S. M. Nolan et al. / Open Journal of Pediatrics 1 (2011) 41-44
Copyright © 2011 SciRes.
43
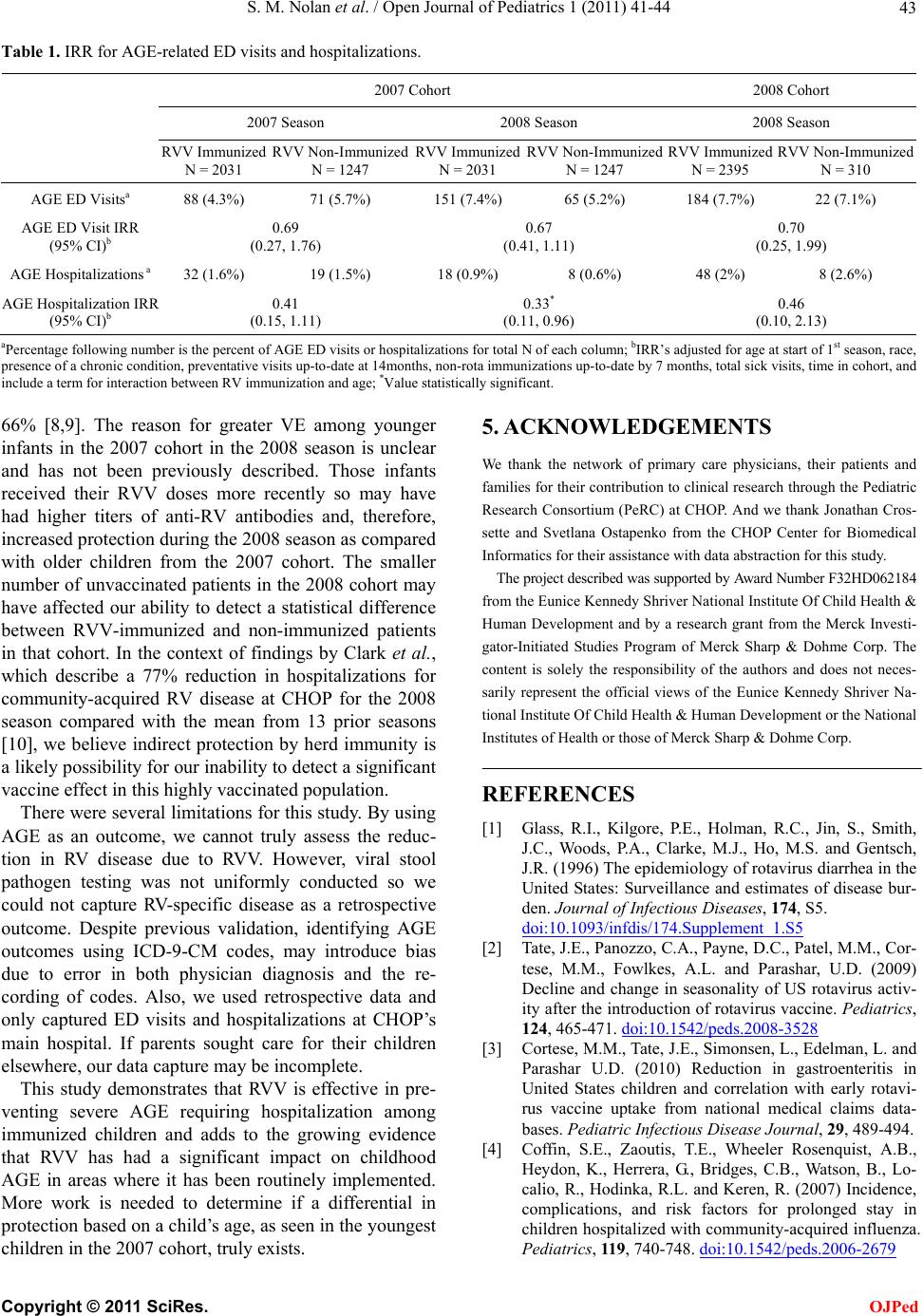
Table 1. IRR for AGE-related ED visits and hospitalizations.
2007 Cohort 2008 Cohort
2007 Season 2008 Season 2008 Season
RVV Immunized
N = 2031 RVV Non-Immunized
N = 1247 RVV Immunized
N = 2031 RVV Non-Immunized
N = 1247 RVV Immunized
N = 2395 RVV Non-Immunized
N = 310
AGE ED Visitsa 88 (4.3%) 71 (5.7%) 151 (7.4%) 65 (5.2%) 184 (7.7%) 22 (7.1%)
AGE ED Visit IRR
(95% CI)b 0.69
(0.27, 1.76) 0.67
(0.41, 1.11) 0.70
(0.25, 1.99)
AGE Hospitalizations a 32 (1.6%) 19 (1.5%) 18 (0.9%) 8 (0.6%) 48 (2%) 8 (2.6%)
AGE Hospitalization IRR
(95% CI)b 0.41
(0.15, 1.11) 0.33*
(0.11, 0.96) 0.46
(0.10, 2.13)
aPercentag e fo llo wing numb er is the p ercent of AGE ED vis it s or hospit ali zati ons for total N of each co lu mn; bIRR’s adjust ed fo r ag e at star t o f 1st s easo n, race,
presence of a chronic condition, preventative visits up-to-date at 14months, non-rota immunizations up-to-date by 7 months, total sick visits, time in cohort, and
include a term for interaction between RV immunization and age; *Value statistically significant.
5. ACKNOWLEDGEMENTS
66% [8,9]. The reason for greater VE among younger
infants in the 2007 cohort in the 2008 season is unclear
and has not been previously described. Those infants
received their RVV doses more recently so may have
had higher titers of anti-RV antibodies and, therefore,
increased protection during the 2008 season as compared
with older children from the 2007 cohort. The smaller
number of unvaccinated patients in the 2008 cohort may
have affected our ability to detect a statistical difference
between RVV-immunized and non-immunized patients
in that cohort. In the context of findings by Clark et al.,
which describe a 77% reduction in hospitalizations for
community-acquired RV disease at CHOP for the 2008
season compared with the mean from 13 prior seasons
[10], we believe indirect protection by herd immunity is
a likely possibility for our inability to detect a significant
vaccine effect in this highly vaccinated population.
We thank the network of primary care physicians, their patients and
families for their contribution to clinical research through the Pediatric
Research Consortium (PeRC) at CHOP. And we thank Jonathan Cros-
sette and Svetlana Ostapenko from the CHOP Center for Biomedical
Informatics for their assistance with data abstraction for this study.
The project described was supported by Award Number F32HD062184
from the Eunice Kennedy Shriver National Institute Of Child Health &
Human Development and by a research grant from the Merck Investi-
gator-Initiated Studies Program of Merck Sharp & Dohme Corp. The
content is solely the responsibility of the authors and does not neces-
sarily represent the official views of the Eunice Kennedy Shriver Na-
tional Institute Of Child Health & Human Development or the National
Institutes of Health or those of Merck Sharp & Dohme Corp.
REFERENCES
There were several limitations for this study. By using
AGE as an outcome, we cannot truly assess the reduc-
tion in RV disease due to RVV. However, viral stool
pathogen testing was not uniformly conducted so we
could not capture RV-specific disease as a retrospective
outcome. Despite previous validation, identifying AGE
outcomes using ICD-9-CM codes, may introduce bias
due to error in both physician diagnosis and the re-
cording of codes. Also, we used retrospective data and
only captured ED visits and hospitalizations at CHOP’s
main hospital. If parents sought care for their children
elsewhere, our data capture may be incomplete.
[1] Glass, R.I., Kilgore, P.E., Holman, R.C., Jin, S., Smith,
J.C., Woods, P.A., Clarke, M.J., Ho, M.S. and Gentsch,
J.R. (1996) The epidemiology of rotavirus diarrhea in the
United States: Surveillance and estimates of disease bur-
den. Journal of Infectious Diseases, 174, S5.
doi:10.1093/infdis/174.Supplement_1.S5
[2] Tate, J.E., Panozzo, C.A., Payne, D.C., Patel, M.M., Cor-
tese, M.M., Fowlkes, A.L. and Parashar, U.D. (2009)
Decline and change in seasonality of US rotavirus activ-
ity after the introduction of rotavirus vaccine. Pediatrics,
124, 465-471. doi:10.1542/peds.2008-3528
[3] Cortese, M.M., Tate, J.E., Simonsen, L., Edelman, L. and
Parashar U.D. (2010) Reduction in gastroenteritis in
United States children and correlation with early rotavi-
rus vaccine uptake from national medical claims data-
bases. Pediatric Infectious Disease Journal, 29, 489-494.
This study demonstrates that RVV is effective in pre-
venting severe AGE requiring hospitalization among
immunized children and adds to the growing evidence
that RVV has had a significant impact on childhood
AGE in areas where it has been routinely implemented.
More work is needed to determine if a differential in
protection based on a child’s age, as seen in the youngest
children in the 2007 cohort, truly exists.
[4] Coffin, S.E., Zaoutis, T.E., Wheeler Rosenquist, A.B.,
Heydon, K., Herrera, G., Bridges, C.B., Watson, B., Lo-
calio, R., Hodinka, R.L. and Keren, R. (2007) Incidence,
complications, and risk factors for prolonged stay in
children hospitalized with community-acquired influenza.
Pediatrics, 11 9, 740-748. doi:10.1542/peds.2006-2679
OJPed