Food and Nutrition Sciences
Vol.5 No.13(2014), Article
ID:48220,9
pages
DOI:10.4236/fns.2014.513140
Organochlorine Pesticides in Infant Milk Formulas Marketed in the South of Mexico City
Rey Gutiérrez Tolentino1, Salvador Vegay León1*, Beatriz Schettino Bermúdez1, Guadalupe Prado Flores1, María de Lourdes Ramírez Vega1, Claudia Radilla Vázquez2, María Radilla Vázquez3, Marcela Vazquez Francisca4
1Departamento de Producción Agrícola y Animal, Universidad Autónoma Metropolitana Unidad Xochimilco, Coyoacán, D.F., México
2Departamento de Atención a la Salud, Universidad Autónoma Metropolitana Unidad Xochimilco, Coyoacán, D.F., México
3Puente de Piedra #150, Tlalpan, D.F., México
4Universidad Autónoma Metropolitana, México D.F., México
Email: *svega@correo.xoc.uam.mx
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/
![]()
![]()

Received 21 May 2014; revised 25 June 2014; accepted 5 July 2014
ABSTRACT
The nature of organochlorine pesticides (OCP) and their physical-chemical properties exert immediate action of control on live systems, which has justified their use in agricultural practices. Their long life makes them a persistent ecological aggressor and biomagnifier. They reach foods by biotic and abiotic means, and are absorbed and accumulate in adipose tissue. In lactation processes, they are excreted in milk through the mobilization of fats. Diverse studies have identified them as neurotoxic, affecting reproductive processes, altering the immunological response and act as endocrine disruptors. An analysis was made of the content of organochlorine pesticides in twenty-one samples of infant milk formulas marketed in the south of Mexico City in 2010. The determinations were made following the protocols of the International Dairy Federation, by means of gas chromatography with electron capture detector, and the majority presence was found of α-HCH (100%), β-HCH (95.2%), γ-HCH (90.5%), aldrin (85.7%), heptachlor (80.9%) and heptachlor epoxide (80.9%) with mean values of 0.24, 0.13, 0.32, 0.62, 0.92 and 0.18 μg/kg of fat, respectively; all below the limits permitted by the Codex Alimentarius. With null or lower recurrence and in lower quantities, the family of DDT, endrin, endrin aldehyde and the endosulphanes were quantified.
Keywords:Organochlorine Pesticides, Infant Milk Formulas, Gas Chromatography, Mexico

1. Introduction
The nature of the organochlorine pesticides (OCP) and their physical-chemical properties exert immediate control action on live systems, which has justified their use in agricultural practices. However, they have long term effects on biota and health, acting as persistent ecological aggressors and biomagnifiers [1] -[4] as well as causing disturbance in animal health [5] . They are transported through biotic and abiotic means to foods, are absorbed for the most part orally, are metabolized and distributed, accumulating in adipose tissue. In lactation processes, they are excreted in milk through the mobilization of fats [1] [6] [7] . Diverse studies identify their presence with metabolic and genetic alterations: they are neurotoxic [8] , affect reproductive processes [9] [10] , alter the immunological response [11] and act as endocrine disruptors [12] . The International Agency for Research on Cancer (IARC) has recognized the mutagenic and carcinogenic activity of DDE, heptachlor, dieldrin, the isomers α, β and γ of HCH. Recently information has been given of genes that are affected by DDT, HCB and β-HCH [8] .
The subjects most vulnerable to their effects are infants, the elderly and the malnourished. Due to the evidence of their damaging effects, the international organisms FAO/WHO and national legislation have established increasingly more restrictive limits in their content [13] .
The consideration that maternal alimentation is the most adequate for infants is sustained in theory and practice. Additional to human history, this right is recognized and legislated by the FAO/WHO [14] , Rights of Children and Mexican norms, which indicate that both human milk and infant formulas should insure the health of infants [15] [16] . Despite the legitimacy of the regimentation, environmental contamination is the cause of disturbances in milk quality and the presence of the OCP offers a warning with respect to its safety. In view of this practice, the market offers substitution options of maternal alimentation by human milk substitutes or infant milk formulas (IMF) for infant populations. These formulas should be of high quality and nutritional specificity and be free of substances that are potentially harmful to the vulnerable condition of the consumers [16] .
These foods are mainly made from powdered milk, with or without de-mineralized lactoserum, vegetable oils or mixtures of vegetable oils and fats, raw materials which are susceptible to contamination. Attention has been given to the quality of animal fat and vegetable oils, as they may register residues of OCP, as has been pointed out by studies of Waliszewski et al., who found α-hexachlorocyclohexane (α-HCH), β-HCH, γ-HCH, aldrin, heptachlor epoxide, dieldrin, endrin, metoxychlorine, mirex and the isomers o,p’ and p,p’ of DDT, DDE and DDD [17] . The analysis of residues of OCP made at the end of the 1960’s in Canadian milk formulas reported the content of lindane (γ-HPH), heptachlor, dieldrin and DDT [18] . In Spain residues have been detected of Ʃ-HCH, hexachlorobenzene (HCB), aldrin, endrin, heptachlor, DDE and DDD [19] . In Venezuela, OCP was found in all infant formulas, with average values of HCB 0.07, Ʃ-heptachlor + heptachlor epoxide 0.015. dieldrin 0.003 and Ʃ-DDT 0.085 mg/kg fat basis [19] [20] .
Because infants are populations that merit high quality in their alimentation and are especially vulnerable to risks from contaminants, the present study was focused on determining by gas chromatography with electron capture detector of the residues of OCP in IMF marketed in the south of Mexico City, because of its high significance in harm to infant health.
2. Materials and Methods
The method of obtaining results of its content consists of a sampling, extraction of the fat (because the OCP is found in this fraction), its purification, dissolution and quantitative determination by means of gas chromatography. The analytical characteristics that sustain this choice are the specificity, sensitivity and degree of resolution of the process and of the equipment (gas chromatography) to achieve repeatability and reproducibility of the information.
2.1. Samples
A monthly sampling was carried out during October, November and December of 2010 of seven commercial brands of dehydrated IMF. These samples were acquired in commercial establishments in the south of Mexico City. A total of 21 samples were obtained, which were maintained at room temperature until their analysis. A code was assigned to each commercial brand of IMF studied: IMF1 for the product corresponding to a formula of zero to six months of age, with iron; IMF2 and IMF3 are continuation formulas with iron for infants of six to twelve months of age; IMF4 responds to a semi-elemental formula with iron and extensively hydrolyzed proteins offered from birth; IMF5 is recommended for babies with gastroesophageal reflux, after four weeks of age; IMF6 is a formula for babies with lactose intolerance, recommended after the fourth week of age and IMF7, which is a formula for babies between six and twelve months with iron and probiotics. All of the IMFs informed in their label the presence of vegetable oils, of palm, soy, coconut and sunflower.
2.2. Extraction of Fat
Each one of the samples was reconstituted according to the specifications of the manufacturer, proceeding to the extraction of fat with phosphate free detergent solution by means of the proposal of Frank et al., obtaining five to six grams of anhydrous fat [18] . The samples were conserved under freezing at −20˚C until their analysis.
2.3. Extraction and Purification of Organochlorine Pesticides
The technique used was the analysis recommended by the International Dairy Federation, which is a procedure of chromatography in a glass column [21] . This column was packed with partially deactivated florisil (Merck), and the elution was made with mixtures of hexane (J.T. Baker) and dichloromethane (J.T. Baker) 4:1 with a flow of 3 mL/min [22] . Then in a vacuum rotary evaporator (Büchi, Germany), the residues of pesticides were concentrated, and dissolved in an exact volume of isooctane (Merck) and a measured volume was injected into the gas chromatograph for its quantitative determination.
2.4. Conditions of the Gas Chromatograph
The gas chromatograph was a Hewlett Packard® model 6890 Series II (US), with electron capture detector (ECD 63Ni) with model 3396 integrator and capillary column SPB-5 (phenylmethylsilicon at 5%) of 30 m × 0.25 mm DI and 0.25 μm of layer thickness of stationary phase. The injected volume was 1 μL. The operation conditions were: temperature of the injector 260˚C; temperature of detector 320˚C; oven temperature programmed from 90˚C (2 min) at 30˚C/min to 180˚C (0 min) at 1˚C/min, to 200˚C (0 min) at 10˚C/min and to 300˚C (6 min). Helium was used as gas carrier with a flow of 1 mL/min. The standards were of Chem ServiceTM PPO-BJM at a concentration of 20 ng/mL in isooctane and the run time was 40 min.
2.5. Quantification of Pesticides
The quantification was made using the method of the external standard from a mixture of sixteen OCP at a concentration of 20 ng/mL. Through the particular registers of retention times of the components of the standard mixture, the chromatographic variables of the samples were compared with a relative standard deviation no higher than 20%. In addition, the linearity of the responses was valuated and confirmed using a calibration curve. The quantitative data was referred by the relationship among the areas of each one of the chromatographic signals. The control employed was the analysis of targets of solvents; the run with fortified samples to assess the percentage of recovery; the injection of the midpoint of the calibration curve that was made with the mixture at a concentration of 20 ng/mL of each compound and the determinations were made in duplicate. The limit of detection of the chromatographic system was evaluated in 0.001 μg/kg fat basis. The OCP under study were α-HCH, β-HCH, γ-HCH, δ-HCH, p,p’-DDT, P,P’-DDD, p,p’-DDE, aldrin, dieldrin, endrin, endrin aldehyde, endosulphan I, endosulphan II, endosulphan sulfate, heptachlor and heptachlor epoxide.
2.6. Statistical Analysis
An exploratory analysis was made, along with a descriptive statistic, sequences graph and analysis of means of Kruskal-Wallis, using the SPSS program for Windows version 20.0.
3. Results and Discussion
Organochlorine pesticides profiles present in standard solution and IMF fat are shown in Figure 1. The chromatographic conditions allowed us to obtain defined peaks for OCP. Sixteen chromatographic signals were identified in the standard solution, as well as five to sample of IMF. IMF sample was characterized by having α-HCH, β-HCH, γ-HCH, heptachlor and heptachlor epoxide.
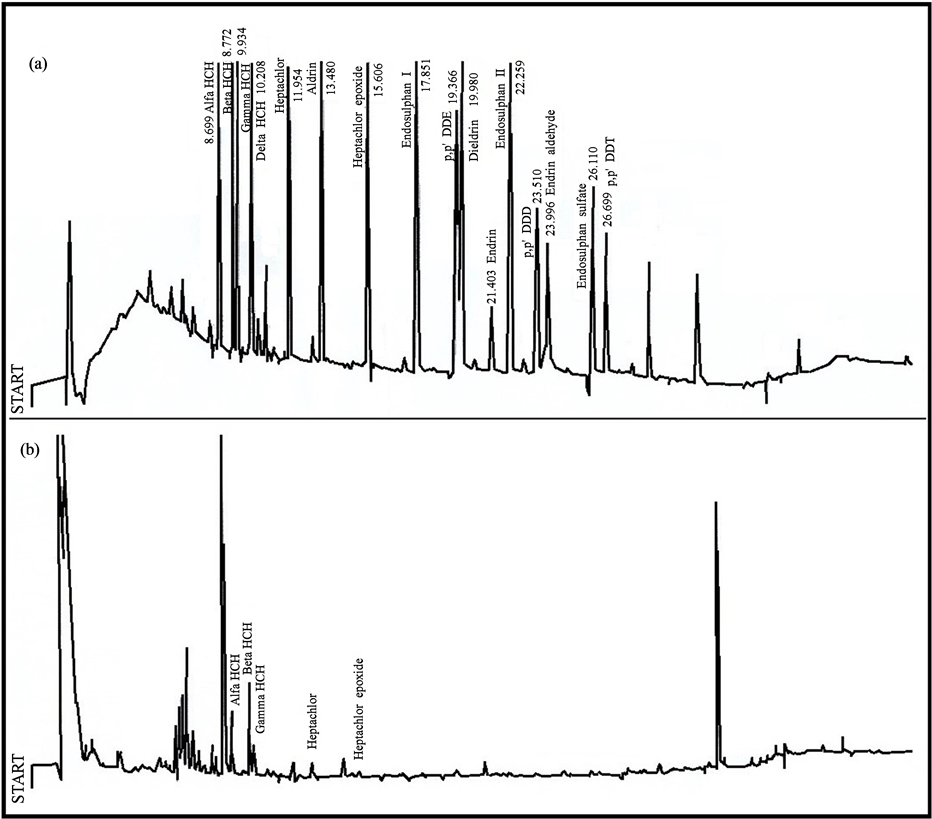
Figure 1. Organochlorine pesticide chromatograms in: (a) standar solution; (b) fat infant milk formula (application volume to gas chromatograph = 1 µL).
Table 1 shows the results of the analysis of means and frequencies of occurrence of the concentrations of organochlorine pesticides present in the samples of infant milk formulas, from October to December, expressed in parts per billion (μg/kg fat basis). The Kruskal-Wallis test did not show significant differences (p = 0.08) among the means of OCP of the IMF of the seven industries studied.
In October, there was no presence of endrin and its aldehyde, DDE and endosulphan sulfate, however, in November and December an OCP was detected in at least one sample (Figure 2), which indicates a persistence in these products.
The following frequencies were found in decreasing order: α-HCH (100%), β-HCH (95.2%), γ-HCH (90.5%), aldrin (85.7%) and heptachlor and heptachlor epoxide (80.9%), endosulphan I (71.4%), δ-HCH (61.9%), endrin aldehyde (42.8%), p,p’-DDT (38.1%), P,P’-DDD (33.3%), dieldrin and endrin (23.8%), endosulphan II (19.0%), p,p’-DDE (14.35) and endosulphan sulfate (9.5%). Therefore, above 60% frequency six pesticides were found, while the aromatics of the DDT family had lower occurrence. The contents higher than 1 μg/kg fat basis in the seven types of formulations were registered in the following scale: β-HCH (6/7), Ʃ-heptachlor + heptachlor epoxide (6/7), aldrin (2/7), endrin aldehyde (1/7) and endosulphan I (1/7). The IMF1 and IMF2 registered the lowest number of OCP, while those classified as IMF3, IML4 and IMF5 had the highest number of residues, although all of the values were lower than the limits permitted by the Codex Alimentarius [13] .
Figure 2 shows the sequences of OCP from October to November. It is observed that the IMF1; 2 and 7
Table 1 . Median and frequencies of organochlorine pesticides (µg/kg fat basis) in infant milk formulas.
†: Maximum residue limits (µg/kg) (Codex Alimentarius, 1993); ¶: Median/frequency of occurrence; §: Not detected.
present lower total concentration than the rest of the IMF (2.55, 5.38 and 8.72 μg/g fat basis, respectively). The IMF with highest total concentration was 3, 5 and 6 with 22.78, 21.76 and 20.62 μg/kg, respectively. Despite the fact that the α-HCH had the highest frequency, the highest concentration was found in β-HCH with a mean of 1.13 μg/kg fat basis.
This work shows values of OCP in IMF marketed in Mexico City at the end of 2010. In all of the IMF it was observed that none of the pesticides exceeded the maximum residue limit (MRL) for pesticides established by the Codex Alimentarius (0.006 - 0.020 μg/kg).
Figure 2 shows that β-HCH had a higher concentration than α-HCH. These isomers of HCH have been found for long periods of time in diverse parts of the planet [23] . In the last decade they are still being identified; they have been found in ten IMF from India, in concentrations that fluctuate between 0.04 - 335.87 μg/kg fat basis, among which β-HCH, α-endosulphan, malathion, dimetoate, dieldrin and aldrin were found present in most of the IMF [6] . Recently the content of these derivates was measured in soils of the La Lorraine Region in France and the highest content was presented by β-HCH with a value of 0.64 ± 0.15 μg/kg [4] . Their presence has also been estimated in the Bay of Abu Qir in Egypt [2] and their presence was reported along with other organochlorine compounds in sediments. The transport and biomagnification of these substances by the trophic chain is well known, reaching the highest links of the chain.
The study of Raghavan et al. in India has ratified the effect of HCH on the production of free radicals and in the response of alteration of the redox state, reducing the cellular antioxidant systems [24] . In the clinical aspect, Dar et al. analyzed the effect of β-HCH and DDT on the immunological response in Lupus eritematosus [11] . The exposure to the isomer β-HCH markedly increased the percentage of lymphocytes T CD3 (+) and CD4 (+) while there was a decrease in CD25 (+).
It is known that only the lindane (γ-HCH) has insecticide properties and that along with the isomer α are
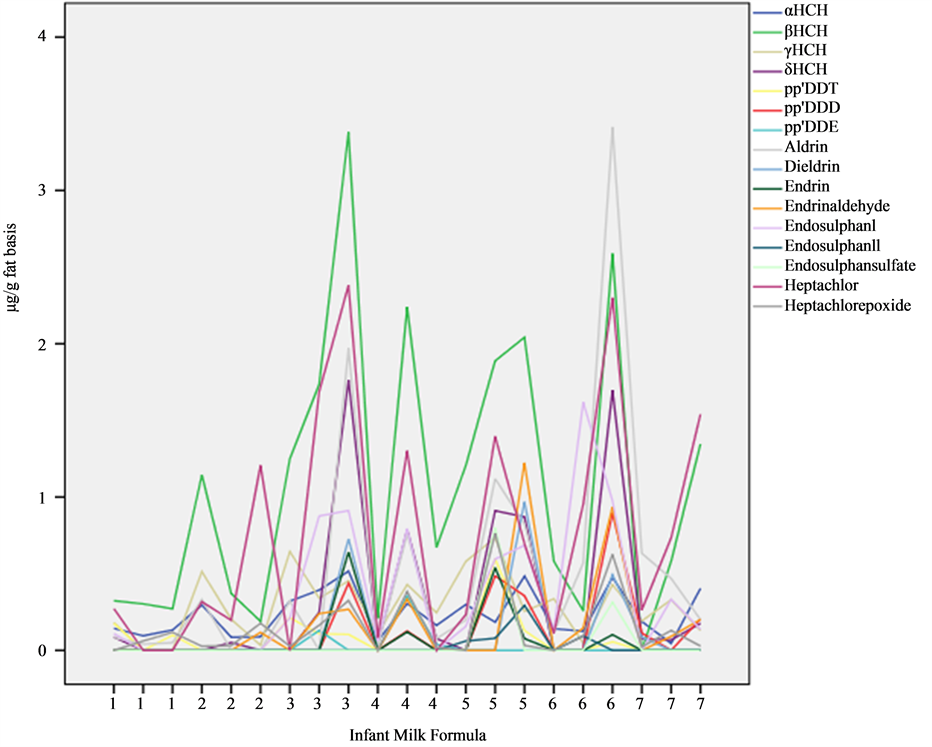
Figure 2. Graphical representation of sequences of organochlorine pesticides (µg/g fat basis) present in infant milk formulas. (111,···, 777 = October, November, December).
transformed by different means to the derivate β, which has greater persistence. Presently studies are being made of the response of absorption of lindane with nanometric particles of fullerene to eliminate it from the medium, although the safety of this combination is still being determined [25] .
The presence of heptachlor merits a careful examination. Although the data found are considered unobjectionable, studies of their adverse effect on health have shown it to be dangerous. One of its action mechanisms is oxidative stress [26] . This activity was evident in the turtles of Magdalena Bay in Southern California, given that antioxidant agents such as glutathione and enzymes such as catalase and dismutase superoxide diminished its capacity to maintain the redox state due to the presence of the pesticides that were found present. The causes of this presence and of the enzymatic activity analyzed were linked with water currents, the habitat and agricultural activities [3] . Its estrogenic activity has also been evaluated with the expression of the vitelogenin gene after an exposure of 96 hours to the percentages of 10, 25, 50 and 75 of the EC50 and this response has been considered a biomarker of endocrine disruption [12] .
The presence of DDT and its derivates continues to be an important motive for study. Although numerous legislations have prohibited its use in many countries, its stability makes it persistent and its derivate DDE has been qualified as highly toxic. In addition to the content of the family of the HCH, heptachlor, heptachlor epoxide, hexachlorobenzene, endosulphan, aldrin and dieldrin, both in sediments of Abu Qir Bay in Egypt and in soils of La Lorraine, Ʃ-DDT was calculated in 27 μg/kg in the Egyptian zone and in soils of the French region studied at 0.2 and 2.3 μg/kg [2] [4] .
Its effects on reproductive health have been an abundant motive of study and the IARC has classified DDE as carcinogenic. Recently, Mahalingaiah et al. measured the residues of DDT, DDE and HCB in serum of women during the follicular phase when expressing spontaneous abortions in fertilization treatments in vitro, and the investigators did not find a significant association with the detectable levels of DDT/DDE; HCB; DDT and DDE; of 0.087 μg/kg for HCB; 1.12 μg/kg for a total value of DDT and 1.04 μg/kg for p,p’-DDE [10] .
A reality which increases the risk on the environment and biotic resources is the diversity of compounds present in the air, soil and water; these mixtures are susceptible to giving synergic responses. Labrada-M. et al have analyzed the synergy between heptachlor and cadmium and have found lipidic peroxidation [3] . The studies of Bräuner et al. reported the analysis of a cohort of 47 053 individuals in relation to the presence of organochlorine pesticides. In addition to the OCP in the environment, other compounds were registered, including the polychlorobiphenyls (PCB) and heavy metals [5] . The synergy among them in many cases is a logical inference, but in the case of making specialized studies between the OCP and PCB, the authors found a prediagnosis of non-Hodking Lymphoma associated with this double presence.
There is abundant information of the metabolic damage of the OCP and there is more and more evidence of their genetic effects. The investigation group of Mitra et al. has identified the expression of the genes ATAD2B, BIVM, CD96, CXorf39, CYTH1, ETNK1, FAM13A, HIRA, INO80B, ODG1, RAD23B, and TSGA14 involved in the exposures to DDT, β-HCH and HCB, thus this evidence recognizes their potential of action on the genetic information. Their effects showed disorders in the connective tissues and skeletal muscle, as well as neurological diseases [8] .
Among the perspectives in this wide field that associates the agricultural practices with health and environment, is sustained research. Hence, the importance of the study of the chemical characteristics of the enantiomers and their characters of chirality [27] makes them responsible for a fraction of their toxicity. Another focus is the bioremediation through the use of bacteria Pseudodomonas putida 9, Stenotrophomonas maltophilia IMV B-7288 [28] and plants such as Phragmites autralis which degrade HCH [29] . The extraction of active principles of medicinal plants is incorporated as a means of partial solution to the contamination by the OCP; one of these is the artemis extracted from Artemisa vulgaris L. which acts with a biopesticide effect. An additional line of study is the absorption of toxic compounds with nanoparticles [25] . Vaccinations are also produced which are tested in zones affected by malaria, a motive for the massive use of DDT. In this way, there is a reduction in the use of insecticides which control the presence of the vectors of Plasmodium falciparum. In the area of predictions, mathematical models are being generated which involve the knowledge and integration of diverse hydrological, climatological variables and human parameters of the potentially affected individuals. Recently the genetically modified mosquitoes Anopholes are another control option of the causes of the use of these OCP.
4. Conclusion
Although the concentrations found in this study do not surpass the limits established by the Codex Alimentarius [13] , it is convenient to maintain vigilance and reduce its contents, as these compounds manifest a danger for infant consumers by their accumulation in target tissue and the toxic potential that has been manifested. The possible synergies among the components of the mixtures present signal the use of resources of prevention and monitoring.
References
- Kutz, F., Wood, P. and Bottimore, D. (1991) Organochlorine Pesticides and Polychlorinated Biphenyls in Human Adipose Tissue. Review Environmental Contamination Toxicology, 120, 1-82. http://dx.doi.org/10.1007/978-1-4612-3080-9_1
- Labrada-Martagón, V., Rodríguez, P., Méndez, L. and Zenteno, T. (2011) Oxidative Stress Indicators and Chemical Contaminants in East Pacific Green Turtles (Chelonia mydas) Inhabiting two Foraging Coastal Lagoons in the Baja California Peninsula. The Online Version of Comparative Biochemistry and Physiology Part C: Toxicology. Pharmacology, 154, 65-75. http://dx.doi.org/10.1016/j.cbpc.2011.02.006
- Thomas, M., Lazartigues, A., Banas, D., Brun-Bellut, J. and Feidt, C. (2012) Organochlorine Pesticides and Polychlorinated Biphenyls in Sediments and Fish From Freshwater Cultured Fish Ponds in Different Agricultural Contexts in North-Eastern France. Ecotoxicology and Environmental Safety, 77, 35-44. http://dx.doi.org/10.1016/j.ecoenv.2011.10.018
- Khairy, M., Kolb, M., Mostafa, A., El-Fiky, A. and Bahadir, M. (2011) Risk Posed by Chlorinated Organic Compounds in Abu Qir Bay, East Alexandria, Egypt. Environmental Science and Pollution Research International, 3, 794-811.
- Bräuner, E., Sørensen, M., Gaudreau, E., LeBlanc, A., Eriksen, K., Tjønneland, A., Overvad, K. and Raaschou-Nielsen, O. (2012) A Prospective Study of Organochlorines in Adipose Tissue and Risk of Non-Hodgkin Lymphoma. Environmental Health Perspectives, 120, 105-111. http://dx.doi.org/10.1289/ehp.1103573
- Mishra, R., Johnson, S. and Vankar, P. (2002) Pesticide Residue Analysis of Infant Formula in India. Bulletin of Environmental Contamination and Toxicology, 69, 667-673. http://dx.doi.org/10.1007/s00128-002-0113-7
- Hernández, M., Vidal, J. and Marrugo, J. (2010) Organochlorine Pesticides in Cow’s Milk Supplemented with Cotton Waste in San Pedro, Colombia. Revista Salud Pública, 12, 982-989.
- Mitra, P., Ghosh, S., Zang, S., Sonneborn, D., Hertz-Picciotto, I., Trnovec, T., Palkovicova, L., Sovcikova, E., Ghimbovschi, S., Hoffman, E. and Dutta, S. (2012) Analysis of the Toxicogenomic Effects of Exposure to Persistent Organic Pollutants (POPs) in Slovakian Girls: Correlations between Gene Expression and Disease Risk. Environment International, 39, 188-199. http://dx.doi.org/10.1016/j.envint.2011.09.003
- International Agency for Research on Cancer. IARC. (2001) Monographs on the Evaluation of Carcinogenic Risks to Human. Vol. 79, IARC, Lyon. http://monographs.iarc.fr/ENG/Monographs/vol79/mono79.pdf
- Mahalingaiah, S., Missmer S. A., Maity, A., Williams, P., Meeker, J., Berry, K., Ehrlich, S., Perry, M., Cramer, D. and Hauser, R. (2012) Association of Hexachlorobenzene (HCB), Dichlorodiphenyltrichloroethane (DDT), and Dichlorodiphenyldichloroethylene (DDE) with in Vitro Fertilization (IVF) Outcomes. Environmental Health Perspectives, 120, 316-320. http://dx.doi.org/10.1289/ehp.1103696
- Dar, S., Das, S., Ramachandran, G., Bhattacharya, S., Mustafa, M., Banerjee, B. and Verma. P. (2012) Alterations in T-Lymphocyte Sub-Set Profiles and Cytokine Secretion by PBMC of Systemic Lupus Erythematosus Patients upon in Vitro Exposure to Organochlorine Pesticides. Journal of Immunotoxicology, 9, 85-95. http://dx.doi.org/10.3109/1547691X.2011.642103
- Chow, W., Chan, K. and Chan, M. (2013) Toxicity Assessment and Vitellogenin Expression in Zebrafish (Danio rerio) Embryos and Larvae Acutely Exposed to Bisphenol A, Endosulfan, Heptachlor, Methoxychlor and Tetrabromobisphenol A. Journal of Applied Toxicology, 33, 670-678. http://dx.doi.org/10.1002/jat.2723
- Codex Alimentarius (2012) Residuos de Plaguicidas en Alimentos y Piensos. Clasificación del Codex de Alimentos y Piensos. Sección 2. 2nd Edition, Período. http://www.codexalimentarius.org/normas-oficiales/lmr-de-plaguicidas/es/
- Codex Alimentarius Commission (2011) Procedural Manual. 20th Edition, Joint FAO/WHO Food Standards Programme. ftp://ftp.fao.org/codex/publications/ProcManuals/Manual_20e.pdf
- UNICEF, CDN (1990) Convención Para los Derechos del Niño. http://www.unicef.org/argentina/spanish/ar_insumos_MNcdn.pdf
- Norma Oficial Mexicana. NOM-131-SSA1-2012, Productos y Servicios. Fórmulas Para Lactantes, de Continuación y Para Necesidades Especiales de Nutrición. Alimentos y Bebidas no Alcohólicas para Lactantes y Niños de Corta Edad. Disposiciones y Especificaciones Sanitarias y Nutrimentales. Etiquetado y Métodos de Prueba (10 Septiembre 2012).
- Waliszewski, S., Sanchez, K., Caba, M., Saldariaga, H., Meza, E., Zepeda, R., Valencia, R. and Infanzon, R. (2012) Organochlorine Pesticide Levels in Female Adipose Tissue from Puebla, Mexico. Bulletin of Environmental Contamination and Toxicology, 88, 296-301. http://dx.doi.org/10.1007/s00128-011-0438-1
- Izquierdo, P., Allara, M., Torres, G., Garcia, A., and Piñero, M. (2004) Organo-Chlorine Pesticide Residues in Infant Formulas. Revista Científica, FCV-LUZ, 14, 147-152.
- Piñero, M., Izquierdo, P., Allara, M., and García, A. (2007) Organochlorine Pesticide Residues in 4 Types of Vegetables Oils. Archivos Latinoamericanos de Nutrición, 57, 397-401.
- Frank, R., Smith, E. H., Holdrinet, M. and McWade, J. (1975) Organochlorine Insecticides and Pollutants in the Milk Supply of the Southern Region of Ontario, Canada. Journal of Milk Food Technology, 38, 65-72.
- International Dairy Federation (1991) Milk and Milk Products. Recommended Methods for Determination of Organochlorine Compounds (Pesticides) FIL-IDF Standard. No. 75C/1991.
- Pinto, M., Vega, S. and Pérez, N. (1996) Métodos de Análisis de la Leche y Derivados. Garantía de Calidad. 1st Edition, Ediciones Universidad Austral de Chile, Valdivia, 531 p.
- Picó, Y., Viana, E., Font, G. and Manes, J. (1995) Determination of Organochlorine Pesticide Content and Human Milk and Infant Formulas Using Solid Phase Extraction and Capillary Gas Chromatography. Journal of Agricultural and Food Chemistry, 43, 1610-1615. http://dx.doi.org/10.1021/jf00054a036
- Raghavan, A.K., Raghavan, K., Khanum, F., Shivanna, N. and Singh, B. (2008) Effect of Sea Buckthorn Leaves Based Herbal Formulation on Hexachlorocyclohexane-Induced Oxidative Stress in Rats. Journal of Dietary Supplements, 5, 33-46. http://dx.doi.org/10.1080/19390210802329022
- Srivastava, M., Abhilash, P. and Singh, N. (2011) Remediation of Lindane Using Engineered Nanoparticles. Journal of Biomedical Nanotechnology, 7, 172-174. http://dx.doi.org/10.1166/jbn.2011.1255
- Prado, G. (2012) Posibles Acciones Tóxicas del Heptacloro y Epóxido de Heptacloro Vinculadas al Estrés Oxidativo. En Fierro, F.F. and Vergara, O.M., Eds., Impacto de la Biología Molecular y Las Nuevas Tecnologías en el Conocimiento de la Función Celular y sus Aplicaciones, 1a Edition, UAM-X, Ciudad de México, 253-266.
- Pavlíková, N., Bláhová, L., Klán, P., Bathula, S., Sklenár, V., Giesy, J. and Bláha, L. (2012) Enantioselective Effects of Alpha-Hexachlorocyclohexane (HCH) Isomers on Androgen Receptor Activity in Vitro. Chemosphere, 86, 65-69. http://dx.doi.org/10.1016/j.chemosphere.2011.08.052
- Iamborko, N. and Pindrus, A. (2011) Toxic and Mutagenic Influence of Hexachlorocyclohexane and Its Microbial Destruction Products on Soil Microbial Cenosis. Mikrobiolohichnyi Zhurnal, 73, 50-57.
- Faure, M, San Miguel, A., Ravanel, P. and Raveton, M. (2012) Concentration Responses to Organochlorines in Phragmites australis. Environmental Pollution, 164, 188-194. http://dx.doi.org/10.1016/j.envpol.2012.01.040
NOTES

*Corresponding author.


