Paper Menu >>
Journal Menu >>
 Materials Sciences and Applications, 2010, 1, 25-31 doi:10.4236/msa.2010.11005 Published Online April 2010 (http://www.SciRP.org/journal/msa) Copyright © 2010 SciRes. MSA Blue-Shift and Enhanced Photoluminescence in BaMgAl10O17:Eu2+ Nanophosphor under VUV Excitation for PDPs Application Raghvendra Singh Yadav1,2, Shiv Kumar Pandey1,3, Avinash Chandra Pandey1,2 1Nanophosphor Application Centre, University of Allahabad, Allahabad, India; 2Physics Department,University of Allahabad, Alla- habad, India; 3Motilal Nehru National Institute of Technology, Allahabad, India. Email: raghvendra_nac@yahoo.co.in Received January 18th, 2010; revised January 28th, 2010; accepted February 9th, 2010. ABSTRACT In this report, five systems of varied diameters viz. 62 nm, 85 nm, 115 nm, 160 nm and 450 nm of BaMgAl10O17:Eu2+ nanorods are prepared by solution combustion approach and with annealing at different temperature in reduced at- mosphere (N2 + H2). An intense broad blue photoluminescence (PL), corresponding to the electronic transition of Eu2+ from the 4f 6 5d excited state to the 4f 7 ground state, is observed. The blue-shift and enhanced photoluminescence is also observed, and found to be highly dependent on the size of diameter of BaMgAl10O17:Eu2+ nanostructures. The change in decay time and color-coordinates with change in size of diameter of BaMgAl10O17:Eu2+ nanostructure have been analysed and thoroughly discussed. Keywords: Quantum Confinement Effect, Nanophosphor, PDPs 1. Introduction Recently, plasma display panels (PDPs) have been used for large screen television at home and for a public in- formation display. However, luminescent properties such as intensity and efficiency of the resulting PDPs are still inferior to those of traditional cathode-ray tube display [1-4]. To overcome these drawbacks, optimization of ph- osphors used in PDPs becomes necessary. Red, blue and green phosphors used in PDPs are key materials to im- prove the performance of PDPs such as brightness, cost-effectiveness and stability. BaMgAl10O17:Eu2+ (BAM) is an important blue-emitting phosphor for plasma display panel (PDP) because it can efficiently absorb the vacuum ultraviolet (VUV) light coming from the resonance radiation line of Xe atoms (147 nm) and from the excited state of molecular Xe (172 nm). BaMgAl10O17:Eu2+ is an insulating host material with a wide band gap of 6.4 eV. Recently, nanocrystals com- posed of wide band gap insulator have attracted a lot of attention, since several interesting results have been ob- served in semiconductor nanocrystal [5-8]. Therefore, it is interesting to study whether a similar behaviour can be observed for wide band gap insulators. It is well known that size-dependent quantum confinement has significant effects on both radiative and non-radiative electronic transitions in nanocrystals. The principal consequences of quantum confinement are an increase in the band gap energy, and an associated increased probability of radia- tive transitions. Confinement of carriers in real space causes their wavefunctions to spread out in momentum space, increasing the probability of radiative processes due to greater wavefunction overlap [9-11]. B. Mercier et al. [12] observed the quantum confinement effect on the wide band gap of a material such as Gd2O3 (5.4 eV), however the observed effect was quite weak. Recently, Guiling Ning et al. [13] succeeded in reducing the size of BaMgAl10O17:Eu2+ phosphor (particle size ~ 400 nm) and observed the increase in the intensity due to quantum size effect of nano-scale phosphor, but they failed to study optical properties with further reduction of particle size. In this paper, a novel solution combustion approach to synthesize BAM nanophosphors has been reported. Our primary aim is to explore and study the photolumines- cence property of BaMgAl10O17:Eu2+ nano particles with reduction of particle size under quantum confinement ef- fect. The synthesized BaMgAl10O17:Eu2+ nanoparticles have enhanced photoluminescence property which has a technological importance in plasma display panels (PDPs). 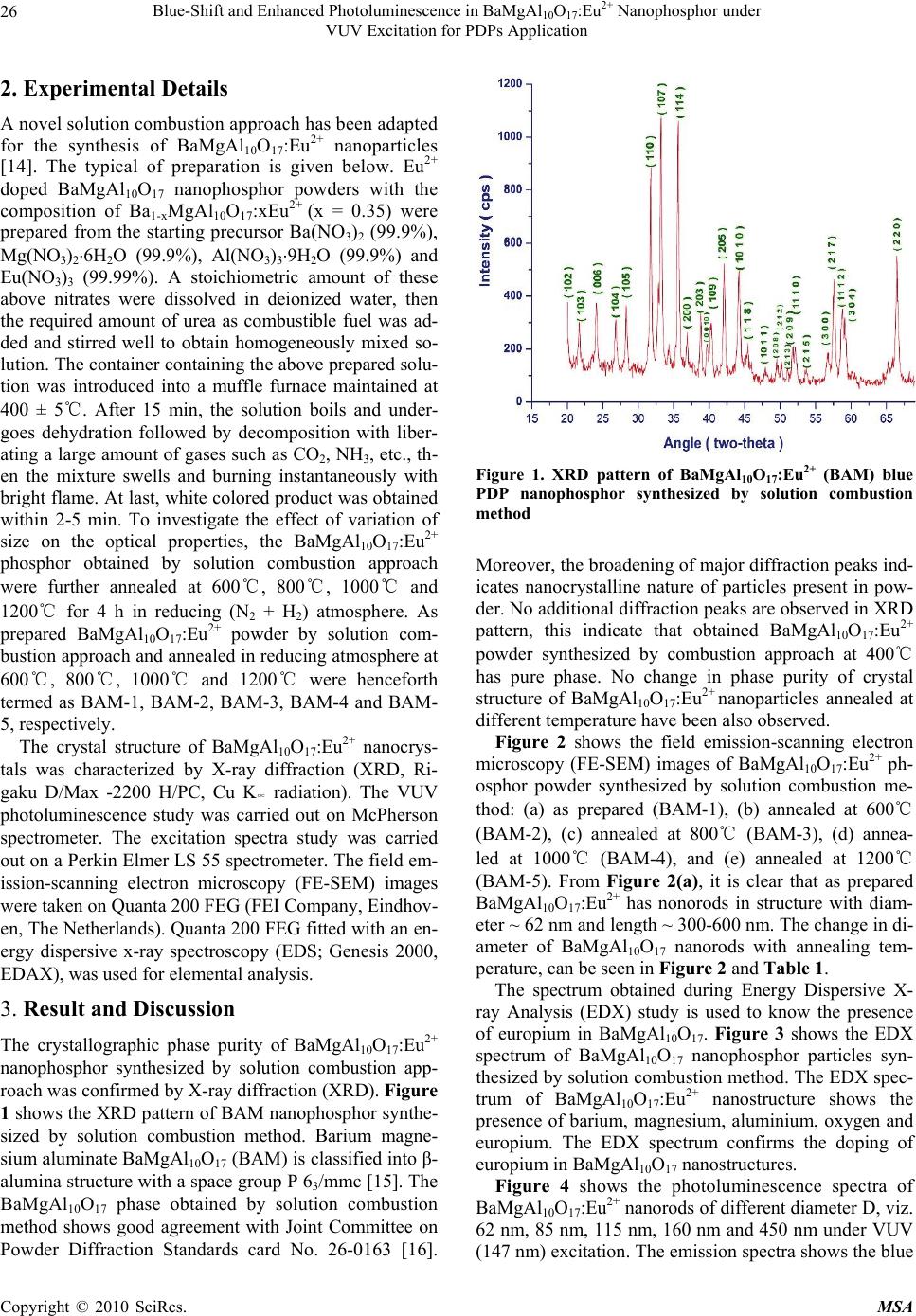 Blue-Shift and Enhanced Photoluminescence in BaMgAlO:Eu2+ Nanophosphor under 26 10 17 VUV Excitation for PDPs Application 2. Experimental Details A novel solution combustion approach has been adapted for the synthesis of BaMgAl10O17:Eu2+ nanoparticles [14]. The typical of preparation is given below. Eu2+ doped BaMgAl10O17 nanophosphor powders with the composition of Ba1-xMgAl10O17:xEu2+ (x = 0.35) were prepared from the starting precursor Ba(NO3)2 (99.9%), Mg(NO3)26H2O (99.9%), Al(NO3)39H2O (99.9%) and Eu(NO3)3 (99.99%). A stoichiometric amount of these above nitrates were dissolved in deionized water, then the required amount of urea as combustible fuel was ad- ded and stirred well to obtain homogeneously mixed so- lution. The container containing the above prepared solu- tion was introduced into a muffle furnace maintained at 400 ± 5℃. After 15 min, the solution boils and under- goes dehydration followed by decomposition with liber- ating a large amount of gases such as CO2, NH3, etc., th- en the mixture swells and burning instantaneously with bright flame. At last, white colored product was obtained within 2-5 min. To investigate the effect of variation of size on the optical properties, the BaMgAl10O17:Eu2+ phosphor obtained by solution combustion approach were further annealed at 600℃, 800℃, 1000℃ and 1200℃ for 4 h in reducing (N2 + H2) atmosphere. As prepared BaMgAl10O17:Eu2+ powder by solution com- bustion approach and annealed in reducing atmosphere at 600℃, 800℃, 1000℃ and 1200℃ were henceforth termed as BAM-1, BAM-2, BAM-3, BAM-4 and BAM- 5, respectively. The crystal structure of BaMgAl10O17:Eu2+ nanocrys- tals was characterized by X-ray diffraction (XRD, Ri- gaku D/Max -2200 H/PC, Cu K∝ radiation). The VUV photoluminescence study was carried out on McPherson spectrometer. The excitation spectra study was carried out on a Perkin Elmer LS 55 spectrometer. The field em- ission-scanning electron microscopy (FE-SEM) images were taken on Quanta 200 FEG (FEI Company, Eindhov- en, The Netherlands). Quanta 200 FEG fitted with an en- ergy dispersive x-ray spectroscopy (EDS; Genesis 2000, EDAX), was used for elemental analysis. 3. Result and Discussion The crystallographic phase purity of BaMgAl10O17:Eu2+ nanophosphor synthesized by solution combustion app- roach was confirmed by X-ray diffraction (XRD). Figure 1 shows the XRD pattern of BAM nanophosphor synthe- sized by solution combustion method. Barium magne- sium aluminate BaMgAl10O17 (BAM) is classified into β- alumina structure with a space group P 63/mmc [15]. The BaMgAl10O17 phase obtained by solution combustion method shows good agreement with Joint Committee on Powder Diffraction Standards card No. 26-0163 [16]. Figure 1. XRD pattern of BaMgAl10O17:Eu2+ (BAM) blue PDP nanophosphor synthesized by solution combustion method Moreover, the broadening of major diffraction peaks ind- icates nanocrystalline nature of particles present in pow- der. No additional diffraction peaks are observed in XRD pattern, this indicate that obtained BaMgAl10O17:Eu2+ powder synthesized by combustion approach at 400℃ has pure phase. No change in phase purity of crystal structure of BaMgAl10O17:Eu2+ nanoparticles annealed at different temperature have been also observed. Figure 2 shows the field emission-scanning electron microscopy (FE-SEM) images of BaMgAl10O17:Eu2+ ph- osphor powder synthesized by solution combustion me- thod: (a) as prepared (BAM-1), (b) annealed at 600℃ (BAM-2), (c) annealed at 800℃ (BAM-3), (d) annea- led at 1000℃ (BAM-4), and (e) annealed at 1200℃ (BAM-5). From Figure 2(a), it is clear that as prepared BaMgAl10O17:Eu2+ has nonorods in structure with diam- eter ~ 62 nm and length ~ 300-600 nm. The change in di- ameter of BaMgAl10O17 nanorods with annealing tem- perature, can be seen in Figure 2 and Table 1. The spectrum obtained during Energy Dispersive X- ray Analysis (EDX) study is used to know the presence of europium in BaMgAl10O17. Figure 3 shows the EDX spectrum of BaMgAl10O17 nanophosphor particles syn- thesized by solution combustion method. The EDX spec- trum of BaMgAl10O17:Eu2+ nanostructure shows the presence of barium, magnesium, aluminium, oxygen and europium. The EDX spectrum confirms the doping of europium in BaMgAl10O17 nanostructures. Figure 4 shows the photoluminescence spectra of BaMgAl10O17:Eu2+ nanorods of different diameter D, viz. 62 nm, 85 nm, 115 nm, 160 nm and 450 nm under VUV (147 nm) excitation. The emission spectra shows the blue Copyright © 2010 SciRes. MSA  Blue-Shift and Enhanced Photoluminescence in BaMgAl10O17:Eu2+ Nanophosphor under VUV Excitation for PDPs Application Copyright © 2010 SciRes. MSA 27 a b c d e Figure 2. FE-SEM image of BaMgAl10O17:Eu2+ (BAM) blue PDP nanophosphor synthesized by solution combustion method: (a) as prepared (BAM-1); (b) annealed at 600℃ (BAM-2); (c) annealed at 800℃ (BAM-3); (d) annealed at 1000℃ (BAM-4); (e) annealed at 1200℃ (BAM-5) 241 193 145 96 48 0 O K EuM AlK BaL EuL MgK BaL EuL Energy - kev 1.00 2.00 3.00 4.00 5.00 6.00 7.00 Figure 3. EDX spectrum of BaMgAl10O17:Eu2+ nanostructure synthesized by solution combustion method  Blue-Shift and Enhanced Photoluminescence in BaMgAlO:Eu2+ Nanophosphor under 28 10 17 VUV Excitation for PDPs Application Table 1. The mean grain size (diameter) D (estimated by FE-SEM, the standard deviation is given in brackets), emission en- ergy (eV), decay time τ ( μs ), color-coordinates and relative intensity (%) Samples D ( nm ) Emission Peak Decay time ( μ s ) Color-coordinate Relative Position (eV ) τ1 τ2 x y Intensity (%) BAM-1 62 ( ±4) 2.775 1.07 7.18 0.1387 0.0784 100 BAM-2 85 (±6) 2.744 2.91 8.89 0.1377 0.0866 87 BAM-3 115 (±8) 2.719 4.33 10.27 0.1356 0.0905 73 BAM-4 160 (±20) 2.702 4.75 10.69 0.1351 0.0936 69 BAM-5 450 (±26) 2.670 2.46 8.51 0.1289 0.1088 96 emission in the region 400-510 nm with strongest peak at ~ 450 nm, corresponding to the electronic transition of Eu2+ from the 4f 65d excited state to the 4f 7 ground state [17]. It can also be clearly seen from the Figure 4 that with the reduction in size of diameter of BaMg Al10O17: Eu2+ nanostructure there is blue-shift in emission peak position as well as change in intensity of luminescence under 147 nm excitation. There are many reports on the blue-shift effect in the luminescence spectra of semicon- ductor nanocrystals [18-19]. However, few reports are available on nano-sized rare earth doped insulating lu- minescent material. The mechanism of luminescence in the semiconductor is the recombination of electron in the conduction band and holes in the valence band. A larger band gap is necessary for quantum confinement to cause the blue-shift in the luminescence. However, the lumi- nescence in BaMgAl10O17:Eu2+ is due to the transition between energy levels of Eu2+ atoms as the luminescence center. Therefore, a different interpretation is required to explain the blue-shift in the luminescence spectra of the BaMgAl10O17:Eu2+ nanorods. Figure 5 shows the excitation spectra of BaMgAl10O17: Eu2+ nanostructures at 450 nm emission peak. The exci- tation spectrum consists of a broad band with a maxi- Figure 4. Photoluminescence spectra of BaMgAl10O17:Eu2+ (BAM) blue PDP nanophosphor under 147 nm excitation mum ~ 328 nm with two shoulders at 270 nm and 380 nm, respectively, which are due to the transitions from the ground state 8S7/2 of Eu2+ with 4f 7 configuration to the different crystal field splitting components of the Eu2+ with (4f 6)5d configuration in the excited states [20]. Recently, S. Zhang et al. [21] reported that when the BaMgAl10O17:Eu2+ phosphor is excited at wavelength 147 nm, the excitation energy is first absorbed by the host and then transferred to Eu2+ ions. In this case, the emission efficiency of Eu2+ ions therefore depends stro- ngly on the host. The change in the particle size will sig- nificantly affect the emission intensity of Eu2+ ions in the indirect excitation. From excitation spectra, it is clear that there is also blue-shift in the broad band corresponding to 4f 651 → 4f7 transition of Eu2+ ions in BaMgAl10O17:Eu2+ nanostruc- ture host with decrease in size. Here, it is noticeable that the BaMgAl10O17:Eu2+ nanostructure with diameter 450 nm has little greater intensity as compare to nanostruc- ture with diameter size 160 nm, and also deviates from the trend of decrease in PL intensity with increase of size, while the trends of decrease of PLE intensity with in- crease in size is maintained in PLE spectra. This can be explained as: the BaMgAl10O17:Eu2+ nanostructure with Figure 5. Excitation spectra of BaMgAl10O17:Eu2+ (BAM) blue PDP nanophosphor Copyright © 2010 SciRes. MSA  Blue-Shift and Enhanced Photoluminescence in BaMgAlO:Eu2+ Nanophosphor under 29 10 17 VUV Excitation for PDPs Application diameter 450 nm have low PLE intensity and also due to larger size it has low surface defects as compare to sma- ller diameter sized nanostructure. The surface defects in nanostructures play an important role in influencing the luminescence properties. The surface defects, mainly res- ponsible for non-radiative transitions, quenchs the PL intensity. Here, in case of BaMgAl10O17:Eu2+ nanostruc- ture with diameter size 450 nm, the abrupt increase in PL intensity with lower PLE intensity is mainly due to decr- ease in surface states which are responsible for non-ra- diative transition. It is also noticeable that the deviation in PL intensity does not support the degradation in PL intensity due to annealing temperature. Therefore the observed change in PL and PLE intensity is mainly due to change in size of diameter of BaMgAl10O17:Eu2+ nanostructures. The observed blue-shift in PLE and con- sequently PL is related to change in position of energy levels of 4f 65d1 →4f 7 transition of Eu2+ ions in BaMgAl10O17:Eu2+ nanostructure host. It is already ob- served that the energy states associated with the lumi- nescent center are influenced by the host lattice material. The degree to which they are influenced depends also on the size and shape of nanostructures [22,23]. Recently, Y. Lin et al. [24] reported preparation of the ultrafine SrAl2O4:Eu,Dy needle-like phosphor and its optical properties. This group observed that the optical absorp- tion edge shifts to the blue as the phosphor particle size decreases. They explained that it may be associated with the quantum size effect of the nanometer phosphor, which increased the kinetic energy of the electrons and resulted in a larger band gap, and thus required higher energy to excite the luminescent powders. Simultane- ously they observed that the emission maximum shifted to shorter wavelength, they explained that it may be caused by the prepared technology, which resulted in some changes of the crystal field around Eu2+. Although the 4f electrons of Eu2+ are not sensitive to lattice envi- ronment due to the shielding function of the electrons in the inner shell, the 5d electron may couple strongly to the lattice. Consequently, the mixed states of 4f and 5d will be split by the crystal field, which may lead to the blue-shift of its emission peak. In our case, the blue-shift in the emission band may be attributed to the changes of the crystal field around Eu2+ arising from the nanosized particles. Since the excited 4f 65d 1 configuration of Eu2+ ion is extremely sensitive to the change in the lattice en- vironment in contrast to the shielded 4f 7 ground con- figurations, the 5d electron may couple strongly with the lattice. Therefore, the mixed states of 4f and 5d will be influenced strongly by the crystal field. On the other hand, the particles with nanometer size make the surface energy increase significantly, which causes the change of the crystal field around the local environment of Eu2+. These reasons lead to the blue-shift of PLE and PL emis- sion peak in BaMgAl10O17:Eu2+ nanophosphor with de- crease in particle size. Table 1 contains information about change in emission peak position, decay time, color coordinate and relative intensity with change in diameter of BaMgAl10O17:Eu2+ nanostructure. The colorimetric coordinate (x, y) for BAM:Eu2+ nanophosphor were calculated using equidistant wave- length method [25]. Table 1 summarizes the comparison of CIE colorimetric coordinates for BaMgAl10O17 nanos- tructure with variation of diameter size of nanostructure. To study the decay behaviours of Eu2+ luminescence in BaMgAl10O17 nanostructures with varied diameter size, fluorescence decay curve for the 4f 65d1 → 4f 7 transition of the Eu2+ were studied [26]. Figure 6 shows the fluo- rescence decay curve of BaMgAl10O17 nanostructure of diameter size 62 nm. The decay curve can be well fitted by double exponential equation: I (t) = I0 + A exp (-t/τ1) + B exp (-t/τ2), where I and I0 is the luminescence intensity, A and B are constants, t is the time, τ1 and τ2 are the de- cay time for the exponential component, respectively. It is found that the life time of BaMgAl10O17 nanostructure is varied as a function of diameter size of nanostructure. Table 1 summarizes the change in decay time of BaMgAl10O17:Eu2+ nanostructure with variation in diam- eter size. From table I, it is clear that the life time varies with the diameter size of BaMgAl10O17:Eu2+ nanorods and decay rate decreases with increase in diameter size. The PL intensity of the 4f 65d 1 → 4f 7 transition is str- ongly related to the decay time of a particular transition. The decrease in decay rate trend is continue to diameter size 62 nm, 85 nm, 115 nm and then 160 nm, however the trend of decrease of decay rate with increase of size deviates in case of BaMgAl10O17:Eu2+ nanostructure with diameter size 450 nm. The increment in decay rate at diameter size 450 nm in case of BaMgAl10O17:Eu2+ nanorods is due to decrease in surface states, responsible for non-radiative transition, with increase of size. In gen- eral, the photoluminescence (PL) decay rate is a sum of Figure 6. Fluorescence-decay curve of 62 nm diameter sized BaMgAl10O17:Eu2+ nanostructure Copyright © 2010 SciRes. MSA  Blue-Shift and Enhanced Photoluminescence in BaMgAlO:Eu2+ Nanophosphor under 30 10 17 VUV Excitation for PDPs Application the radiative and non-radiative decay rates: 111 P LRNR where τPL, τR, and τNR are the photoluminescence, rad- iative and non-radiative decay time constants, respecti- vely. Therefore, the origion of the decrease in PL decay rate versus size may be radiative and non-radiative. The possibility may be invoked for the non-radiative process in origion is surface states, responsible for non-radiative decay, which changes as surface-to-volume ratio varies with size. Other possibility for radiative in origion, may be considered for the size dependence as quantum confi- nement effect may lead to size dependent oscillator str- ength. The quantum confinement effect in nanoparticles predicts a decreased radiative decay rate as the size in- creases [27]. As PL and PLE spectra are dependent on the size of diameter of BAM nanostructures indicating that the quantum confinement effect as well as surface effect both is responsible for change in decay rate. These finding provide glimpse for detailed study of quantum confinement effect for rare earth doped wide band gap insulating material for enhancement of emission intensity, emission energy, improvement in color-coordinates and decay time etc. for better results of phosphor in LEDs and PDPs. 4. Conclusions In summary, we have successfully synthesized BaMg Al10O17: Eu2+ nanorods with variation in size of diameter of nanostructure by solution combustion approach and annealing at different temperature under reducing atom- sphere (H2 + N2). Enhanced blue photoluminescence emission and blue-shift is observed under 147 nm excita- tion, and found to be highly dependent on the size of diameter of BaMgAl10O17:Eu2+ nanostructures. Decre- ased radiative decay rate with increase in the diameter size of BaMgAl10O17:Eu2+ is also observed. 5. Acknowledgements The authors gratefully acknowledge the financial support of the Council of Scientific and Industrial Research (CSIR), India under New Millennium Indian Technology Leadership Initiative (NMITLI) project and Dr. D. Hara- nath, National Physical Laboratory, India for discussions and annealing of nanophosphor at different temperature in reducing atmosphere. REFERENCES [1] B. M. J. Smets, “Phosphors Based on Rare-Earths, a New Era in Fluorescent Lighting,” Materials Chemistry and Physics, Vol. 16, 1987, pp. 283-299. [2] C. R. Ronda, “Recent Achievements in Research on Phosphors for Lamps and Displays,” Journal of Lumi- nescence, Vol. 72-74, 1997, pp. 49-54. [3] C. R. Ronda, “Phosphors for Lamps and Displays: An Applicational View,” Journal of Alloys and Compounds, Vol. 225, 1995, pp. 534-538. [4] C. H. Kim, I. I. Eok Kwon, C. H. Park, Y. J. Hwang, H. S. Bae, B. Y. Yu, C. H. Pyun and G. Y. Hing, “Phosphors for Plasma Display Panels,” Journal of Alloys and Com- pounds, Vol. 311, 2000, pp. 33-39. [5] Z. R. Tian, J. A. Voigt, J. Liu, B. Mckenzie, M. J. Mcdermott, M. A. Rodriguez, H. Konishi and H. Xu, “Complex and Oriented ZnO Nanostructures,” Nature Materials, Vol. 2, 2003, pp. 821-826. [6] Z. L. Wang, “Zinc Oxide Nanostructures: Growth, Prop- erties and Applications,” Journal of Physics: Condensed Matter, Vol. 16, 2004, pp. R829-R858. [7] S. Kumar, V. Gupta and K. Sreenivas, “Synthesis of Photoconducting ZnO Nano-Needles Using an Unbal- anced Magnetron Sputtered ZnO/Zn/ZnO Multilayer Structure,” Nanotechnology, Vol. 16, 2005, pp. 1167- 1171. [8] A. D. Yoffe, “Low-Dimensional Systems: Quantum Size Effects and Electronic Properties of Semiconductor Mi- crocrystallites (Zero-Dimensional Systems) and Some Quasi-Two-Dimensional Systems,” Advances in Physics, Vol. 42, 1993, pp. 173-262. [9] R. N. Bhargava, D. Gallagher, X. Hong and A. Nurmikko, “Optical Properties of Manganese-Doped Nanocrystals of ZnS,” Physical Review Letters, Vol. 72, 1994, pp. 416- 419. [10] A. Henglein, “Small-Particle Research: Physicochemical Properties of Extremely Small Colloidal Metal and SemiConductor Particles,” Chemical Review, Vol. 89, 1989, pp. 1861-1873. [11] P. Alivisatos, “Perspectives on the Physical Chemistry of Semiconductor Nanocrystals,” Journal of Physics and Chemistry, Vol. 100, 1996, pp. 13226-13239. [12] Mercier, C. Dujardin, G. Ledoux, C. Louis, O. Tillement and P. Perriat, “Confinement Effect in Sesquioxydes,” Journal of Luminescence, Vol. 119-120, 2006, pp. 224- 227. [13] J. Wang, G. Ning, W. Pan, X. Yang and Y. Lin, “A Novel Route to BaMgAl10O17:Eu Blue Phosphor by Nano- coating Method,” Material Science and Engineering B, Vol. 147, 2008, pp. 43-46. [14] Z. Chen and Y. Yan, “Nano-sized PDP Phosphors Pre- pared by Solution Combustion Method,” Journal of Ma- terials Science, Vol. 41, 2006, pp. 5793-5796. [15] H. Lu, C. T. Chen and B. Bhattacharjee, “Sol-Gel Prepa- ration and Luminescence Properties of BaMgAl10O17: Eu2+ Phosphors,” Journal of Rare Earth, Vol. 24, 2006, pp. 706-711. [16] ICDD Powder Diffraction File, Card No. 26-0163. [17] Z. Chen and Y. Yan, “Morphology Control and VUV Photoluminescence Characteristics of BaMgAl10O17:Eu2+ Phosphors,” Physica B, Vol. 392, 2007, pp. 1-6. Copyright © 2010 SciRes. MSA  Blue-Shift and Enhanced Photoluminescence in BaMgAl10O17:Eu2+ Nanophosphor under VUV Excitation for PDPs Application Copyright © 2010 SciRes. MSA 31 [18] A. D. Yoffe, “Low-Dimensional Systems: Quantum Size Effects and Electronic Properties of Semiconductor Mi- crocrystallites (Zero-Dimensional Systems) and Some Quasi-Two-Dimensional Systems,” Advances in Physics, Vol. 42, 1993, pp. 173-266. [19] D. Andrea and R. Del Sole, “Excitons in Semiconductor Confined Systems,” Solid State Communications, Vol. 74, 1990, pp. 1121-1124. [20] Y. Zhou and J. Lin, “Morphology Control and Lumines- cence Properties of BaMgAl10O17:Eu2+ Phosphors Pre- pared by Spray Pyrolysis,” Journal of Solid State Chem- istry, Vol. 178, 2005, pp. 441-447. [21] S. Zhang, T. Kono, A. Ito, T. Yasaka and H. Uchiike, “Degradation Mechanisms of the Blue-Emitting Phosphor BaMgAl10O17:Eu2+ under Baking and VUV-Irradiating Treatments,” Journal of Luminescence, Vol. 106, 2004, pp. 39-46. [22] P. D. Rack, J. C. Heikenfeld and A. J. Steckl, “Handbook of Luminescence, Display Materials, and Devices, Vol. 3, In: H. S. Nalwa and L. S. Rohwer, Eds., Display Devices, American Scientific Publishers, 2003. [23] S. W. Mhin, J. H. Ryu, K. M. Kim, G. S. Park, H. W. Ryu, K. B. Shim, T. Sasaki and N. Koshizaki, “Pulsed-La- ser-Induced Simple Synthetic Route for Tb3Al5O12:Ce3+ Colloidal Nanocrystals and Their Luminescent Proper- ties,” Nanoscale Research Letters, Vol. 4, 2009, pp. 888- 895. [24] Y. Lin, Z. Zhang, F. Zhang, Z. Tang and Q. Chen, “Preparation of the Ultrafine SrAl2O4:Eu, Dy Nee- dle-like Phosphor and its Optical Properties,” Materials Chemistry and Physics, Vol. 65, No. 1, 2000, pp. 103- 106. [25] R. S. Yadav, R. K. Dutta, M. Kumar and A. C. Pandey, “Improved Color Purity in Nano Size Eu3+-doped YBO3 Red Phosphor,” Journal of Luminescence, Vol. 129, 2009, pp. 1078-1082. [26] Z. Chen, Y. W. Yan, J. M. Liu, Y. Yin, H. Wen, G. Liao, C. Wu, J. Zao, D. Liu, H. Tian, C. Zhang and S. Li, “Mi- crostructure and Luminescence of Surface-Coated Nano- BaMgAl10O17:Eu2+ Blue Phosphor,” Journal of Alloys and Compounds, Vol. 478, 2009, pp. 679-683. [27] A. D. Andreev and E. P. O’Reilly, “Optical Transitions and Radiative Lifetime in GaN/AlN Self-organized Quan- tum Dots,” Applied Physics Letters, Vol. 79, 2001, p. 521. |

