M. C. Schwartz et al. / Open Journal of Pediatrics 1 (2011) 87-89
88
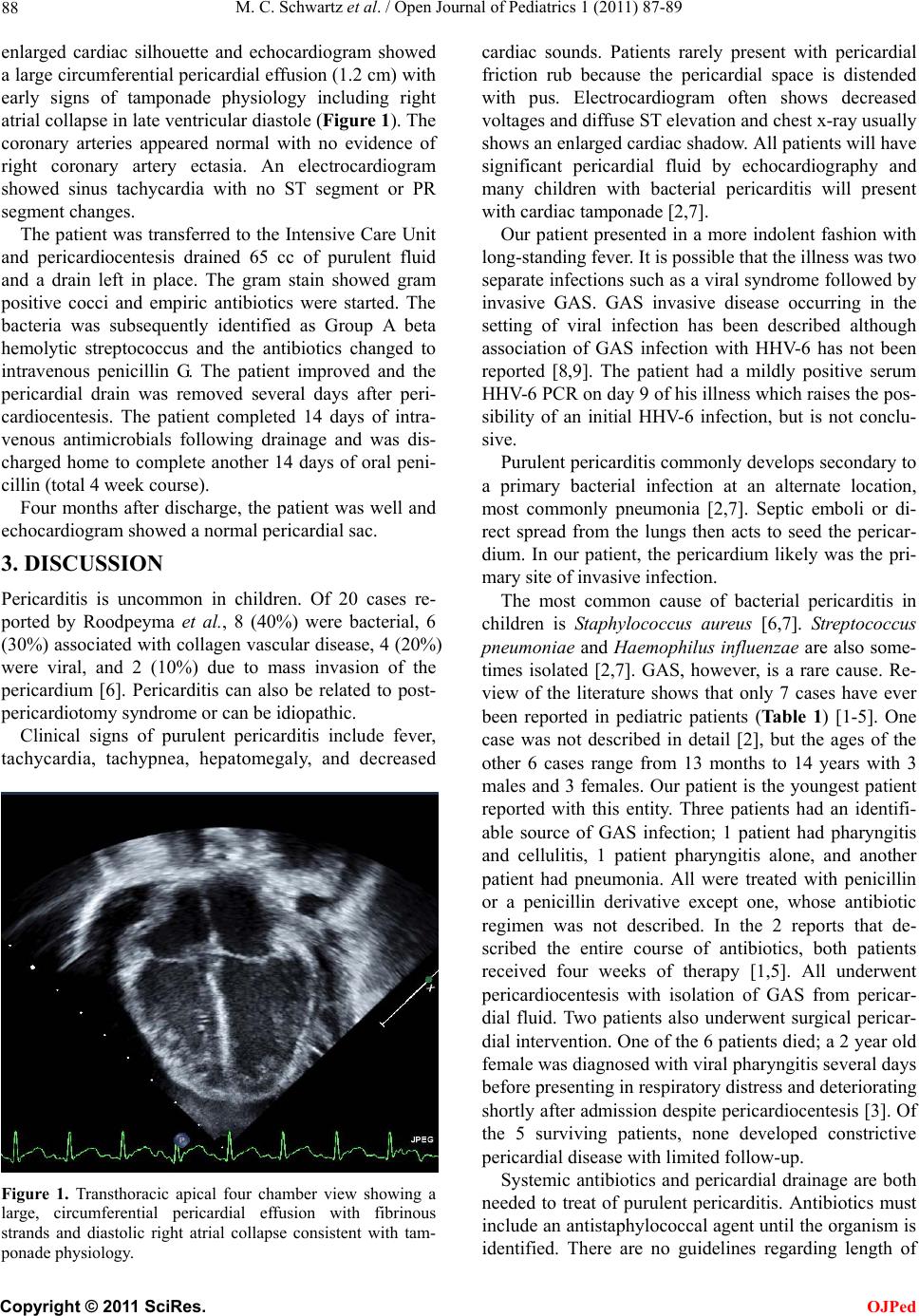
enlarged cardiac silhouette and echocardiogram showed
a large circumferential pericardial effusion (1.2 cm) with
early signs of tamponade physiology including right
atrial collapse in late ventricular diastole (Figure 1). The
coronary arteries appeared normal with no evidence of
right coronary artery ectasia. An electrocardiogram
showed sinus tachycardia with no ST segment or PR
segment changes.
The patient was transferred to the Intensive Care Unit
and pericardiocentesis drained 65 cc of purulent fluid
and a drain left in place. The gram stain showed gram
positive cocci and empiric antibiotics were started. The
bacteria was subsequently identified as Group A beta
hemolytic streptococcus and the antibiotics changed to
intravenous penicillin G. The patient improved and the
pericardial drain was removed several days after peri-
cardiocentesis. The patient completed 14 days of intra-
venous antimicrobials following drainage and was dis-
charged home to complete another 14 days of oral peni-
cillin (total 4 week course).
Four months after discharge, the patient was well and
echocardiogram showed a normal pericardial sac.
3. DISCUSSION
Pericarditis is uncommon in children. Of 20 cases re-
ported by Roodpeyma et al., 8 (40%) were bacterial, 6
(30%) associated with collagen vascular disease, 4 (20%)
were viral, and 2 (10%) due to mass invasion of the
pericardium [6]. Pericarditis can also be related to post-
pericardiotomy syndrome or can be idiopathic.
Clinical signs of purulent pericarditis include fever,
tachycardia, tachypnea, hepatomegaly, and decreased
Figure 1. Transthoracic apical four chamber view showing a
large, circumferential pericardial effusion with fibrinous
strands and diastolic right atrial collapse consistent with tam-
ponade physiology.
cardiac sounds. Patients rarely present with pericardial
friction rub because the pericardial space is distended
with pus. Electrocardiogram often shows decreased
voltages and diffuse ST elevation and chest x-ray usually
shows an enlarged cardiac shadow. All patients will have
significant pericardial fluid by echocardiography and
many children with bacterial pericarditis will present
with cardiac tamponade [2,7].
Our patient presented in a more indolent fashion with
long-standing fever. It is possible that the illness was two
separate infections such as a viral syndro me followed by
invasive GAS. GAS invasive disease occurring in the
setting of viral infection has been described although
association of GAS infection with HHV-6 has not been
reported [8,9]. The patient had a mildly positive serum
HHV-6 PCR on day 9 of his illness which raises the pos-
sibility of an initial HHV-6 infection, but is not conclu-
sive.
Purulent pericarditis co mmonly develops seco ndary to
a primary bacterial infection at an alternate location,
most commonly pneumonia [2,7]. Septic emboli or di-
rect spread from the lungs then acts to seed the pericar-
dium. In our patient, the pericardium likely was the pri-
mary site of invasive infection.
The most common cause of bacterial pericarditis in
children is Staphylococcus aureus [6,7]. Streptococcus
pneumonia e and Haemophilus influenzae are also some-
times isolated [2,7]. GAS, however, is a rare cause. Re-
view of the literature shows that only 7 cases have ever
been reported in pediatric patients (Ta b l e 1) [1-5]. One
case was not described in detail [2], but the ages of the
other 6 cases range from 13 months to 14 years with 3
males and 3 females. Our patient is the youngest patient
reported with this entity. Three patients had an identifi-
able source of GAS infection; 1 patient had pharyngitis
and cellulitis, 1 patient pharyngitis alone, and another
patient had pneumonia. All were treated with penicillin
or a penicillin derivative except one, whose antibiotic
regimen was not described. In the 2 reports that de-
scribed the entire course of antibiotics, both patients
received four weeks of therapy [1,5]. All underwent
pericardiocentesis with isolation of GAS from pericar-
dial fluid. Two patients also underwent surgical pericar-
dial intervention. One of the 6 patients died; a 2 year old
female was diagnosed with viral pharyngitis several days
before presenting in respiratory distress and deteriorating
shortly after admission despite pericardiocentesis [3]. Of
the 5 surviving patients, none developed constrictive
pericardial disease with limited follow-up.
Systemic antibiotics and pericardial drainage are both
needed to treat of purulent pericarditis. Antibiotics must
include an antistaphylococcal agent until the organism is
identified. There are no guidelines regarding length of
C
opyright © 2011 SciRes. OJPed