Paper Menu >>
Journal Menu >>
 Materials Sciences and Applications, 2010, 1, 36-38 doi:10.4236/msa.2010.11007 Published Online April 2010 (http://www.SciRP.org/journal/msa) Copyright © 2010 SciRes. MSA Preparation of Star-Shaped Polylactic Acid Drug Carrier Nanoparticles Michele Marini Mazal-TOV S. A. S., Via Carmelitane Scalze – Modena, Italy. Email: marini.michele@email.it Received February 13th, 2010; revised March 13th, 2010; accepted March 15th, 2010. ABSTRACT Drug carrier biocompatible and biodegradable nanoparticles of about 15 nm were prepared by solvent evaporation technique from star-shaped poly(D,L-lactide) synthesized using dipentaerythritol as core and Tin (II) ethylhexanoate as catalyst. Keywords: PLA, Nanoparticles, Drug-Carriers 1. Introduction It is well known that the penetration of substances into the brain is limited by the blood-brain barrier (BBB) wh- ich is formed by the endothelium of the brain vessels, the basal membrane and neuroglial cells [1]. Because of this most drugs do not pass the BBB. It is widely accepted that only compounds which are unionized at physiologi- cal pH, are lipophilic, and of low molecular mass can cross the BBB by diffusion mechanisms, and other com- pounds, such as amino acids, neuropeptides, and hexoses, need specific carrier proteins to pass into the brain [1]. To overcome the limited access of drugs to the brain, several methods have been employed to achieve BBB penetration. The use of drug carriers such as liposomes [2] and nanoparticles for targeted drug delivery has been examined. Biodegradable polymers have long been of interest in drug carriers and controlled release technology because of the ability of these polymers to be reabsorbed by the body [3]. This alleviates the need for removal, often sur- gically, of a drug release device. The most widely used and studied class of biodegradable polymers is the poly- esters, including poly(lactic acid), poly(glycolic acid), and their copolymers and poly(lactic acid) (henceforth referred to as PLA) was investigated as a drug delivery material as early as 1971 [3]. There are a large number of research groups, world- wide, examining PLA in the form of microparticles and nanoparticles, for use in controlled drug delivery systems using a solvent evaporation technique, or modification thereof [4,5]. Although these references, there isn’t any work in literature which reports the preparation of star- shaped PLA drug carrier nanoparticles. In this work the synthesis of star-shaped PLA and the preparation of nanoparticles using a modified solvent evaporation method is reported. 2. Experimental Part Star-shaped PLA was synthesized using dipentaerythritol (DPE) as core, D,L-dilactide (LA) as monomer and Tin (II) ethylhexanoate (Sn(Oct)2) as catalyst [6] as reported in Scheme 1. Monomer (5 g, 0.035 mol) was added under nitrogen to a flame dried, 50 mL round-bottomed flask containing a magnetic stir bar and sealed with a rubber septum. The system was purged with nitrogen. Dipentaerythritol (0.247 g, 0.00097 mol) in toluene (1 ml) was added via syringe under nitrogen. The reaction flask was immersed in a 130℃ oil bath and sufficient time was allowed for the lactide and DPE to melt. A catalytic amount of Sn(Oct)2 (0.162 g, 0.0004 mol) in toluene (1 ml) was added, and the reaction mixture was allowed to stir for 10 min. The reaction temperature was decreased to 120℃, and the reaction was allowed to proceed for 24 h. The product was dissolved in chloroform, precipitated in a hexane/methanol mixture (90:10), and dried in vacuo at 60℃ for 24 h. Molecular weight and chemical structure of the star-shaped PLA were analyzed by 1H-NMR. Nuclear magnetic spectra, performed with a Bruker FT Avance DPX200 instrument using CDCl3 as solvent and tetrame- thylsilane as internal reference, is reported in the follow- ing Figure 1. Mn values was calculated by comparison of multiplet at 5 ppm (a, 1H) with respect to the singolet at 4 ppm (e, 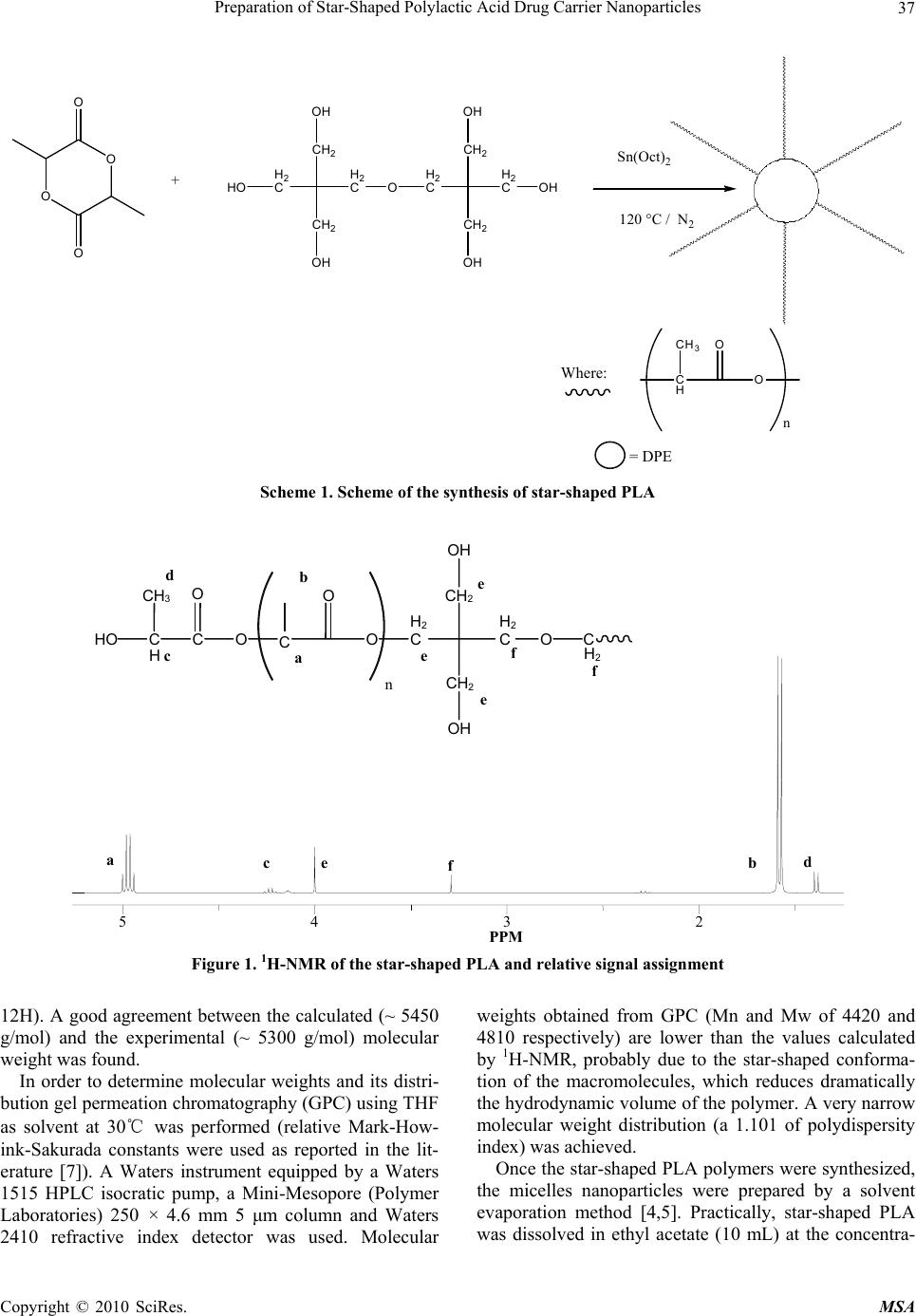 Preparation of Star-Shaped Polylactic Acid Drug Carrier Nanoparticles37 = DPE O O O O HO H2 C CH2 OH CH2 OH H2 CO H2 C CH2 OH CH2 OH H2 COH + Sn(Oct)2 120 °C / N2 CH3 C H O O n Where: Scheme 1. Scheme of the synthesis of star-shaped PLA a b fd c e 5 PPM 432 HO C H c CH 3 C O O O O H 2 C CH 2 OH O n a b d f f e C CH 2 OH e e H 2 CC H 2 Figure 1. 1H-NMR of the star-shaped PLA and relative signal assignment 12H). A good agreement between the calculated (~ 5450 g/mol) and the experimental (~ 5300 g/mol) molecular weight was found. In order to determine molecular weights and its distri- bution gel permeation chromatography (GPC) using THF as solvent at 30℃ was performed (relative Mark-How- ink-Sakurada constants were used as reported in the lit- erature [7]). A Waters instrument equipped by a Waters 1515 HPLC isocratic pump, a Mini-Mesopore (Polymer Laboratories) 250 × 4.6 mm 5 μm column and Waters 2410 refractive index detector was used. Molecular weights obtained from GPC (Mn and Mw of 4420 and 4810 respectively) are lower than the values calculated by 1H-NMR, probably due to the star-shaped conforma- tion of the macromolecules, which reduces dramatically the hydrodynamic volume of the polymer. A very narrow molecular weight distribution (a 1.101 of polydispersity index) was achieved. Once the star-shaped PLA polymers were synthesized, the micelles nanoparticles were prepared by a solvent evaporation method [4,5]. Practically, star-shaped PLA was dissolved in ethyl acetate (10 mL) at the concentra- Copyright © 2010 SciRes. MSA 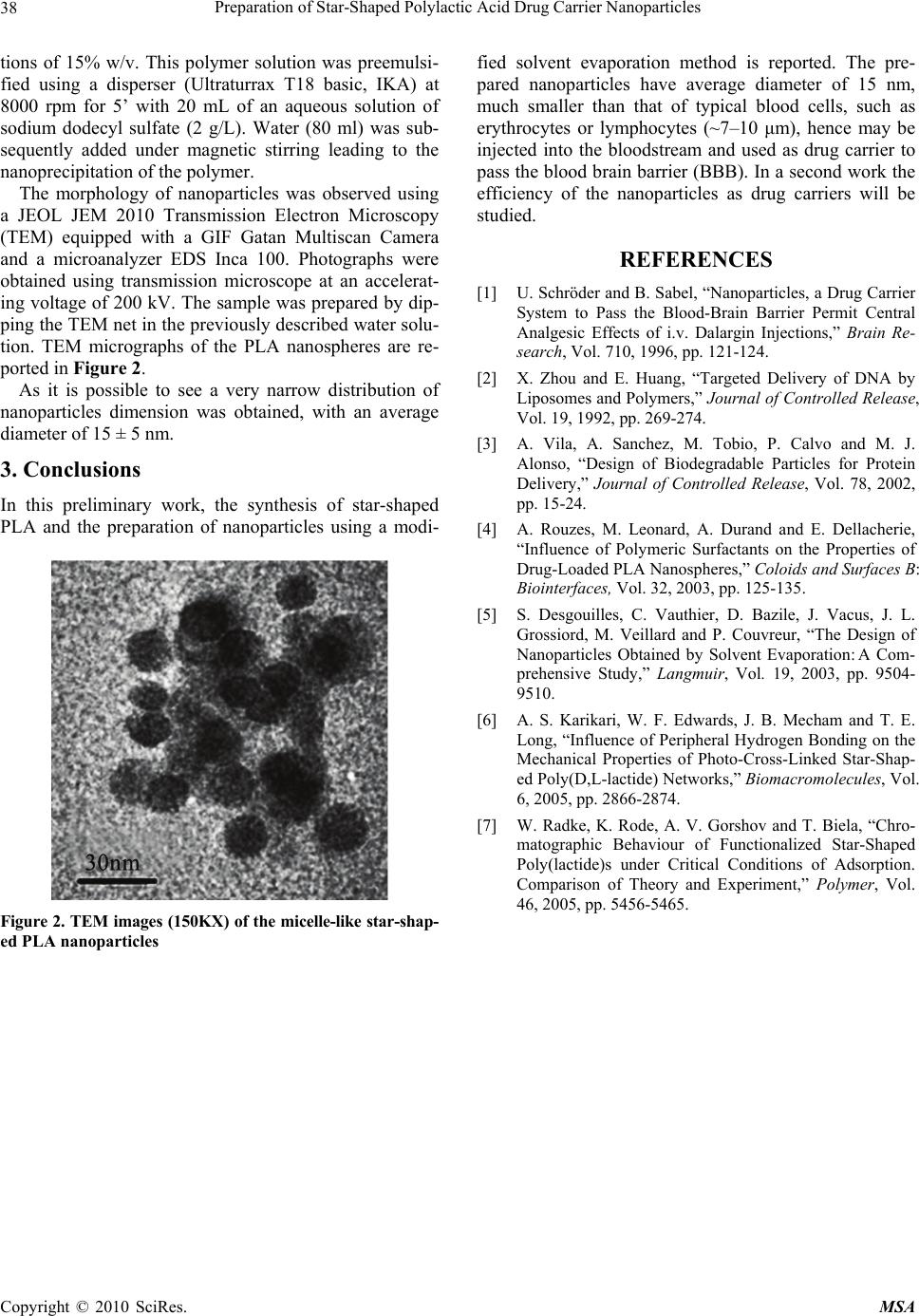 Preparation of Star-Shaped Polylactic Acid Drug Carrier Nanoparticles 38 tions of 15% w/v. This polymer solution was preemulsi- fied using a disperser (Ultraturrax T18 basic, IKA) at 8000 rpm for 5’ with 20 mL of an aqueous solution of sodium dodecyl sulfate (2 g/L). Water (80 ml) was sub- sequently added under magnetic stirring leading to the nanoprecipitation of the polymer. The morphology of nanoparticles was observed using a JEOL JEM 2010 Transmission Electron Microscopy (TEM) equipped with a GIF Gatan Multiscan Camera and a microanalyzer EDS Inca 100. Photographs were obtained using transmission microscope at an accelerat- ing voltage of 200 kV. The sample was prepared by dip- ping the TEM net in the previously described water solu- tion. TEM micrographs of the PLA nanospheres are re- ported in Figure 2. As it is possible to see a very narrow distribution of nanoparticles dimension was obtained, with an average diameter of 15 ± 5 nm. 3. Conclusions In this preliminary work, the synthesis of star-shaped PLA and the preparation of nanoparticles using a modi- Figure 2. TEM images (150KX) of the micelle-like star-shap- ed PLA nanoparticles fied solvent evaporation method is reported. The pre- pared nanoparticles have average diameter of 15 nm, much smaller than that of typical blood cells, such as erythrocytes or lymphocytes (~7–10 μm), hence may be injected into the bloodstream and used as drug carrier to pass the blood brain barrier (BBB). In a second work the efficiency of the nanoparticles as drug carriers will be studied. REFERENCES [1] U. Schröder and B. Sabel, “Nanoparticles, a Drug Carrier System to Pass the Blood-Brain Barrier Permit Central Analgesic Effects of i.v. Dalargin Injections,” Brain Re- search, Vol. 710, 1996, pp. 121-124. [2] X. Zhou and E. Huang, “Targeted Delivery of DNA by Liposomes and Polymers,” Journal of Controlled Release, Vol. 19, 1992, pp. 269-274. [3] A. Vila, A. Sanchez, M. Tobio, P. Calvo and M. J. Alonso, “Design of Biodegradable Particles for Protein Delivery,” Journal of Controlled Release, Vol. 78, 2002, pp. 15-24. [4] A. Rouzes, M. Leonard, A. Durand and E. Dellacherie, “Influence of Polymeric Surfactants on the Properties of Drug-Loaded PLA Nanospheres,” Coloids and Surfaces B: Biointerfaces, Vol. 32, 2003, pp. 125-135. [5] S. Desgouilles, C. Vauthier, D. Bazile, J. Vacus, J. L. Grossiord, M. Veillard and P. Couvreur, “The Design of Nanoparticles Obtained by Solvent Evaporation:A Com- prehensive Study,” Langmuir, Vol. 19, 2003, pp. 9504- 9510. [6] A. S. Karikari, W. F. Edwards, J. B. Mecham and T. E. Long, “Influence of Peripheral Hydrogen Bonding on the Mechanical Properties of Photo-Cross-Linked Star-Shap- ed Poly(D,L-lactide) Networks,” Biomacromolecules, Vol. 6, 2005, pp. 2866-2874. [7] W. Radke, K. Rode, A. V. Gorshov and T. Biela, “Chro- matographic Behaviour of Functionalized Star-Shaped Poly(lactide)s under Critical Conditions of Adsorption. Comparison of Theory and Experiment,” Polymer, Vol. 46, 2005, pp. 5456-5465. Copyright © 2010 SciRes. MSA |

