Journal of Modern Physics
Vol.06 No.15(2015), Article ID:62519,6 pages
10.4236/jmp.2015.615234
Quantum Carnot Heat Engine Efficiency with Minimal Length
A. Purwanto, H. Sukamto, B. A. Subagyo
Theoretical Physics Laboratory, Sepuluh Nopember Institute of Technology, Surabaya, Indonesia
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 12 November 2015; accepted 28 December 2015; published 31 December 2015
ABSTRACT
In this paper, the effects of the minimum lengths ( ) to the efficiency of a quantum heat engine are considered. A particle in infinite one-dimensional potential well is used as the “working substance”. We obtain quantized energy of particle in the presence of minimal length, and then we do the isoenergetic cycle. We calculate heat exchanged between the system and reservoir, and then we get the efficiency of the engine. We observe that the minimum length increases efficiency of the engine at the small width of the potential well.
) to the efficiency of a quantum heat engine are considered. A particle in infinite one-dimensional potential well is used as the “working substance”. We obtain quantized energy of particle in the presence of minimal length, and then we do the isoenergetic cycle. We calculate heat exchanged between the system and reservoir, and then we get the efficiency of the engine. We observe that the minimum length increases efficiency of the engine at the small width of the potential well.
Keywords:
Isoenergetic Efficiency, Minimal Length, Quantum Heat Engine

1. Introduction
A deformed quantum mechanics with a generalized Heisenberg Uncertainty (GUP) has been introduced by Kemp et al. [1] [2] . As a consequence, there exist smallest distance limitations in spacetime, known as minimal lengths. This minimal lengths change quantum mechanics that have been established. As an example, there has been calculated Schrodinger equation in the presence of minimal length [3] [4] , the effect of the minimal length on the energy spectrum of Coulomb potential [5] [6] , Casimir effect [7] -[10] , and Dirac Oscillator [11] -[14] .
The minimum length also affects the quantum thermodynamics, quantum generalization of the classical thermodynamics, for instance, quantum heat engine. In the quantum thermodynamics, there is isoenergetic process that is analogous to the isothermal process; and isoentropic process that is analogous to adiabatic process in classical thermodynamics. The cycle composed of two isoenergetic and two isoentropic trajectories is called isoenergetic cycle [15] . The efficiency of quantum heat engine has been calculated in [15] -[17] . The results show that the efficiency depends only on the expansion parameter .
.
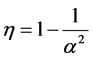 (1)
(1)
The point is that the width of the potential well has no effect on the value of efficiency. In this paper, we compute the effect of the minimum length on the quantum heat engine efficiency.
This paper is organized as follows. In Section 2 we derive quantized particle energy in infinite one-dimen- sional potential well in the presence of minimal length. In Section 3 we determine inward and outward heat through the system by isoenergetic and isoentropic process, and then we compute the efficiency of Carnot Quantum heat engine with two-level state. Finally, in Section 4 we present a discussion of our results and our conclusions.
2. Schrodinger Equation with Minimal Length
The general form one-dimensional Schrodinger equation is as follows
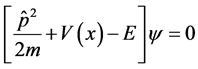 (2)
(2)
with operator . In order to incorporate minimal lengths in our equation, we used literature [3] about the position space representations as follows
. In order to incorporate minimal lengths in our equation, we used literature [3] about the position space representations as follows
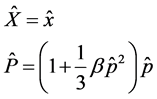 (3)
(3)
where 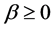 is a small parameter. With the representation above, we obtain Schrodinger equation with minimal lengths as follows
is a small parameter. With the representation above, we obtain Schrodinger equation with minimal lengths as follows
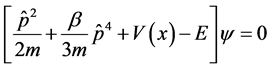 (4)
(4)
We choose one-dimensional infinite potential well as a simple model, with potential energy
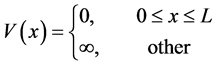 (5)
(5)
So, particle in potential well can be described by one-dimensional time independent Schrodinger as follows
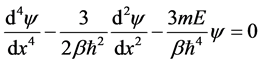 (6)
(6)
The equation can be solved by first determine the roots of equation
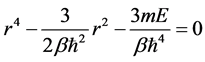 (7)
(7)
And we get
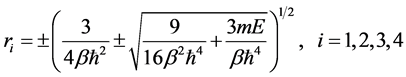 (8)
(8)
We only have two boundary condition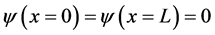 . It is impossible to find solutions of the equation by using all four roots. So, in order to obtain exact energy particle that can be applied to boundary conditions, we only use two roots. Then we propose the solution as follows
. It is impossible to find solutions of the equation by using all four roots. So, in order to obtain exact energy particle that can be applied to boundary conditions, we only use two roots. Then we propose the solution as follows
 (9)
(9)
By applying the boundary conditions and nornalization condition, we obtain quantized wave functions as follows
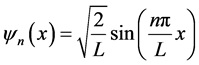 (10)
(10)
and energy

which when we take

3. Isoenergetic Cycle Process with Minimum Length
The system is assumed to be driven by reversible quasi-static process. That means the wall is moved very slowly by an applied external forced [15] . Because we work on quantum thermodynamics, it is necessary to introduced the ensemble average energy of the system as

The change of the energy during the moving is given by

The above equation is analogous to the first law of thermodynamics. The term 


For practical reason, we choose the system with two-level energy state
Let us consider first the isoenergetic process. The isoenergetic process analogous to isothermal process in classical thermodynamics, so



Because the initial state entirely to 

Figure 1. Carnot circle for two-level system.

By using (11), we get

As noted earlier, that during the isoenergetic process, the total energy remains constant. Then we get


The work done to the system, can be obtain by
At the second, we arrive at isoentropic process. For isoentropic process, the probability is unchanged through von Neumann entropy

The heat exchange during isoentropic process equal to zero. As a Figure 1, we expand the width of the potential well, from 



Similar with isoenergetic process, we can calculate the heat exchanged from 


The last path along the cycle is isoentropic process, which return fully to the initial condition. The work performed during this process from 


We obtain that work along two isoentropic process cancel each other, that is

By substituting Equation (18) and Equation (21), we obtain the explicit analytical expression

with

Then we plot the graph between the efficiency versus the width of potential as Figure 2. From Figure 2, we obtain that the efficiency value depends on the initial value of potential width. We get interesting result that the efficiency value increase above classical result with the decreasing the width of potential. The efficiency is also affected by the size of minimal length. If we approximate

And at large width of potential well, the efficiency value approaches classical result.
At Figure 3, we plot the graph with 

Figure 2. The efficiency versus initial potential width, with 

Figure 3. The efficiency versus initial potential width, with 

Schrodinger limit as

Efficiency value returns to the quantum engine efficiency without the presence of minimal length.
4. Discussion and Conclusion
In this work, we have studied the consequences of the minimal length on the quantum thermodynamics. This minimal length modifies Schrodinger equation to be fourth order differential equation. We choose periodic solutions in order to obtain the exact solutions. After that, we calculate the efficiency of heat engine with procedure in Reference [15] . We obtain for the width of potential smaller than

We conclude that the minimal length affects the efficiency of the quantum heat engine at small size of potential well. This effect can be explained by considering the particle as a ball-point having a finite size which is of order of the minimal length [1] .
Acknowledgements
This work is supported by LPPM ITS.
Cite this paper
A.Purwanto,H.Sukamto,B. A.Subagyo, (2015) Quantum Carnot Heat Engine Efficiency with Minimal Length. Journal of Modern Physics,06,2297-2302. doi: 10.4236/jmp.2015.615234
References
- 1. Kempf, A., Mangano, G. and Mann, R.B. (1995) Physical Review D, 52, 1108.
http://dx.doi.org/10.1103/PhysRevD.52.1108 - 2. Maziashvili, M. and Megrelidze, L. (2013) Progress of Theoretical and Experimental Physics, Article ID: 123B06.
- 3. Haouat, S. (2014) Physics Letters B, 729, 33-38.
http://dx.doi.org/10.1016/j.physletb.2013.12.060 - 4. Hassanabadi, H., Molaee, Z. and Zarrinkamar, S. (2014) Advances in High Energy Physics, Article ID: 459345.
- 5. Pedram, P. (2013) Europhysics Letters, 101, Article ID: 30005.
http://dx.doi.org/10.1209/0295-5075/101/30005 - 6. Nouicer, K. (2012) Journal of Mathematical Physics, 48, Article ID: 112104.
http://dx.doi.org/10.1063/1.2809267 - 7. Harbach, U. and Hossenfelder, S. (2008) Physics Letters B, 632, 379-383.
http://dx.doi.org/10.1016/j.physletb.2005.10.045 - 8. Nouicer, K. (2005) Journal of Physics A: Mathematical and General, 38, Article ID: 10027.
http://dx.doi.org/10.1088/0305-4470/38/46/009 - 9. Panella, O. (2007) Physical Review D, 76, Article ID: 045012.
http://dx.doi.org/10.1103/PhysRevD.76.045012 - 10. Frassino, A.M. and Panella, O. (2012) Physical Review D, 85, Article ID: 045030.
http://dx.doi.org/10.1103/PhysRevD.85.045030 - 11. Chang, L.N., Minic, D., Okamura, N. and Takeuchi, T. (2002) Physical Review D, 65, Article ID: 125027.
http://dx.doi.org/10.1103/PhysRevD.65.125027 - 12. Menculini, L., Panella, O. and Roy, P. (2015) Physical Review D, 91, Article ID: 045032.
http://dx.doi.org/10.1103/PhysRevD.91.045032 - 13. Hassanabadi, H., Molaee, Z. and Zarrinkamar, S. (2012) The European Physical Journal C, 72, 2217.
http://dx.doi.org/10.1140/epjc/s10052-012-2217-5 - 14. Betrouche, M., Maamache, M. and Choi, J.R. (2013) Scientific Reports, 3, Article No. 3221.
http://dx.doi.org/10.1038/srep03221 - 15. Bender, B.C.M., Brody, D.C. and Meister, B.K. (2000) Journal of Physics A, 33, 4427-4436.
http://dx.doi.org/10.1088/0305-4470/33/24/302 - 16. Quan, H.T., Liu, Y., Sun, C.P. and Nori, F. (2007) Physical Review E, 76, Article ID: 031105.
http://dx.doi.org/10.1103/PhysRevE.76.031105 - 17. Latifah, E. and Purwanto, A. (2011) Journal of Modern Physics, 2, 1366-1372.
http://dx.doi.org/10.4236/jmp.2011.211169




