Journal of Diabetes Mellitus
Vol. 2 No. 2 (2012) , Article ID: 19366 , 7 pages DOI:10.4236/jdm.2012.22040
Amelioration of type 1 diabetes following treatment of non-obese diabetic mice with INGAP and lisofylline
![]()
1Department of Pediatrics, Indiana University, Indianapolis, USA; *Corresponding Authors: rmirmira@iupui.edu, nadlerjl@evms.edu
2Herman B Wells Center for Pediatric Research, Indiana University, Indianapolis, USA
3Department of Medicine, University of Virginia, Charlottesville, USA
4Department of Surgery, McGill University, Montreal, Canada
5Department of Medicine and the Strelitz Diabetes Center, Eastern Virginia Medicial School, Norfolk, USA
6Department of Medicine, Indiana University, Indianapolis, USA
7Department of Cellular and Integrative Physiology, Indiana University, Indianapolis, USA
Received 7 December 2011; revised 13 January 2012; accepted 15 February 2012
Keywords: INGAP; lisofylline; non-obese diabetic mice; type 1 diabetes; insulin
ABSTRACT
Type 1 diabetes mellitus results from the autoimmune and inflammatory destruction of insulinproducing islet ß cells, rendering individuals devoid of insulin production. Recent studies suggest that combination therapies consisting of anti-inflammatory agents and islet growthpromoting factors have the potential to cause sustained recovery of ß cell mass, leading to amelioration or reversal of type 1 diabetes in mouse models. In this study, we hypothesized that the combination of the anti-inflammatory agent lisofylline (LSF) with an active peptide fragment of islet neogenesis associated protein (INGAP peptide) would lead to remission of type 1 diabetes in the non-obese diabetic (NOD) mouse. We treated groups of spontaneously diabetic NOD mice with combinations of LSF, INGAP peptide, or control saline parenterally for up to 6 weeks. Our results demonstrate that the mice receiving combined treatment with LSF and INGAP peptide exhibited partial remission of diabetes with increased plasma insulin levels. Histologic assessment of pancreata in mice receiving combined therapy revealed the presence of islet insulin staining, increased ß cell replication, and evidence of Pdx1-positivity in ductal cells. By contrast, diabetic animals showed severe insulitis with no detectible insulin or Pdx1 staining. We conclude that the novel combination treatment with LSF and INGAP peptide has the potential to ameliorate hyperglycemia in the setting of established type 1 diabetes via the recovery of endogenous ß cells and warrant further studies.
1. INTRODUCTION
Type 1 diabetes mellitus (T1DM) is an autoimmune disorder characterized by inflammatory damage to pancreatic ß cells resulting in loss of insulin secretion. The lack of insulin producing ß cells results in disrupted glucose homeostasis, which leads to microand macrovascular complications. Combining interventions that block the relevant inflammatory reactions with one that restores ß cell mass is required for reversing T1DM [1].
Interleukin 12 (IL-12) regulates T cell differentiation and Th1 cell activation [2]. Because activated T cells directly cause cytotoxcitity in ß cells, IL-12 may directly or indirectly facilitate the development of T1DM [3]. Lisofylline (LSF) is an immunomodulator and anti-inflammatory small molecule that reduces IL-12-mediated STAT4 signaling, effectively suppressing T-cell activation and differentiation [4]. LSF also maintains b cell insulin secretory function in the presence of inflammatory cytokine insult and regulates immune cellular function to suppress autoimmunity and prevent the onset of T1DM in non-obese diabetic (NOD) mice [4]. Whereas LSF also enhances insulin secretion in isolated islets and in transformed ß cell lines in response to glucose stimulation in vitro [5], LSF alone was not capable of reversing autoimmune diabetes in NOD mice [6]. In recent studies, it was shown that combinations of LSF with the islet growth GLP-1 receptor agonist exendin-4 reversed diabetes in NOD mice, and even maintained euglycemia for up to 145 days after treatment withdrawal [6]. These studies suggest that combinations of inflammation-modulating agents with islet trophic factors may be one approach in the treatment or reversal of T1DM.
Islet neogenesis associated protein (INGAP) is a member of the Reg3 family of pancreatic proteins and regulated by transcriptional factors involved in neuroendocrine lineage commitment [7]. In studies of normal hamsters, administration of an INGAP-derived peptide (corresponding to amino acids 104 - 118 of INGAP) appeared to stimulate islet neogenesis, as evidenced by small foci of insulin-positive islet-like clusters budding from intralobular and terminal ductules [8]. In streptozotocin-induced diabetes in mice, INGAP peptide administration also induced new islet formation and restored euglycemia [8]. We hypothesized that combination therapy with LSF and INGAP peptide would ameliorate or reverse T1DM by providing protection from inflammatory destruction (via LSF) and stimulation of islet ß cell regeneration (via INGAP peptide). In this preliminary study, we show that this combination therapy has promise in reducing hyperglycemia in the NOD mouse model of T1DM, but requires additional studies.
2. METHODS
2.1. Reagents
LSF (1-(5-R-hydroxyhexyl)-3,7-dimethylxanthine) was provided by DiaKine Therapeutics, Inc. (Charlottesville, VA). INGAP peptide was provided by Kinexum Metabolics, Inc. (Harper’s Ferry, WV). LSF was dissolved in injectable normal saline and INGAP peptide was dissolved in sterile phosphate buffer solution.
2.2. Mice
Eight-week-old female NOD/ShiLTJ (NOD) mice were obtained from Jackson Laboratories (Bar Harbor, ME) and maintained in a pathogen-free colony in the Center for Comparative Medicine under protocols approved by the Institutional Animal Care and Use Committee using AALAAC guidelines at the University of Virginia. NOD mice were monitored for diabetes by measuring blood glucose levels using AccuChek Advantage Glucose Monitors (Roche, Indianapolis, IN) with tail vein blood samples. Non-fasting blood glucose values > 250 mg/dL for 3 consecutive days were considered as evidence of diabetes onset.
2.3. Treatments
Spontaneously diabetic NOD mice, between 12 - 25 weeks of age, were implanted with sustained release bovine insulin pellets (LinShin, Ontario, Canada). The insulin pellets have a release rate of ~0.1 U/24hr/pellet for 30 days and were used at 1/4, 1/2, and single doses. Insu lin pellets were immersed briefly in 2% povidone-iodine solution before being implanted subcutaneously using a 12-gauge trocar while under anesthesia. Insulin pellets were supplied to the mice to maintain blood glucose values around 200 mg/dL. Blood glucose values were monitored a minimum of twice a week to ensure euglycemia. If a mouse had a biweekly reading above 300 mg/dL the mouse received another dose of insulin pellet to help maintain euglycemia, if the biweekly reading was below 125 mg/dL, insulin pellets were removed. All treatments started within 3 - 5 days after diabetes was initially diagnosed (defined as a blood glucose >250 mg/dL on three consecutive readings). LSF was delivered at 27 mg/kg/day or saline control via Alzet osmotic mini-pumps (DURECT Co., Cupertino, CA). Each minipump was placed subcutaneously on the back of a mouse that was under general anesthesia; mini-pumps were replaced every two weeks. INGAP was administered by intraperitoneal (IP) injection at 500 µg/day once daily for 6 weeks. Groups were as follows: Group A mice received saline pumps for four weeks and saline IP injections for 6 weeks; Group B mice received LSF pumps for 4 weeks and saline IP injections for 6 weeks; Group C mice received saline pumps for 4 weeks and INGAP IP injections for 6 weeks; Group D mice received LSF pumps for 4 weeks and INGAP IP injections for 6 weeks; Group E mice received LSF pumps for 6 weeks and INGAP IP injections for 6 weeks, INGAP was started one week after the initial LSF pump (see Figure 1(a)). All treatment groups were kept on insulin pellets during the course of treatment. After six weeks of treatment (mice in Group E were treated for a total of 7 weeks), insulin pellets were surgically removed and mice were monitored one week before euthanasia. Final blood glucose values were measured the day of euthanasia between 9 - 10 am in the morning.
2.4. Immunohistochemisty and Immunofluorescence
Pancreas isolation and tissue embedding proceeded as previously described [9]. Antibodies and dilutions were as follows: insulin (1:5000, Dako, Carpinteria, CA), Pdx1 (1:800, Millipore, Temecula, CA), Ki67 (1:500, Abcam, Cambridge, MA), anti-rabbit Alexa Fluor 555 (1:500, Invitrogen, Carlsbad, CA), and anti-guinea pig Alexa Fluor 488 (1:200, Invitrogen, Carlsbad, CA). All sections were stained with Hoechst Dye (5 µl/mg; Invitrogen, Carlsbad, CA) to visualize the nucleus. Digital images were acquired on an Axio-Observer Z1 microscope (Zeiss, Thornwood, NY) fitted with an AxioCam high resolution color camera. Florescence sections were acquired on an Olympus BX51 WI fluorescence microscope (Olympus, Tokyo, Japan) fitted with a Hamamatsu
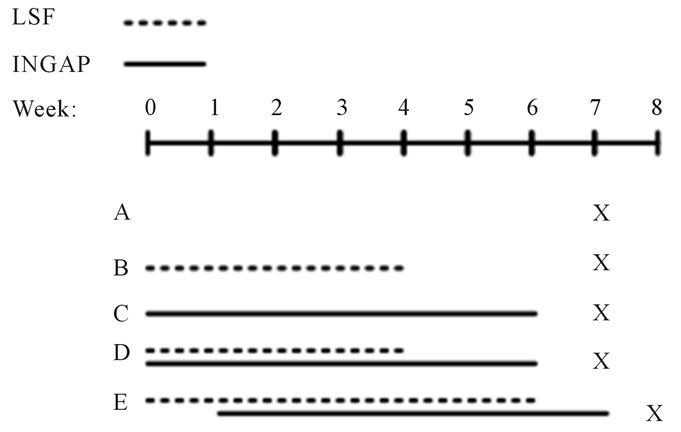 (a)
(a)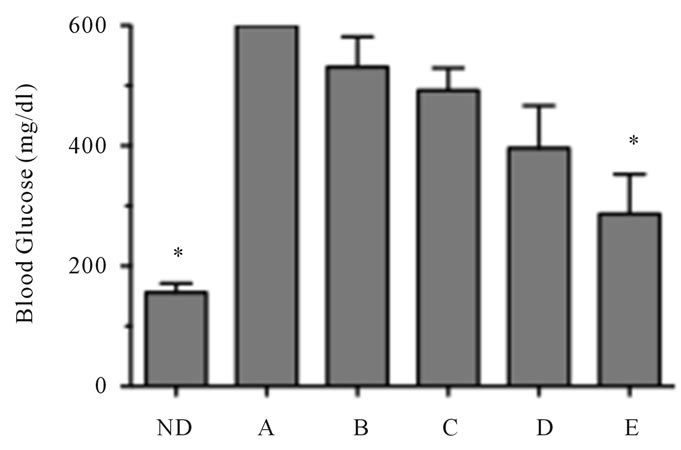 (b)
(b)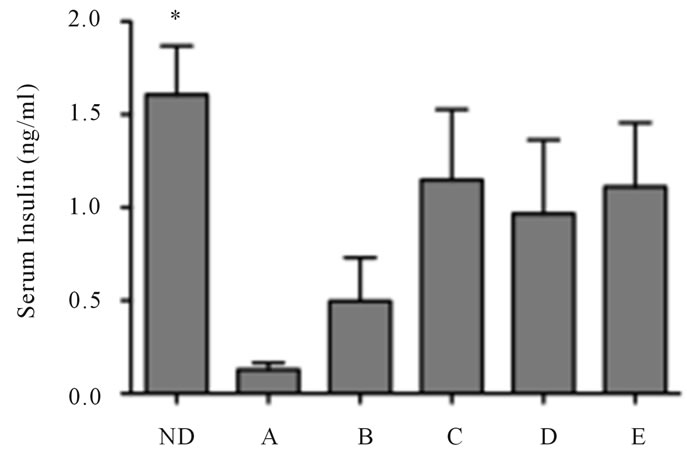 (c)
(c)
Figure 1. Reversal of diabetes in mice treated with LSF and INGAP. (a) Schematic representation of the treatment protocol and group identification; (b) Non-fasting final blood glucose values of treated mice at the end of the study. The detection limit for the glucometer is 600 mg/dlL; (c) Non-fasting serum insulin levels of treated mice one week after stopping treatment (n = 6 - 8 per group). Non-diabetic NOD mice (ND) were used as non-diabetic controls. The “*” indicates that the values are statistically different (P < 0.05) compared to the value for the saline-treated diabetic mice (Group A).
ORCA-ER monochrome charged-coupled device camera (Hamamatsu Photonics, Iwata City, Japan).
2.5. Insulin Measurements
At time of euthanasia, blood was obtained by cardiac puncture. Serum insulin concentrations were determine by mouse insulin ELISA (Mercodia, Winston Salem, NC) and quantitated against a standard curve.
2.6. Statistics
Data are shown as mean ± SEM. One-way ANOVA (with Bonferroni post-test) was used for data analysis using GraphPad Prism (version 5.0) software. P values of less than 0.05 were considered significant.
3. RESULTS
3.1. Glycemic Control Is Improved in Animals Treated with Combinations of LSF and INGAP Peptide
Groups of 6 - 8 female NOD mice were started on one of five treatments after being diagnosed with diabetes (defined as a blood glucose >250 mg/dl on three consecutive readings). There was no difference in starting blood glucose values between all treatment groups (Table 1). Diabetic mice were randomly assigned into one of five groups: saline/normal control (Group A), LSF alone (Group B), INGAP alone (Group C), INGAP plus LSF (Group D), and LSF pretreatment plus INGAP (Group E; treatment for 7 days with LSF, followed by dual treatment with both LSF and INGAP; see Figure 1(a)). All mice received a sustained release insulin pellet during the treatment period to reduce the complications associated with severe hyperglycemia. Blood glucose values were maintained at an average of 223 ± 7.1 mg/dL throughout the course of treatment for all groups.
After initiation of drug treatment, mice in the combination treatment groups, D and E required fewer insulin pellets to maintain near normal glycemia compared to any other group, suggestive of partial recovery from
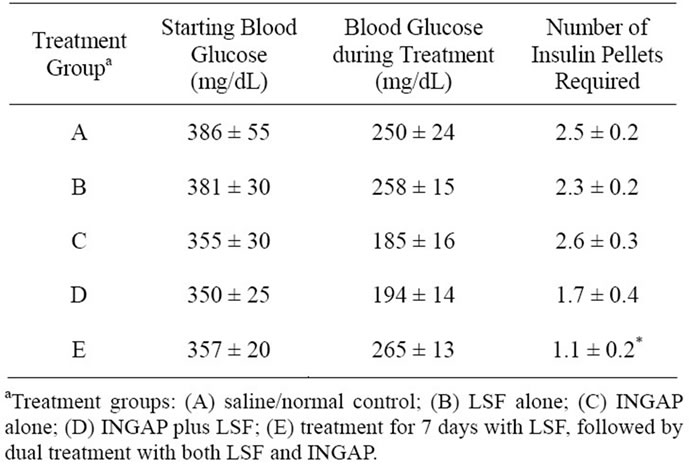
Table 1. Blood glucoses at the start and during treatment of NOD mice.
diabetes. Only in Group E did the reduction in pellets achieve statistical significance (Table 1). At the end of treatment, insulin pellets were removed and animals were monitored for seven days to determine their glycemic state. As shown in Figure 1(b), mice in Groups B-D averaged blood random glucoses in excess of 400 mg/dL upon removal of the insulin pellets, and were statistically no different from saline treated control animals (Group A). Importantly, however, animals in Group E (which were pretreated one week with LSF) averaged 280 mg/dL, a value that while modest, was significantly lower than saline-treated controls (Group A) (Figure 1(b)). Importantly, 5 out of 7 animals in Group E exhibited random glucoses under 250 mg/dL (defined a priori as remission) during the entire final week post treatment. Two of two mice in Group E remained euglycemic for two months post treatment (data not shown).
Upon completion of the study, mice were euthanized and random plasma mouse insulin levels were measured (Figure 1(c)). Although not statistically significant, there was a trend toward higher insulin levels in mice from any treatment group with INGAP (Groups C-E) (Figure 1(c)) compared to saline controls.
3.2. Immunohistochemical Analysis Shows Evidence of Islet Regeneration with Combination Therapy
To assess islet growth and regeneration in treated animals, pancreatic tissue was harvested and stained for insulin, Pdx1, and a marker of cell proliferation (Ki67). Notwithstanding evidence of insulitis with mononuclear cellular infiltrate around the islets, control non-diabetic NOD mice exhibited insulin staining in islets of pancreatic sections (Figure 2). By striking contrast, pancreatic sections from diabetic (saline control, Group A) NOD mice exhibited severe islet infiltration of mononuclear cells and minimal to no apparent insulin staining (Figure 2), consistent with active islet b cell destruction. In mice treated with combination LSF pretreatment and INGAP (Group E), insulin-positive cell clusters were widely distributed throughout the pancreas despite evidence of persistent insulitis, similar to non-diabetic NOD mice.
Consecutive tissue sections were stained for insulin and Pdx1, a key transcription factor involved in the differentiation of progenitor cells into insulin-producing cells. As expected, in control non-diabetic NOD mice, we found positive staining for Pdx1 in insulin-positive islets (data not shown), and no Pdx1 staining in the insulin-negative Group A islets. Interestingly, in group E mice, whereas insulin-positive cells were also positive for Pdx1, we also observed Pdx1-positivity in many pancreatic ductal cells (Figure 3). Pdx1 staining in pancreatic ducts was not observed in non-diabetic controls or in pancreata from Group A.
3.3. Combination Therapy Led to Increased Islet ( Cell Proliferation
Cellular proliferation was examined using immunofluorescence staining for Ki67 and insulin. As shown in Figure 4, there were no detectable Ki67/insulin costaining cells in non-diabetic NOD mice or in Group A mice, although Ki67 positivity was abundant in the dense
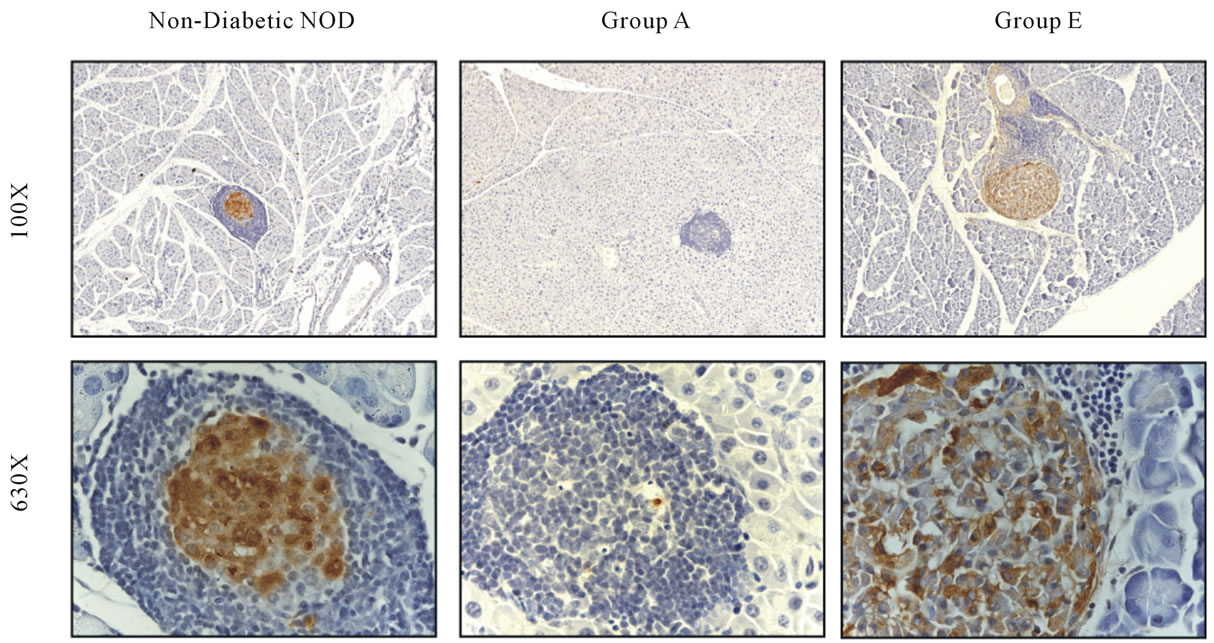
Figure 2. Treatment of NOD mice with LSF and INGAP restored islet insulin positivity. Pancreata were stained for insulin (brown) and counterstained with hematoxylin (blue). Sections from representative pancreata from non-diabetic NOD, saline-treated diabetic Group A, and Group E mice are shown at low (original magnification, × 100; top panels) and high (original magnification, × 630; bottom panels) magnification.
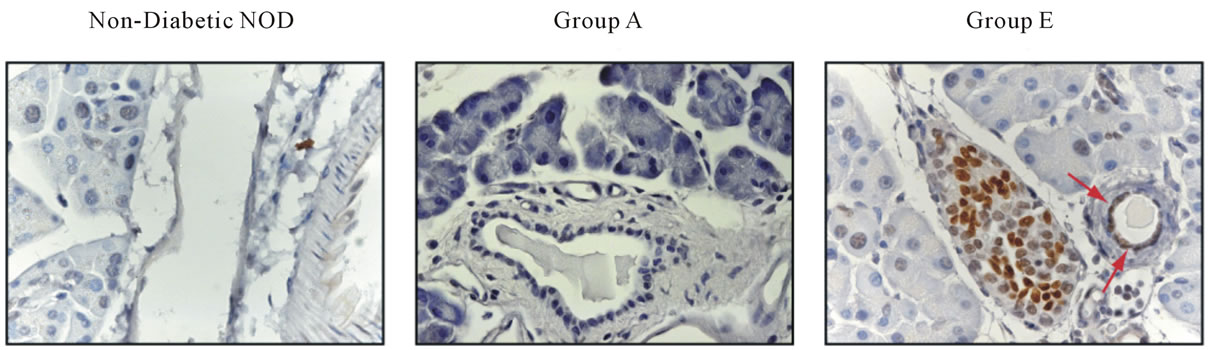
Figure 3. Mice treated with LSF and INGAP displayed Pdx1+ cells in the ducts. Sections of pancreata were stained with Pdx1 (original magnification, × 630). Pdx1 + cells in the duct are indicated by arrows.
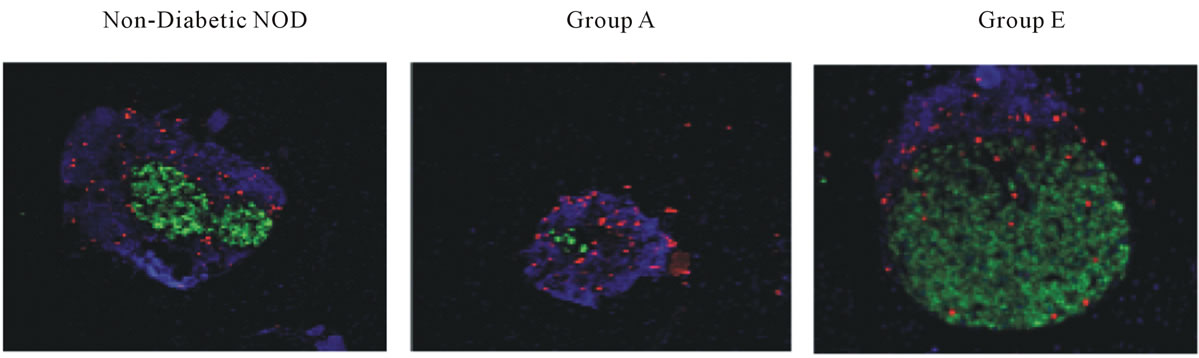
Figure 4. Islet β cell proliferation is increased with LSF and INGAP combination therapy. Pancreata were harvested from the groups of mice indicated, and representative sections stained for Ki67 (red), insulin (green), and DAPI (blue) and are shown (original magnification, × 630).
peri-islet mononuclear infiltrate. By contrast, frequent Ki67/insulin co-staining cells were observed within insulin positive cells in mice from Group E, indicating the presence of proliferating b cells.
4. DISCUSSION
T1DM is caused by autoimmune activation of T cells and macrophages, leading to the inflammatory destruction of islet b cells. Although suppression of the inflammatory and autoimmune responses is crucial in any approach to cure T1DM, it is also clear that islet trophic factors will be necessary to augment the islet mass that is severely compromised in long-standing T1DM [1,10]. Recent studies support this approach in NOD mice. Studies of Yang, et al. demonstrated successful reversal of T1DM in this model using combinations of the antiinflammatory compound LSF and the growth factor exendin-4 [6]. More recently, studies of Tian et al. demonstrated that successful reversal of T1DM in NOD mice by use of a dipeptidyl-peptidase IV inhibitor was likely though a combination effect of the drug on T regulatory cells and islet proliferation [11]. In this study, we tested the hypothesis that a combined administration of the anti-inflammatory agent LSF with the islet neogenesis factor INGAP peptide would ameliorate T1DM in diabetic NOD mice.
LSF appears to target inflammatory pathways by inhibiting IL-12-mediated STAT4 signaling [4]. IL-12 is important in activating Th1 cells and promoting their release of pro-inflammatory cytokines [3]. Prior studies have shown that LSF can prevent the development of autoimmune diabetes in NOD mice [4] and protect transplanted islets from destruction [12]. However, and consistent with prior studies [6], we show here that LSF alone was not capable of reversing diabetes in NOD mice.
INGAP peptide has been shown to stimulate proliferation and growth of islets, and to protect against the development of hyperglycemia in the streptozotocin model of diabetes in mice [8]. The administration of INGAP peptide in combination with immunosuppressants (tacrolimus and sirolimus) in NOD mice led to improvements in glycemic control [13], but its utility as a single agent in NOD mice has not been studied. We show here that a 6 week treatment with either LSF or INGAP peptide alone is not sufficient to reverse hyperglycemia in this model, a finding that likely suggests that growth factors for this period alone may be insufficient to reverse diabetes in this model. In a clinical trial INGAP increased arginine-stimulated C-peptide release during the treatment phase of type 1 diabetic subjects, but this effect was lost after 30 days of drug wash-out [14]. The authors raised the possibility that this was due to persistent autoimmune destruction of newly-formed b cells. To overcome this limitation, we combined use of INGAP peptide with LSF as a modulator of the inflammation process.
Both of our treatment groups involving combined LSF and INGAP peptide administration appeared to reduce non-fasting glucose levels compared to control animals, but this result was statistically significant only in the mice that were pre-treated for one week with LSF prior to starting combination therapy with the two drugs (Group E). Our rationale for this pre-treatment phase was to reduce systemic inflammation prior to the induction of islet neogenesis with INGAP peptide. A particularly notable observation in our studies was that the remission in Group E occurred independent of the level of starting serum glucose, suggesting that amelioration of T1DM was possible even when animals were severely hyperglycemic (>350 mg/dl) prior to the start of treatment.
Insulin, Pdx1, and Ki67 staining was utilized to examine cell differentiation and proliferation in the treated NOD mice. We found insulin/Ki67 co-expressing cells in pancreata of Group E animals, a finding suggestive of b cell proliferation. Pdx1 is a b cell-specific transactivator of the insulin genes and is necessary for the development of the pancreas and for the maintenance of b cells in the adult (for review, see [15]). Activation of Pdx1 has been seen in the duct cells of the pancreas following partial pancreatectomy in mice [16]. Prior studies showed that INGAP enhanced the differentiation of pancreatic duct cells in vitro into islet-like clusters [8,17]. Here, we show increased foci of Pdx1 staining, mostly within ducts (although some Pdx1 staining was also seen in the acinar compartment). Although the significance of this finding is inconclusive, it is possible that INGAP treatment combined with lisofylline could allow for potential neogenesis from these Pdx1-positive/insulin-negative cells.
This study represents an initial attempt at combining the inflammatory modulator, LSF and the growth factor, INGAP in the treatment of T1DM in NOD mice. Through a limited series of experiments our results suggest that combination therapies involving inflammatory modulators and growth factors have the potential to ameliorate or reverse T1DM in mice. More importantly, our studies suggest the efficacy of a novel combination of drugs, both of which have undergone early safety studies in humans. These early results are modest, but encouraging and suggest a more in-depth study to eliminate the limitations of this study. More animals per group and a standard glucose tolerance test including fasting blood glucoses and insulin levels are needed to determine the rate of remission with this treatment in NOD mice. Quantitative b cell mass and Ki67 staining is necessary to determine if indeed LSF plus INGAP is increasing b cell proliferation, as suggested by our preliminary results.
5. ACKNOWLEDGEMENTS
This study was supported in part by grants R01 DK083583 (to RGM) and R01 DK55240 (to JLN) from the NIH, and grants from the Canadian Institute for Health Research (to LR), the Farish Foundation, the Ella Fitzgerald Foundation, and the Iacocca Foundation (all to JLN). We thank Ms. Elizabeth Kropf and Mr. James Garmey for help with immunohistochemisty.
![]()
![]()
REFERENCES
- Bresson, D. and Von Herrath, M. (2007) Moving towards efficient therapies in type 1 diabetes: To combine or not to combine? Autoimmunity Reviews, 6, 315-322. doi:10.1016/j.autrev.2006.09.013
- Trinchieri, G. (1995) Interleukin-12 and interferon-gamma. Do they always go together? American Journal of Pathology, 147, 1534-1538.
- Trembleau, S., Penna, G., Bosi, E., Mortara, A., Gately, M. and Adorini, L. (1995) Interleukin 12 administration induces T helper type 1 cells and accelerates autoimmune diabetes in NOD mice. The Journal of Experimental Medicine, 181, 817-821. doi:10.1084/jem.181.2.817
- Yang, Z., Chen, M., Fialkow, L., Ellett, J., Wu, R. and Nadler, J. (2003) Inhibition of STAT4 activation by lisofylline is associated with the protection of autoimmune diabetes. Annals of the New York Academy Sciences, 1005, 409-411. doi:10.1196/annals.1288.069
- Bleich, D., Chen, S., Bursten, S. and Nadler, J.L. (1996) Lisofylline, an inhibitor of unsaturated phosphatidic acid generation, ameliorates interleukin-1 beta-induced dysfunction in cultured rat islets. Endocrinology, 137, 4871- 4877. doi:10.1210/en.137.11.4871
- Yang, Z., Chen, M., Carter, J., Nunemaker, C., Garmey, J., Kimble, S. and Nadler, J. (2006) Combined treatment with lisofylline and exendin-4 reverses autoimmune diabetes. Biochemical and Biophysical Research Communications, 344, 1017-1022. doi:10.1016/j.bbrc.2006.03.177
- Hamblet, N., Shi, W., Vinik, A. and Taylor-Fishwick, D.A. (2008) The reg family member INGAP is a marker of endocrine patterning in the embryonic pancreas. Pancreas, 36, 1-9. doi:10.1097/MPA.0b013r318148c8e6
- Rosenberg, L., Lipsett, M., Yoon, J., Prentki, M., Wang, R., Jun, H., Pittenger, G., Taylor-Fishwick, D. and Vinik, A. (2004) A pentadecapeptide fragment of islet neogenesis-associated protein increases beta-cell mass and reverses diabetes in C57BL/6J mice. Annals of Surgery, 240, 875-884. doi:10.1097/01.sla.0000143270.99191.10
- Evans-Molina, C., et al. (2009) Peroxisome proliferator-activated receptor gamma activation restores islet function in diabetic mice through reduction of endoplasmic reticulum stress and maintenance of euchromatin structure. Molecular and Cellular Biology, 29, 2053-2067. doi:10.1128/MCB.01179-08
- Yang, Z., Chen, M., Ellett, J., Carter, J., Brayman, K. and Nadler, J. (2005) Inflammatory blockade improves human pancreatic islet function and viability. American Journal of Transplantation, 5, 475-483. doi:10.1111/j.1600-6143.2005.00707.x
- Tian, L., Gao, J., Hao, J., Zhang, Y., Yi, H., O’brien, T., Sorenson, R., Luo, J. and Guo, Z. (2010) Reversal of new-onset diabetes through modulating inflammation and stimulating beta-cell replication in nonobese diabetic mice by a dipeptidyl peptidase IV inhibitor. Endocrinology, 151, 3049-3060. doi:10.1210/en.2010-0068
- Yang, Z., Chen, M., Ellett, J., Fialkow, L., Carter, J. and Nadler, J. (2004) The novel anti-inflammatory agent lisofylline prevents autoimmune diabetic recurrence after islet transplantation. Transplantation, 77, 55-60. doi:10.1097/01.TP.0000104844.48064.81
- Lipsett, M., Hanley, S., Castellarin, M., Austin, E., SuarezPinzon, W.L., Rabinovitch, A. and Rosenberg, L. (2007) The role of islet neogenesis-associated protein (INGAP) in islet neogenesis. Cell Biochemistry and Biophysics, 48, 127-137. doi:10.1007/s12013-007-0028-3
- Dungan, K.M., Buse, J. and Ratner, R. (2009) Effects of therapy in type 1 and type 2 diabetes mellitus with a peptide derived from islet neogenesis associated protein (INGAP). Diabetes Metabolism Research and Reviews, 25, 558-565. doi:10.1002/dmrr.999
- Babu, D.A., Deering, T. and Mirmira, R. (2007) A feat of metabolic proportions: Pdx1 orchestrates islet development and function in the maintenance of glucose homeostasis. Molecular Genetics and Metabolism, 92, 43-55. doi:10.1016/j.ymgme.2007.06.008
- Peshavaria, M., Larmie, B., Lausier, J., Satish, B., Habibovic, A., Roskens, V., Larock, K., Everill, B., Leahy, J. and Jetton, T. (2006) Regulation of pancreatic beta-cell regeneration in the normoglycemic 60% partial-pancreatectomy mouse. Diabetes, 55, 3289-3298. doi:10.2337/db06-0017
- Li, J., et al. (2009) Islet neogenesis-associated proteinrelated pentadecapeptide enhances the differentiation of islet-like clusters from human pancreatic duct cells. Peptides, 30, 2242-2249. doi:10.1016/j.peptides.2009.09.003

