Advances in Bioscience and Biotechnology
Vol.2 No.4(2011), Article ID:6865,6 pages DOI:10.4236/abb.2011.24043
Low molecular fucoidan and its macromolecular complex with bee venom melittin
![]()
1Graduate School of Biotechnology, Kyung Hee University, Suwon, Republic of Korea;
2Center of Genomic Technologies, Institute of Genetics and Plant Experimental Biology, Academy Sciences of Uzbekistan, Tashkent, Uzbekistan;
Email: *genomics@uzsci.net
Received 21 May 2011; revised 15 July 2011; accepted 21 July 2011.
Keywords: Fucoidan; Free Radical Depolymerization; Melittin; Anti-inflammatory Activity
ABSTRACT
Low molecular weight (LMW) fucoidan, obtained by free radical depolymerization of high molecular polysaccharide extract of brown algae Hizikia fusiformis, was complexed with HPLC purified bee venom melittin. Water soluble form of the LMW fucoidan:melittin complex shows increased anti-inflammatory activity, inhibiting the production of nitric oxide in murine macrophage cell line Raw 264.7. The LMW fucoidan: melitin complex obtained in this study showed good biological activities, resulting in 2-fold reduction of the melittin toxicity. The fucoidan:melittin macromolecular complex obtained should be useful in future therapeutic applications.
1. INTRODUCTION
The clinical importance of many venomous compounds underlies the development of innovative approaches to minimize their cytotoxicity level during therapeutic applications in disease treatments [1]. In this context, a screening of efficient macromolecular complexes with therapeutic venoms, helping to reduce a generic toxicity, is one of the expanding research areas to exploit venomous molecules of animal and plant origin [1].
Fucoidan, a fucose-containing highly sulfated nontoxic polysaccharide, is produced by many brown algae. Molecular mass of fucoidan is heterogenic (10 - 1000 kDa) and difficult for making measurement standards in studying of its biological activity. The molecular mechanism of fucoidan’s anti-tumor [2], LDL cholesterol lowering [3], blood anti-coagulating [4], anti-oxidant and anti-inflammatory [5], anti-viral [6] and contraceptive [7] activities have not yet been revealed in detail. It has been reported that fucoidan is absorbed in an undigested form and displays thereafter all beneficial activities. There is no evidence of fucoidan’s toxicity in the literature and purified fucoidan is nontoxic in cell and animal test systems [8].
A number of studies revealed that fucoidan preserves its biological activities after partial hydrolysis with sulfuric acid [9] and free radical depolymerization [10]. These features of the fucoidan call interest to formulate biologically active complexes with a priori known therapeutic agents for efficient delivery and action.
Melittin, a major bee venom constituent and a cationic polypeptide, is known as potential therapeutic agent with a wide range of activities including anti-inflammatory [11] and antimicrobial [12] effects. Here, we investigated the biological activity of a fucoidan:melittin macromolecular complex. We report the comparison of physicochemical and biological activity of low molecular weight (LMW) variant of fucoidan and its complexes with HPLC-purified melittin.
2. MATERIALS AND METHODS
2.1. Extraction of Fucoidan
500 g of air dried powder of edible brown algae Hizikia fusiformis was defatted by 3 times washing with 500 ml of acetone. Residue was kept at 40˚C in vacuum oven, for adequate time to remove acetone and treated with 2.5 L of warm water (35˚C - 40˚C) containing 2% CaCl2. Water extract was filtered through 4× layer of gauze tissue and residue was extracted one more time in the same conditions. Combined filtrates were centrifuged for 15 min at 3500 rpm. (Combi-514R centrifuge, Hanil Science Industrial, Korea). The water soluble polysaccharides were precipitated by adding 2.5 volumes of ethanol. Ethanol pellet was collected by centrifugation, dissolved in 500 ml of 0.1 M HCl and insoluble matter was removed by centrifugation for 15 min at 3500 rpm. Acid-soluble fraction was neutralized up to pH 7.5 - 7.8 with 1 M Tris-base and loaded into 2.5 × 15 cm Q-sepharose column (catalog No. Q1126, Sigma, USA), equilibrated in 50 mM Tris-HCl pH 7.8, 0.1 M NaCl. Column was washed with 500 ml equilibrating buffer, then 500 ml of 0.4 M NaCl; both fractions contained neutral and mild acidic polysaccharides that were discarded. Fucoidan was eluted from column with 150 - 200 ml of 1 M and then 3 M NaCl in water. Yield of high acidic fractions was controlled on 230 nm absorption or on refraction (Tunable absorption 486 and RI 410 detectors, Waters, USA). High molecular weight, native fucoidan (HMW) fractions were dialyzed against water and lyophilized. Experiments with “native” fucoidan were performed with 3 M NaCl eluate from Q-sepharose. Yield was 3 g or 0.6% for high acidic 3 M NaCl eluate from Q-sepharose.
2.2. Partial Hydrolysis of Fucoidan with Sulfuric Acid
2 g of HMW fucoidan was dissolved in 50 ml of water. A concentrate of sulfuric acid was added up to 2 M, and acidic fucoidan solution was partially hydrolyzed at 60˚C for 3 hours. Reaction mixture was neutralized with 10 M NaOH, dialyzed against water and lyophilized.
2.3. Free Radical Depolymerization of Fucoidan
0.5 g of native fucoidan was dissolved in 50 ml of water, and then, 300 mg of cupper sulfate pentahydrate was added. Mixture was kept at 60˚C and total of 10 ml of 9% H2O2 solution was slowly added using dropping funnel during 2 hours; pH of reaction mixture was maintained between 7 - 8 by addition of 4 M NaOH. Reaction mixture was dialyzed for a short time against 50 mM Tris-HCl pH 7.8, 0.1 M NaCl and loaded into 1 × 10 cm QAE-Sephadex A50 column (catalog No. Q50120, Sigma, USA); a chromatography separation conditions were as suggested for native fucoidan isolation. The separated LMW fucoidan fraction separated was carefully dialyzed against water and lyophilized.
2.4. Isolation of Melittin from Bee Venom
1 g of bee venom (collected by electrical stimulation) was dissolved in 2 ml of 0.1% TFA and fractionated on 2 × 25 cm YMC-Pack ODS-A column (YMC Co., Ltd, Japan) by means of Waters 600E preparative system, using 0.1% TFA and 80% acetonitrile as mobile phase. HPLC conditions were applied as described in reported protocol [13]. Melittin peak was collected and a major acetonitrile component was concentrated by vacuum evaporation until 80% of starting volume. HPLC fraction of melittin was further purified on cation exchange chromatography according to Ziyavitdinov et al. [13] with minor modifications. A concentrate of melittin fraction (40 ml) was diluted with 80 ml 50 mM Na-acetate pH 4.0, containing 2% Triton X-100 and loaded into SP-Sephadex C50 column (1 × 10 cm), equilibrated in 50 mM Naacetate pH 4.0. Column was washed with 10 ml equilibrating buffer containing 2% Triton X-100 and 0.1 M NaCl, 20 ml of 0.1 M NaCl in equilibration buffer, and then melittin was eluted with 15 ml stepwise gradient of NaCl: 0.150 M, 0.300 M and 0.450 M in 50 mM Naacetate pH 4.0. Melittin was eluted in 0.450 M NaCl fraction. Fraction was desalted on small C18-silica gel cartridge (1 × 2 cm) and lyophilized after initial evaporation of acetonitrile.
2.5. Gel-Filtration of Fucoidan
Gel-filtration of fucoidan fractions was carried out on column 2.8 × 60 cm with Toyopearl HW-55F (ToyoSoda, Japan) using 50 mM Tris-HCl pH 7.5, 0.2 M NaCl at flow rate 1 ml/min. Elution profile was controlled by means of tunable 486 (at 230 nm) or RI 410 detectors (Waters, USA) and Servogor 120 dual-pen recorder (Lab Extreme Inc., USA) sensitivity 100 mV, paper speed 0.1 cm/min. Fractions were collected on 15 ml volume. Column was calibrated using genomic DNA (>103 kDa), dextranes (molecular mass 100 and 6 kDa from Leuconostoc spp., Sigma, USA, detection with RI 410 detector) and polyvinyl pyrrolidones (40 and 20 kDa molecular mass, Sigma, USA).
2.6. Mass Spectrometric Analysis of LMW Fucoidan
MALDI-TOF spectrum of the depolymerized free radical LMW fucoidan was analyzed on 4700 MALDI TOF/ TOF analyzer (Applied Biosystem, USA) by using 2,5- dihydroxybenzoic acid as matrix.
2.7. Preparation of Melittin LMW Fucoidan Complexes
Optimal proportions of melittin and LMW fucoidan for the formation of soluble macromolecular complex were assessed by turbidimetric method similar as described for fucoidan—snake venom myotoxic phospholipase interaction [1,14]. To 50 μl of LMW fucoidan solution (2 μg/μl, dissolved in the buffer containing 20 mM TrisHCl pH 7.0 and 0.14 M NaCl), 50 μl of 0 to 2 μg/μl melittin solution in the same buffer was added to give the different final ratio of fucoidan: melittin. Alternatively, 100 μg of melittin solution in the buffer was complexed in the same manner as above with 0 - 100 μg of LMW fucoidan solutions. Macromolecular complex turbidity was determined at 340 nm by means of Versamax microplate reader (Molecular devices, USA) in 96 well plates.
2.8. Lysis of Erythrocytes by Melittin and Melittin:Fucoidan Complex
Red blood from the heparinized blood of laboratory rats were precipitated by centrifuging for 10 min at 1500 rpm (Combi-514R centrifuge, Hanil Science Industrial, Korea). Additionally, cells were washed 3 times with TNbuffer (50 mM Tris-HCl pH 7, 0.14 M NaCl), and cells were suspended in same buffer at concentration 4% (v/v). The erythrocytes suspension was kept at +5˚C until utilization. Melittin or melittin:fucoidan complex samples, preliminarily diluted in a final volume of 300 μl, were allocated in test tubes and an erythrocyte lysis test was performed after adding 100 μl of the suspension of washed red blood (final concentration of 1%). The test was carried out for 30 min at 37˚C, then mixture was centrifuged for 5 min at 10,000 rpm. 200 ml of supernatant was transferred to 96 well microplate. The lysis of red cells was determined on haemoglobin release, where zero hemolysis (blank) and 100% of hemolysis controls were determined by using centrifugate of erythrocytes suspended in TN-buffer and 1% Triton X-100, respectively [15]. The optic density of lysate was registered at 414 nm by means of Versamax microplate reader (Molecular devices, USA).
2.9. Cell Culture
Raw 264.7 cells, a mouse leukaemic monocyte macrophage cell line from ATCC (Manassas, USA), were cultured in Dulbecco’s Eagle Medium supplemented with 10% noninactivated fetal bovine serum, 100U/ml penicillin, and 100 μg/ml streptomycin at 37˚C in a humidified atmosphere of 95% air and 5% CO2. During the experiment, cells were monitored under microscopic observation to detect any morphological change.
2.10. Measurement of Nitrite Production by Griess Reagent
Raw 264.7 cells (2.5 × 104 cells/well) were plated and allowed to stabilize for 24 h. Following this period, treatment with low molecular fucoidan and melittin for 12 h was performed by the addition of 0.5 μg/ml lipopolysaccharide (LPS) for 24 h. The production of nitric oxide (NO) was measured by the accumulation of nitrite in the culture supernatants using a colorimetric reaction with the Griess reagent. Briefly, 170 μl of culture supernatants was diluted with equal volumes of the Griess reagent [0.1% (w/v) N-(1-naphthyl)-ethylenediamine dihydrochloride and 1% (w/v) sulphanilamide containing 5% (w/v) H3PO4] and were kept during 15 min in the dark. The absorbance at 530 nm was measured with microplate reader. Culture medium was used as blank and a nitrite concentration was determined from a regression analysis using serial dilutions of sodium nitrite as the standard.
3. RESULTS AND DISCUSSION
Gel-filtration of native fucoidan and its low molecular forms, obtained by partial sulfuric acid hydrolysis and free radical depolymerization, provided an evidence of molecular heterogeneity of native fucoidan fraction and sulfuric acid hydrolysate, whereas a free radical depolymerized form consisted of symmetric peak with molecular mass of 20 ± 3 kDa (Figure 1). The tertiary conformation of fucoidan, high sulfated polysaccharide, was different with used molecular mass ladders, and gelfiltration data were used only for the characterization of heterogeneity. The correct molecular mass of free radical depolymerized low molecular fucoidan was determined by MALDI-TOF/TOF (Figure 2). It’s noteworthy to mention that the molecular masses of LMW fucoidan (3145) and melittin (2840) are comparable, which allows calculating a molar ratio from weight concentration.
Melittin is a high cationic polypeptide and it forms macromolecular complex with sulfated polysaccharides, showing strong Coulomb interaction with high binding constant [16,17]. We studied a macromolecular complex formation process by determining the turbidity of melittin:fucoidan mixture (Figure 3). The process of complex formation was studied in two variants: 1) by titration of constant quantity of melittin (100 µg) with serial dilutions of LMW fucoidan (0 - 100 µg) and 2) by titration of constant quantity of LMW fucoidan (100 µg) with serial dilutions of melittin (0 - 100 µg). Both melittin and fucoidan consist of multiple charged residues and complex formation curve was different from the abovementioned two experimental variants of titration. The turbidity curve of melittin:fucoidan mixture showed that
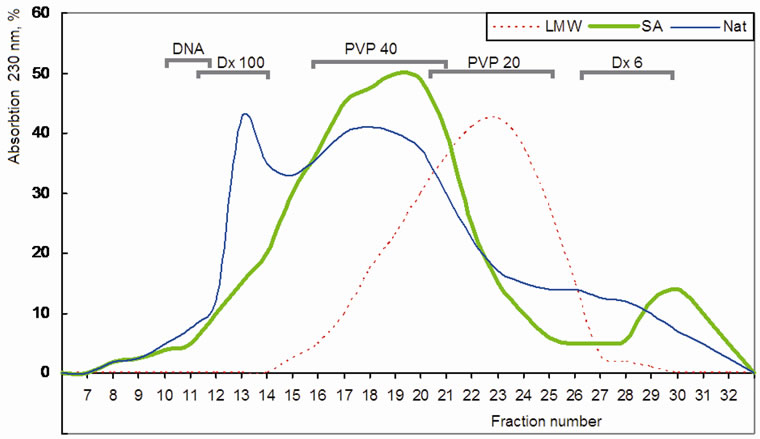
Figure 1. A molecular heterogeneity of fucoidan and its low molecular forms on Toyopearl HW-55 column. “Nat”-native fucoidan, “SA”-low molecular form obtained by sulfuric acid hydrolysis, ”LMW”-low molecular form obtained by free radical depolymerization method. Elution volume of used molecular size markers are indicated as: DNA—genomic DNA (>103 kDa); Dx 100 and Dx 6—dextranes 100 and 6 kDa; PVP 40 and PVP 20 - 40 and 20 kDa polyvinyl pyrrolidones.
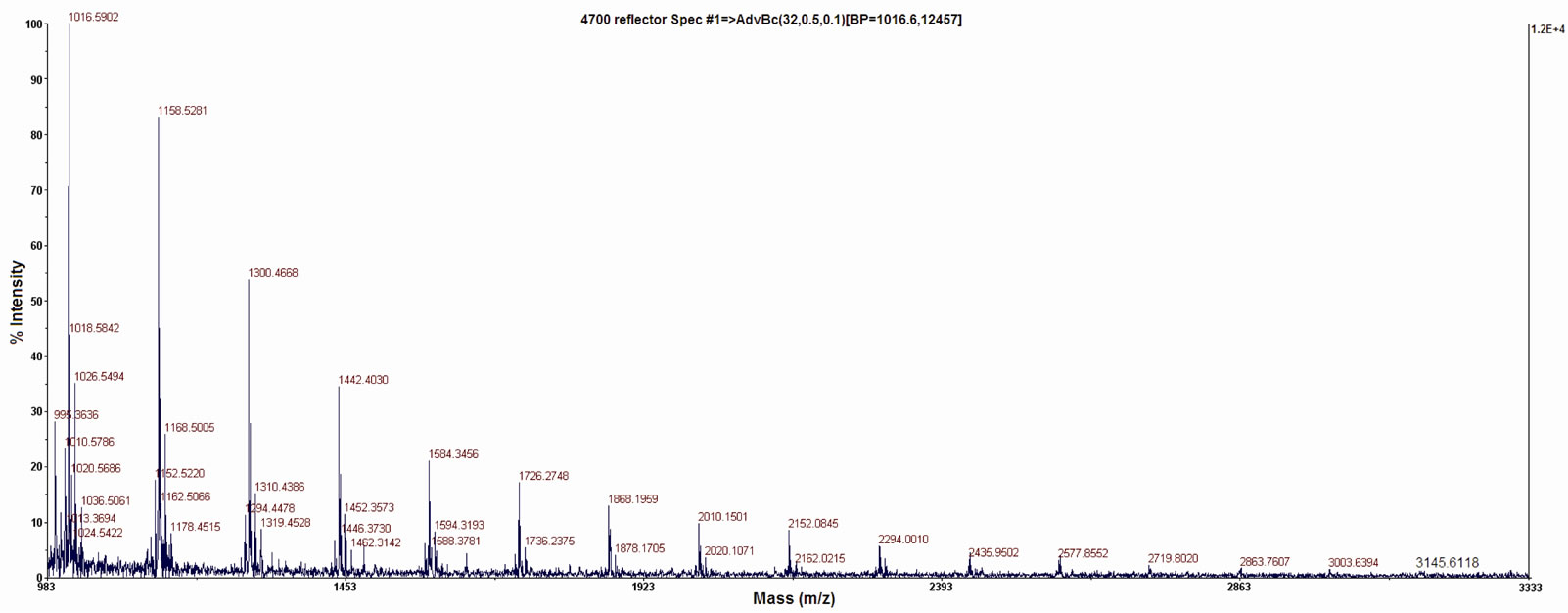
Figure 2. MALDI-TOF/TOF spectrum of LMW fucoidan obtained by free radical depolymerization.
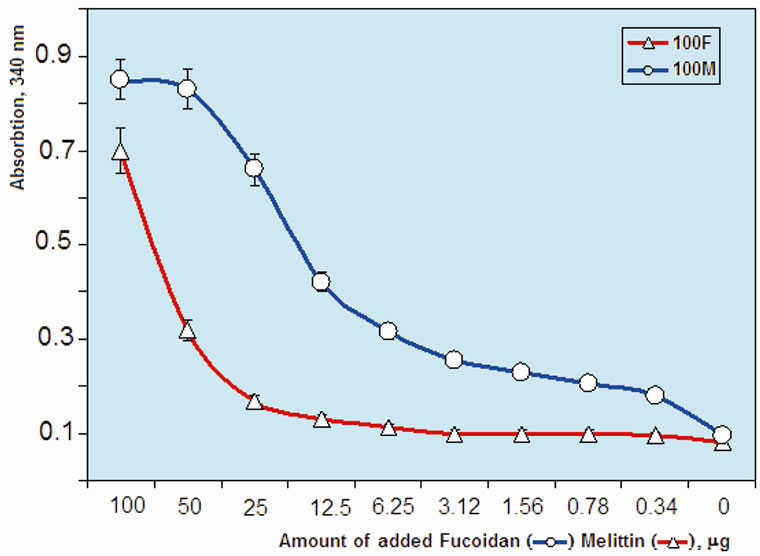
Figure 3. Change of turbidity of LMW fucoidan:melittin complex: 100 μl of 0.1% fucoidan solution mixed with equal volume of indicated amount melittin solution (line “100 F”) and 100 μl of 0.1% melittin solution mixed with equal volume of indicated amount fucoidan solution (line “100 M”).
the ratio approximately 1:10 was critical for solubility of the molecular complex (in both variants). At the ratio close to stoichiometric (1:1), the solubility of complex was minimal and the turbidity of mixture has increased. Based on the turbidity curve of melittin—fucoidan complex, we have selected two type of water soluble complex with different proportion of ingredients: 1) high melittin content (melittin:fucoidan = 10:1) and 2) high fucoidan content (melittin:fucoidan = 1:10).
Further, a possible unwanted toxicity of melittin:fucoidan complexes have been studied on erythrocytes lysis test (Figure 4). Melittin could hemolyze erythrocytes at the concentration of 0.5 mg/ml. However, the melittin:fucoidan complex with ratio of 1:10 decreased toxicity of melittin. This effect has been observed by concen-
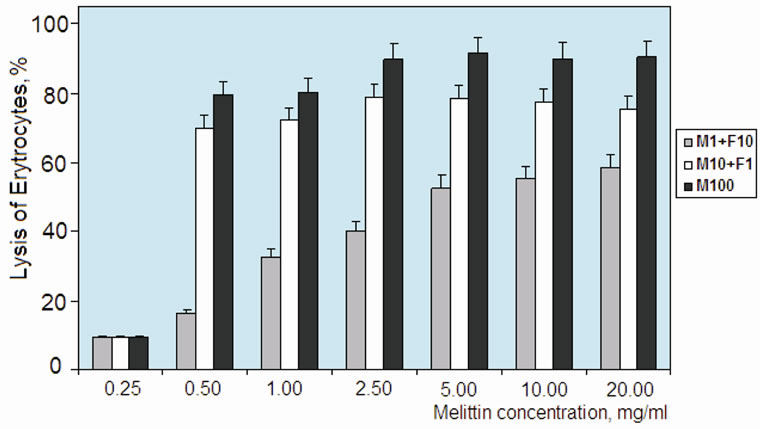
Figure 4. Hemolytic activity of LMW fucoidan:melittin complexes (melittin:fucoidan relation 1:10 “M1 + F10” and 10: 1 “M10 + F1”) and free melittin (“M100”). Concentration of complexes indicated as melittin content. Hemolytic activity was calculated relative to lytic activity of Triton X-100 (0.05%).
tration of up to 20 mg/ml of melittin in complex form. Hemolytic activity of melittin-rich complex (melittin:fucoidan ratio 10:1) showed that it decreased the lysis capacity for only 10% - 15% compared to free melittin. In the literature, cytotoxicity of melittin is discussed in connection with its membrane-acting (hydrophobic sites of peptide) and strong cationic properties (i.e., influence on membrane permeability because of high positive charge) [18]. LMW fucoidan obtained by free radical depolymerization method was preferable in our experiments, because of its good solubility (>10%) and relatively narrow molecular mass parameters. Molecular complex was stable in wide ranges of pH (4 - 8) and ionic strengths (0.05 - 1.0 M NaCl). Two types of complexes show different solubility: a complex with high melittin content (10 melittin: 1 fucoidan) formed a precipitate during storage at concentration >10 mg/ml. Therefore, complex with low melittin content (1 melittin: 10 fucoidan) was more convenient form for our biological tests. Furthermore, a macromolecular complex with low melittin content showed two times lower lysis activity than free melittin. Previously, property of several compounds (e.g. heparin/heparan and fucoidan) in reduction of the toxicity level of snake venoms has been investigated [1,14,19,20]. In particular, fucoidan’s inhibition of cytotoxic and myotoxic effects of a group of phospholipase A2 from crotaline snake venoms has been well documented in the literature [1,14]. Similarly, in our study, LMW fucoidan effectively reduced the cytotoxic effect of melittin within the macromolecular complex.
On the next experiment, we have tested the influence of macromolecular complexes obtained on production of nitric oxide (NO). Results showed that LMW fucoidan and melittin inhibits LPS-induced NO production (Figure 5). The effect on NO production was analyzed by measuring accumulation of nitrite in the culture medium. Because melittin has been known for anti-tumor activity, we first examined the cytotoxicity of the fucoidan:melittin complex against Raw 264.7. Below the concentration of 5 ppm, cytotoxicity was not observed. Therefore, the combination of melittin of 5 ppm and LMWF fucoidan was used to investigate the effect on inflammation. After cell activation with LPS for 24 h, the NO production has increased by 4.8 fold, while in macrophage pretreatment with LMW fucoidan and melittin, the LPS-induced NO production has significantly (p < 0.05 - 0.01) has decreased. The NO reduction activity of the LMW fucoidan (LMWF) and melittin (MLT) complex was 10% more effective than those of single ingredients. Although the increase and decrease of NO production may have various influences [21], NO regulates many important processes (e.g. vascular tone, blood flow, insulin sensitivity and resistance) in the entire body and plays a key role in many pathologies like hypertension, atherosclerosis and angiogenesis-associated disorders [21,22]. NO also regulates many neurological processes influencing behavior and cognitive function. Its over-production results in neurodegeneration [23]. In this context, NO reduction activity of the LMW fucoidan and melittin complex obtained should be useful to overcome unwanted reactions caused by NO during its therapeutic applications.
4. CONCLUSIONS
Taken together, low molecular fucoidan obtained by free radical depolymerization, may be used for the development of macromolecular complexes with bee venom melittin. The LMW fucoidan:melitin complex obtained in this study showed good biological activities such anti-inflammation, resulting in 2-fold reduction of the melittin toxicity and significant decrease of NO production. These results suggest the usefulness of fucoidan:melittin macromolecular complex in future therapeutic applications and might be of great interest in optimization of clinical tests.
5. ACKNOWLEDGEMENTS
This research was supported by Technology Development Program for Fisheries Ministry for Food, Agriculture, Forestry and Fisheries Republic of Korea, grant No. 109203-03-2-CG000. We thank the Acad-
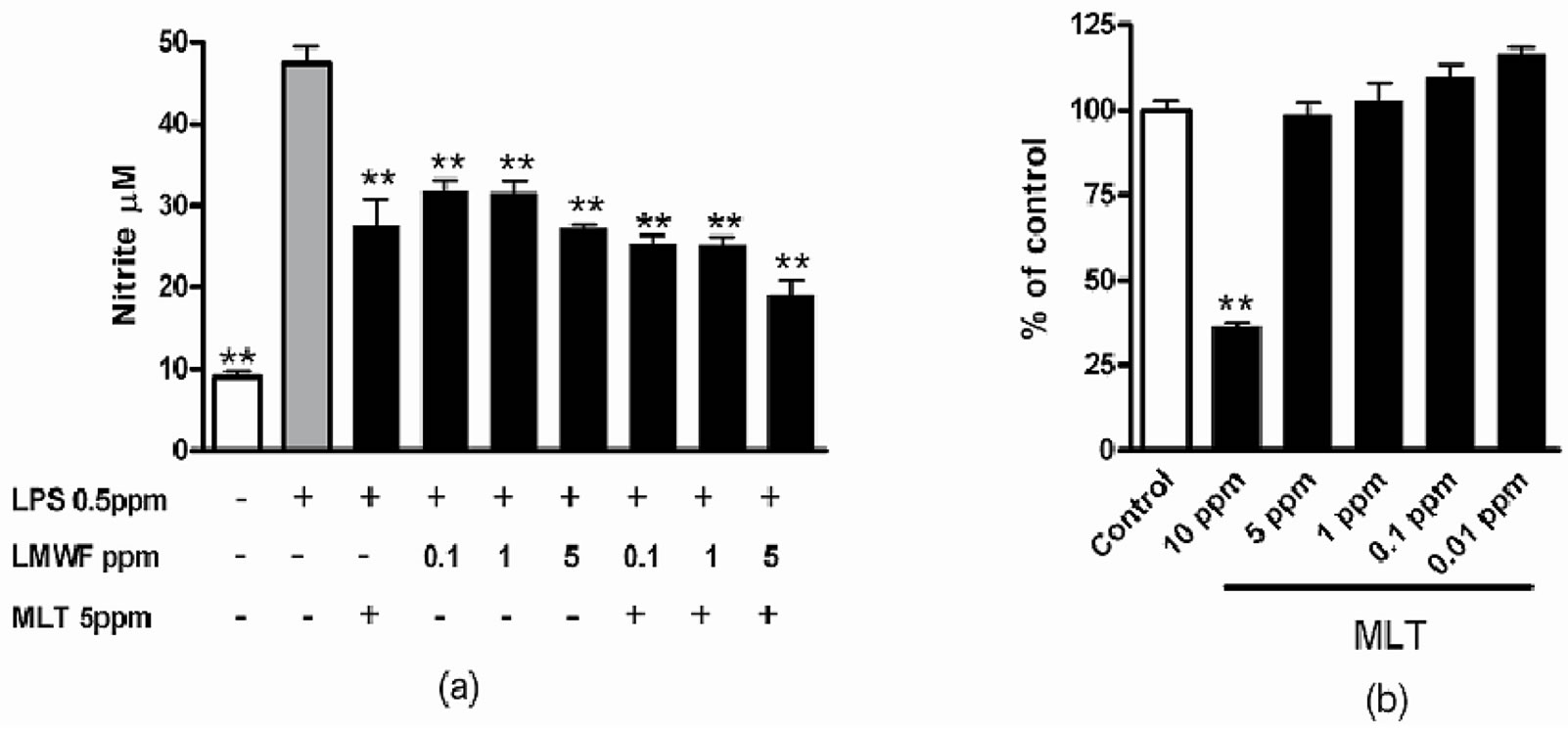
Figure 5. Inhibition of lipopolysaccharide (LPS)-induced production of nitric oxide (a) by LMW fucoidan and melittin and cytotoxicity of melittin on macrophage of Raw 264.7 (b). As indicated, macrophage cell line RAW 264.7 was treated with LPS, LMW fucoidan and melittin. After 24 hours incubation, the amounts of NO in the media were measured as nitrite by Griess reagent. *P < 0.05; **P < 0.01.
emy of Science of Uzbekistan for encouraging the international collaborations.
REFERENCES
- Angulo, Y. and Lomonte, B. (2003) Inhibitory effect of fucoidan on the activities of crotaline snake venom myotoxic phospholipase A2. Biochemical Pharmacology, 66, 1993-2000. doi:10.1016/S0006-2952(03)00579-3
- Kim, E.J., Park, S.Y., Lee, J.Y. and Park, J.H. (2010) Fucoidan present in brown algae induces apoptosis of human colon cancer cells. BMC Gastroenterology, 22, 10-96.
- Suzuki, K., Sakiyama, Y., Usui, M., Obama, T., Kato, R., Itabe, H. and Yamamoto, M. (2010) Oxidized low-density lipoprotein increases interleukin-8 production in human gingival epithelial cell line Ca9-22. Journal of Periodontal Research, 45, 488-495.
- Kim, W.J., Koo, Y.K., Jung, M.K., Moon, H.R., Kim, S.M., Synytsya, A., Yun-Choi, H.S., Kim, Y.S., Park, J.K. and Park, Y.I. (2010) Anticoagulating activities of low-molecular weight fuco-oligosaccharides prepared by enzymatic digestion of fucoidan from the sporophyll of Korean Undaria pinnatifida. Archives of Pharmacal Research, 33, 125-131. doi:10.1007/s12272-010-2234-6
- Cui, Y.Q., Luo, D.Z. and Wang, X.M. (2010) Fucoidan: Advances in the study of its anti-inflammatory and anti-oxidative effects. Acta Pharmaceutica Sinica, 43, 1186-1189.
- Sinha, S., Astani, A., Ghosh, T., Schnitzler, P. and Ray, B. (2010) Polysaccharides from Sargassum tenerrimum: Structural features, chemical modification and anti-viral activity. Phytochemistry, 71, 235-242. doi:10.1016/j.phytochem.2009.10.014
- Mahony, M.C., Clark, G.F., Oehninger, S., Acosta, A.A. and Hodgen, G.D. (1993) Fucoidan binding activity and its localization on human spermatozoa. Contraception, 48, 277-289. doi:10.1016/0010-7824(93)90146-X
- Li, N., Zhang, Q. and Song, J. (2005) Toxicological evaluation of fucoidan extracted from Laminaria japonica in Wistar rats. Food and Chemical Toxicology, 43, 421-426. doi:10.1016/j.fct.2004.12.001
- Jouault, S.C., Chevolot, L., Helley, D., Ratiskol, J., Bros, A., Sinquin, C., Roger, O. and Fisher, A.M. (2001) Characterization, chemical modifications and in vitro anticoagulant properties of an exopolysaccharide produced by Alteromonas infernus. Biochimica et Biophysica Acta, 1528, 141-151.
- Nardella, A., Chubet, F., Boisson-Vidal, C.C., Blondin, C., Durand, P. and Josefonvicz, J. (1996) Anticoagulant low molecular weight fucans produced by radical process and ion exchange chromatography of high molecular weight fucans extracted from the brown seaweed Ascophyllum nodosum. Carbohydrate Research, 289, 201- 208. doi:10.1016/0008-6215(96)00110-3
- Kim, J.I., Yang, E.J., Lee, M.S., Kim, Y.S., Huh, Y., Cho, I.H., Kang, S. and Koh, H.K. (2011) Bee venom reduces neuroinflammation in the MPTP-induced model of Parkinson’s disease. International Journal of Neuroscience, 121, 209-217. doi:10.3109/00207454.2010.548613
- Jeong, N., Kim, J.Y., Park, S.C., Lee, J.K., Gopal, R., Yoo, S., Son, B.K., Hahm, J.S., Park, Y. and Hahm, K.S. (2010) Antibiotic and synergistic effect of Leu-Lys rich peptide against antibiotic resistant microorganisms isolated from patients with cholelithiasis. Biochemical and Biophysical Research Communications, 399, 581-586. doi:10.1016/j.bbrc.2010.07.118
- Ziyavitdinov, Zh.F., Inogamov, U.K., Sagdiev, N.Zh. and Salikhov, Sh.I. (1995) Development of a method for the complex isolation of physiologically active components from bee venom. Chemistry of Natural Compounds, 31, 726-730. doi:10.1007/BF01386189
- Azofeifa, K., Angulo, Y. and Lomonte, B. (2008) Ability of fucoidan to prevent muscle necrosis induced by snake venom myotoxins: Comparison of highand low-molecular weight fractions. Toxicon, 51, 373-380. doi:10.1016/j.toxicon.2007.10.008
- Blondelle, S.E. and Houghten, R.A. (1991) Hemolytic and antimicrobial activities of the twenty-four individual omission of melittin. Biochemistry, 30, 4671-4687. doi:10.1021/bi00233a006
- Klocek, G. and Seelig, J. (2008) Melittin interaction with sulfated cell surface sugars. Biochemistry, 47, 2841-2849. doi:10.1021/bi702258z
- Cai, S., Dufner-Beattie, J.L. and Prestwich, G.D. (2004) A selective protein sensor for heparin detection. Analytical Biochemistry, 326, 33-41. doi:10.1016/j.ab.2003.11.017
- Pratt, J.P., Ravnic, D.J., Huss, H.T., Jiang, X., Orozco, B.S. and Mentzer, S.J. (2005) Melittin-induced membrane permeability: A nonosmotic mechanism of cell death. In Vitro Cellular & Developmental Biology— Animal, 41, 349-355.
- Lomonte, B., Moreno, E., Tarkowski, A., Hanson, L.A. and Maccarana, M. (1994) Neutralizing interaction between heparins and myotoxin II, a lysine 49 phospholipase A2 from Bothrops asper snake venom. Identification of a heparin-binding and cytolytic toxin region by the use of synthetic peptides and molecular modeling. Journal of Biological Chemistry, 269, 29867-29873.
- Lomonte, B., Tarkowski, A., Bagge, U. and Hanson, L.A. (1994) Neutralization of the cytolytic and myotoxic activities of phospholipases A2 from Bothrops asper snake venom by glycosaminoglycans of the heparin/heparan sulfate family. Biochemical Pharmacology, 47, 1509-1518. doi:10.1016/0006-2952(94)90525-8
- Luiking, Y.C., Engelen, M.P. and Deutz, N.E. (2010) Regulation of nitric oxide production in health and disease. Current Opinion in Clinical Nutrition & Metabolic Care, 13, 97-104. doi:10.1097/MCO.0b013e328332f99d
- Chen, K., Pittman, R.N. and Popel, A.S. (2008) Nitric oxide in the vasculature: Where does it come from and where does it go? A quantitative perspective. Antioxid Redox Signal, 10, 1185-1198. doi:10.1089/ars.2007.1959
- Moncada, S. and Bolanos, J.P. (2006) Nitric oxide, cell bioenergetics and neurodegeneration. Journal of Neurochemistry, 97, 1676-1689. doi:10.1111/j.1471-4159.2006.03988.x

