound showed a higher antibacterial and fungal activity. Grafting compound markedly inhibited the gr- owth of most bacteria and fungal tested although their inhibitory effects differed. 9. Acknowledgements This work was supported by Bio-Lab (A-41, Janpath, Ashok Nagar, Bhubaneswar, India-751009). REFERENCES [1] P. L. Nayak, “Grafting of Vinyl Monomers onto Wool Fi- bers,” Journal of Macromolecular Science—Reviews in Macromolecular Chemistry & Physics, Vol. 14, No. 2, 1996, pp. 193-213. [2] P. L. Nayak, “Grafting of Vinyl Monomers onto Nylon,” Journal of Macromolecular Science—Reviews in Macro- molecular Chemistry & Physics, Vol. 17, No. 2, 1979, pp. 149-267. [3] P. L. Nayak, “Vinyl and Graft Copolymerization Initiated by Potassium Peroxydiphosphate,” Journal of Macromo- lecular Science—Reviews in Macromolecular Chemistry & Physics, Vol. 25, No. 2, 1985, pp. 157-189. [4] P. L. Nayak, S. Lenka and N. C. Pati, “Grafting Vinyl Monomers onto Wool Fibers:I:Garfting Copolymeriza- tion of Methyl Methacrylate onto Wool Using V(v)- Thiourea Redox System,” Journal of Applied Polymer Science, Vol. 22, No. 11, 1978, pp. 3301-3309. doi:10.1002/app.1978.070221124 [5] P. L. Nayak, R. K. Samal and M. C. Nayak, “Garfting of Vinyl Monomers onto Nylon-6:I:Graft Co-Polymerizetion of Acryl Amide onto Nylon-6Mn(III) as Initiator Angew,” Copyright © 2011 SciRes. MSA  Grafting Vinyl Monomers onto Chitosan:IV:Graft Copolymerized of Acrylicacid onto Chitosan 1747 Using Ceric Ammonium Nitrate as the Initiator—Characterization and Antimicrobial Activities Macromolecular Chemistry and Physics, Vol. 13, No. 2, 1979, pp. 261-271. doi:10.1080/00222337908066601 [6] P. L. Nayak, M. K. Mishra and A. K. Tripathy, “Graft- ing Vinyl Monomer onto Polyster Fibres:I:Graft Copoly- merization of Methyl Methacrylate onto PET Using He- xavalent Chromium,” Journal of Applied Polymer Sci- ence, Vol. 26, No. 6, 1981, pp. 2109-2111. doi:10.1002/app.1981.070260634 [7] A. K. Pradhan, N. C. Pati and P. L. Nayak, “Grafting Vinyl Monomers onto Polyster Fibers:III:Graft Copoly- merization of Methyl Methaacrylate onto PET Using KMnO4-Oxalic Acid Redox System,” Journal of Applied Polymer Science, Vol. 27, No. 6, 1982, pp. 2131-2138. doi:10.1002/app.1982.070270624 [8] S. Lenka and P. L. Nayak, “Grafting of Vinyl Monomers onto Polyster Fibers:IV: Graft Copolymerization of Ethyl Methacrylate on PET Using Acetylacetonate Complex of Mn(III), Co(III) and Fe(III),” Journal of Applied Polymer Science, Vol. 19, 1982, p. 987. [9] S. Lenka, P. L. Nayak and M. R. Dash, “Photo Induced Graft Copolymerization:VI:Graft Copolymerization of Methyl Methacrylate onto Cellulose in Presence of Pyri- dine-Bromine Charge-Transfer Complex as Initiator,” In: S. lenka, P. L. Nayak and M. R. Dash, Eds., Polym, Pho- tochemistry, Vol. 3, 1983, p. 109. [10] S. Sasmal, G. Sahu and P. L. Nayak, “Grafting Vinyl Monomers onto Chemically Modified Wool Fibres: XI: Graft Copolymerization of Methyl Methacrylate onto Reduced Wool Fiber Using Acetylacetonate Complex of Manganese (III),” Journal of Macromolecular Science, Vol. 20, No. 2, 1983, pp. 153-167. doi:10.1080/00222338308069956 [11] P. L. Nayak, S. Lenka and A. P. Das, “Grafting of Vinyl Monomers onto Natural Rubber: V: Graft Copolymeriza- tion of Methyl Methacrylate onto Natural Rubber Using Pottasium Peroxydisulphate,” Journal of Polymer Science Part A: Polymer Chemistry, Vol. 30, No. 7, 1985, pp. 2753- 2759. [12] P. L. Nayak, “Photo Induced Graft Copolymerzation onto selected Fibers,” Journal of Macromolecular Science—Re- views in Macromolecular Chemistry & Physics, Vol. 31, No. 1, 1991, pp. 91-116. [13] P. L. Nayak, S. Lenka and N. C. Pati, “Grafting Vinyl Monomers onto Silk Fibers V: Graft Copolymerization of Methyl Methacrylate onto Sil Fibers Using Potassium Permanganate as Intiator,” Journal of Macromolecular Science, Vol. 13, 1979, pp. 1157-1169. doi:10.1080/00222337908056707 [14] P. L. Nayak, N. C. Pati and A. K. Pradhan, “Grafting Vinyl Monomers onto Nylon-6:III:Graft Copolymeriza- tion of Methyl Methacrylate on Nylon-6 Using Fe(III)- Thiourea Redox System,” Journal of Polymer Science— Polymer Chemistry Edition, Vol. 19, No. 3, 1981, pp. 831- 834. doi:10.1002/pol.1981.170190321 [15] P. L. Nayak and S. Lenka, “Photo-Induced Graft Copoly- merization IV: Photo Induced Graft Coploymerization of Methyl Methacrylate Using N-Bromo Succinamide as the Initiator,” Journal of Applied Polymer Science, Vol. 27, 1982, p. 3625. [16] P. L. Nayak and S. Lenka, “Grafting Vinyl Monomers onto CelluloseII:Graft-Copolymerization of Methyl Metha- crylate onto Cellulose Using V(V)-Thiourea Redox Sys- tem,” Journal of Polymer Science—Polymer Chemistry Edition, Vol. 19, 1981, p. 1561. [17] D. Sahoo, S. Sahoo, P. Mohanty, S. Sasmal and P. L. Nayak, “Chitosan: A New Versatile Bio-Polymer for Various Applications,” Designed Monomers and Poly- mers, Vol. 12, No. 5, 2009, pp. 377-404. doi:10.1163/138577209X12486896623418 [18] M. N. V. Ravi Kumar, R. A. A. Muzzarelli, C. Muzzarelli, H. Sashiwa and A. J. Domb, “Synthesis of a Chito- san-Dendrimer Hybrid and Its Biodegradation,” Chemical Reviews, Vol. 104, No. 12, 2004, pp. 6017-6084. [19] M. N. V. R. Kumar, “Grafting Vinyl Monomers onto Natural and Synthetic Polymers,” Reactive and Func- tional Polymers, Vol. 46, No. 1, 2000, pp. 1-27. [20] O. Felt, P. Buri and R. Gurny, “Thiolated Chitosans: Novel Polymers for Mucoadhesive,” Drug Delivery and Industrial Pharmaceuticals, Vol. 24, No. 4, 1998, pp. 979-993. doi:10.3109/03639049809089942 [21] S. Hirano, H. Seino, I. Akiyama and I. Nonaka, “Chitin Nanowhisker and Chitosan Nanoparticles in Protein Im- mobilization for Biosensor,” Applications Polymer Engi- neering Science, Vol. 59, 1989, pp. 897-901. [22] P. A. Sanford, G. Skjak-Braek, T. Anthonsen and P. A. Sanford, Eds., “Chitin and Chitosan-Sources, Chemistry, Biochemistry, Physical Properties and Applications,” El- sevier, London, 1989, pp. 51-70. [23] R. A. A. Muzzarelli, “Human Enzymatic Activities Re- lated to the Therapeutical Administration of Chitin De- rivatives,” Cell Molecular and Life Science, Vol. 53, No. 1, 1997, pp. 131-140. doi:10.1007/PL00000584 [24] O. Felt, P. Buri and R. Gurny, “Chitosan: A Unique Poly- saccharide for Drug Delivery,” Drug Delivery and Indus- trial Pharmaceuticals, Vol. 24, No. 4, 1998, pp. 979-993. [25] P. C. Bersch, B. Nies and A. Liebendorfer, “Graft Co- polymerized Chitosan-Present Status and Applications,” Journal of Material Science, Materials in Medicine, Vol. 6, No. 2, 1995, pp. 231-240. doi:10.3109/03639049809089942 [26] M. Sugimoto, M. Morimoto and H. Sashiwa, “Synthesis and Bioactivities of Poly (Ethylene Glycol)-Chitosan Hy- brids,” Carbohydrate Polymers, Vol. 36, No. 1, 1998, pp. 49-59. doi:10.1016/S0144-8617(97)00235-X [27] K. Kurita, T. Kojima, T. Munakata, H. Akao, T. Mori, Y. Nishiyama, et al., “Preparation of Nonnatural Branched Chitin and Chitosan,” Chemistry Letters, Vol. 27, No. 4, 1998, pp. 317-318. doi:10.1246/cl.1998.317 [28] H. Sashiwa and Y. Shigemasa, “Chitosan, a Biodegrad- able and Biocompatible Polymer,” Carbohydrate Poly- mers, Vol. 39, No. 2, 1999, pp. 127-138. doi:10.1016/S0144-8617(98)00167-2 Copyright © 2011 SciRes. MSA  Grafting Vinyl Monomers onto Chitosan:IV:Graft Copolymerized of Acrylicacid onto Chitosan Using Ceric Ammonium Nitrate as the Initiator—Characterization and Antimicrobial Activities Copyright © 2011 SciRes. MSA 1748 [29] N. Terada, M. Morimoto, H. Saimoto, Y. Okamato, S. Minami and Y. Shigemasa, “Graft Copolymerized Chito- san—Present Status and Applications,” Chemistry Letters, Vol. 28, No. 12, 1999, pp. 1285-1286. doi:10.1246/cl.1999.1285 [30] V. Alexandrova, G. V. Obukhova, N. S. Dominina and D. A. Topchiev, “Modification of Chitosan for Construction of Efficient Antioxidant Biodegradable Macromolecular Systems,” Macromolecular Symposia, Vol. 144, No. 2, 1999, pp. 413-418. doi:10.1002/masy.19991440138 [31] T. R. Sridhari and P. K. Dutta, “Ceric Ammonium Nitrate Induced Grafting of Polyacrylamide onto Chitosan,” In- dian Journal of Chemical Technology, Vol. 7, No. 1, 2000, pp. 198-204. [32] A. Heras, N. M. Rodriguez and V. M. Ramos, “N-me- thylene. Phosphonic Chitosan: A Novel Soluble Deriva- tive,” Carbohydrate Polymers, Vol. 44, No. 1, 2001, pp. 1-8. doi:10.1016/S0144-8617(00)00195-8 [33] Y. C. Wang, M. C. Lin, D. M. Wang and H. J. Hsieh, “Fabrication of a Novel Porous PGA-Chitosan Hybrid Matrix for Tissue Engineering,” Biomaterials, Vol. 24, No. 6, 2003, pp. 1047-1057. doi:10.1016/S0142-9612(02)00434-9 [34] Z. K. Yang and Y. Yuan, “Ceric Ammonium Nitrate In- duced Grafting of Polyacrylamide onto,” Journal of Ap- plied Polymer Science, Vol. 82, No. 8, 2001, pp. 1838- 1843. doi:10.1002/app.2026 [35] S. Chen and Y. Wang, “Study on β-Cyclodextrin Grafting with Chitosan and Slow Release of Its Inclusion Complex with Radioactive Iodine,” Journal of Applied Polymer Science, Vol. 82, No. 10, 2001, pp. 2414-2421. doi:10.1002/app.2092 [36] B. O. Jung, C. H. Kim, K. S. Choi, Y. M. Lee and J. J. Kim, “Results, Phosphorous Containing Chitosan Beads for Controlled Oral Drug,” Journal of Applied Polymer Science, Vol. 72, No. 13, 1999, pp. 1713-1719. doi:10.1002/(SICI)1097-4628(19990624)72:13<1713::AI D-APP7>3.0.CO;2-T [37] A. R. Kotze, H. L. Lueben, B. J. De Leeuw, A. G. De Boer, J. C. Verhoef and H. E. Junginger, “Synthesis of Novel Chitosan Derivatives for Micellar Solubilization of Chitosan,” Pharmaceutical Research, Vol. 14, No. 9, 1997, pp. 1197-1202. doi:10.1023/A:1012106907708 [38] M. Thanou, J. C. Verhoef and H. E. Junginger, “Morden Drug Delivery Application of Chitosan,” Advanced Drug Delivery Reviews, Vol. 52, No. 2, 2001, pp. 117-126. doi:10.1016/S0169-409X(01)00231-9 [39] A. S. Hoffman, G. Chen, X. Wu, Z. Ding, B. Kabra, K. Randeri, et al., “Design and Evaluation of Mucoadhesive Controlled Release Oral Tablets of Terbutaline Sulphate using some Natural Materials,” Polymer Preparations, Vol. 38, No. 2, 1997, pp. 524-525. [40] R. A. Tasker, B. J. Connell, S. J. Ross and C. M. Elson, “Reduction of Postoperative Adhesions by N,O-Car- boxymethylchitosan and Spermine NONOate in Rats,” Laboratory Animals, Vol. 32, No. 2, 1998, pp. 270-275. doi:10.1258/002367798780559220 [41] D. K. Singh and A. R. Ray, “Study on Radiation-Induced Grafting of Styrene onto Chitin and Chitosan,” Carbohy- drate Polymers, Vol. 36, No. 2-3, 1998, pp. 251-255. doi:10.1016/S0144-8617(97)00260-9 [42] J. K. F. Suh and H. W. T. Matthew, “Chitosan-Based Hyaluronic Acid Hybrid Polymer Fibers as a Scaffold Biomaterial for Cartilage Tissue Engineering,” Biomate- rials, Vol. 21, No. 24, 2000, pp. 2589-2598. [43] M. N. V. R. Kumar, R. A. A. Muzzarelli, C. Muzzarelli, H. Sashiwa and A. J. Domb, “Chemical Modification of Chitosan: Preparation and Lectin Binding Properties of α-Galactosyl-Chitosan Conjugates. Potential Inhibitors in Acute Rejection Following Xenotransplantation,” Chemi- cal Reviews, Vol. 104, No. 12, 2004, pp. 6017-6084. doi:10.1021/cr030441b [44] J. Berger, M. Reist, J. M. Mayer, O. Belt and R. Gurny, “Fabric Structure Influence in the Deposition of Flavour Microcapsules,” European Journal of Pharamaceutics and Biopharmaceutics, Vol. 57, No. 1, 2004, pp. 35-52. doi:10.1016/S0939-6411(03)00160-7 [45] S. Senel and S. J. McClure, “Potential Applications of Chitosan in Veterinary Medicine,” Advanced Drug De- livery Reviews, Vol. 56, No. 10, 2004, pp. 1467-1480. doi:10.1016/j.addr.2004.02.007 [46] A. J. Verma, S. V. Deshpande and J. K. Kennedy, “Graft Copolymerization of Vinyl Monomers onto Chitosan,” Carbohydrate Polymers, Vol. 55, No. 1, 2004, pp. 77-93. [47] E. Guibal, “Chemical and Radiation Induced Grafting of Indole onto Chitin,” Separation and Purification Tech- nology, Vol. 38, No. 1, 2004, pp. 43-74. doi:10.1016/j.seppur.2003.10.004 [48] K. Kurita, “Perspectives of Chitin and Chitosan Nanofi- brous Scaffolds in Tissue,” Progress in Polymer Science, Vol. 26, No. 9, 2001, pp. 1921-1971. doi:10.1016/S0079-6700(01)00007-7 [49] M. Prabaharan and J. F. Mano, “Preparation of Chitosan Scaffolds for Tissue Engineering,” Drug Delivery, Vol. 12, No. 1, 2005, pp. 41-57. doi:10.1080/10717540590889781
|