Food and Nutrition Sciences
Vol.5 No.2(2014), Article ID:42188,5 pages DOI:10.4236/fns.2014.52028
Preliminary Evaluations of a Complex Amino Acid Supplement, Fatigue RevivaTM, to Reduce Fatigue in a Group of Professional Male Athletes and a Group of Males Recruited from the General Public
School of Environmental and Life Sciences, University of Newcastle, Newcastle, Australia.
Email: hugh.dunstan@newcastle.edu.au
Received October 16th, 2013; revised November 16th, 2013; accepted November 23rd, 2013
ABSTRACT
Fatigue Reviva™ was developed to provide a source of amino acids for rapid uptake by the body while avoiding the need for digestion of proteins. Complex amino acid formulations have significant palatability issues and thus new products require significant development and testing. An initial pilot study with 18 professional male athletes and 21 males recruited from the general public was undertaken to evaluate product palatability and tolerance over a 30 day period. This investigation found that Fatigue Reviva™ was well tolerated in terms of palatability and usage across 39 participants with only two of the 39 subjects reporting an issue with taste and five reporting an issue with flatulence. The professional athlete cohort reported a significantly lower level of fatigue compared with the general public group prior to commencement of supplementation. The general public group reported a significant reduction in fatigue following the use of Fatigue Reviva™ over the 30-day period. Compliance was extremely poor amongst the professional athlete group and as a result, changes in fatigue could not be statistically assessed for this group over the study period. Preliminary assessment of the product indicated that it has the potential to significantly reduce fatigue. Minor modifications to the product were identified for future development.
Keywords:Fatigue; Amino Acid; Dietary Supplement
1. Introduction
An amino acid based nutritional supplement Fatigue RevivaTM has been developed to counteract the symptoms associated with sub-health. Sub-health is a condition described as falling between “health” and “illness” that is associated with fatigue, loss of vitality, sleeping problems and increased incidence of viral infections [1]. Conditions characterised by fatigue such as cancer-related fatigue and chronic fatigue syndrome have previously been found to be associated with altered amino acid homeostasis by our research team [2,3]. It has been proposed that in sub-health, deficits in amino acids may be explained by the presence of a chronic catabolic state with resultant cellular malnutrition [1]. The project’s goal was to determine whether supplementation with Fatigue RevivaTM would assist in counteracting symptoms of sub-health by addressing possible deficiencies in amino acids. Essential amino acids must be obtained in the diet as the human body is unable to synthesise them. However, it has been suggested that some amino acids may become “conditionally” essential if the body cannot produce them in sufficient amounts to meet physiological demands under certain conditions of physical activity or pathogenic challenge [4]. The supplement contained both essential and potential “conditionally essential” amino acids. Unlike protein supplements, amino acids can be easily absorbed after ingestion without the requirement for digestion and thus becoming rapidly available for utilisation. The supplement Fatigue RevivaTM was produced under the restrictions of the Foods Standards Code (FSANZ) [5], the NSW Food Act 2003 [6] and the NSW Food Regulation 2010 [7]. The dosages were governed by the NSW Food Authority restrictions.
Fatigue RevivaTM has been produced as a sachet of powder which is combined with water just prior to consumption. The development of a nutritional support formulation containing complex combinations of amino acids involves addressing significant issues relating to solubility and palatability. Therefore, this initial pilot study focused on an intensive evaluation of small numbers of volunteers aiming at investigating palatability and compliance issues with taking Fatigue RevivaTM. The project incorporated two participant groups, a group of males recruited from the general public and a group of professional athletes who experienced fatigue following regular extensive training regimes. The results have provided important information confirming product palatability and usage potential which was necessary before planning more comprehensive placebo-controlled trials.
2. Materials and Methods
2.1. Study Design
The current project was designed to evaluate the palatability and tolerability of the amino acid based supplement Fatigue RevivaTM in relation to a potential reduction in fatigue in a group of otherwise healthy men. Due to the preliminary nature of the study, the study was not placebo-controlled. It was envisaged that if evidence was found to support the efficacy of the supplement, a future placebo-based trial would be carried out. The current participant groups were part of a larger ongoing study from which sub-sets of professional athletes and members of the general public were drawn. In this investigation, data were collected from a team of professional athletes training regularly under tightly controlled regimes as well as a group of males recruited from the general public for comparison. All participants were required to provide informed written consent before taking part in the study. The project was approved by the University of Newcastle Human Research Ethics Committee (H-2010-1313) and was registered with the Australian New Zealand Clinical Trials Registry (ACTRN12611000403932). General public participants comprised adult males (18 years and older) who had reported symptoms of sub-health including fatigue, but who were otherwise healthy. Potential participants reporting a current diagnosis with any significant medical or psychological condition were excluded from the study.
2.2. Treatment and Measures
Each participant was instructed to take 20 g of the amino acid based dietary supplement, Fatigue RevivaTM, dissolved in 100 ml of water daily for 30 days. The commercially available nutritional supplement comprised a proprietary blend amino acid complex, containing both essential and non-essential amino acids, carbohydrates (including fructooligosaccharide, FOS) and vitamins and minerals. Comparisons were then made between preand post-supplement levels of fatigue using the Chalder fatigue scale [8] (11-items, Likert scored).
At the conclusion of the trial period, using a self-report questionnaire, participants were asked to indicate whether they had taken the supplement for the full 30 days. Only those participants who indicated they had complied with this requirement were included in a final small subgroup analysis in which athlete and general public participants were also age-matched. Participants were asked to indicate whether they would continue to take the supplement if given the opportunity. Finally, participants were also asked in an open-ended question to comment on their experience of using the supplement.
2.3. Statistical Analysis
Comparisons were made between the preand post-supplement levels of fatigue of both the larger cohort and of the age-matched subgroups. The current study was performed as part of an initial phase of product assessment and it was therefore not considered appropriate to include a placebo group at this stage. Open-ended questions regarding supplement experience were assessed using NVivo 9 (QSR International Pty Ltd.) for qualitative data. Other statistical analyses were performed using Statistica™ release 7.0 (Statsoft Inc., Tulsa, USA). Chalder fatigue scale scores were analysed using Mann-Whitney U test. Levels of statistical significance were set at P < 0.05.
3. Results and Discussion
Data were initially analysed for a group of 18 professional athletes and 21 general public recruits. The general public group had a large variance in age and had a significantly higher mean age compared with the professional athlete group (Table 1). These group datasets were used for an initial appraisal of self-reported fatigue responses before and after a 30 day period of Fatigue Reviva™ supplementation. The groups were then assessed for compliance with the daily intake of the Fatigue Reviva™ and it was revealed that the professional athletes had an extremely poor compliance rate where only four of the 18 athletes indicated that they took the product on a daily basis for the full 30 days. In contrast, 20 of the 21
Statistical test: Mann-Whitney U test and *ANOVA, P < 0.05; NS = nonsignificant.
the general public group members indicated that they took the supplement for 30 days. An age-matched general public dataset with full compliance was then constructed from the general public group for comparison with a compliant athlete sub-group. The age characteristics and BMI data for the groups have been summarized in Table1
Participants were provided with a self-report questionnaire and were required to answer a number of questions at the end of the 30 day trial period regarding their experience of using the supplement. When asked to indicate whether they would continue to take the supplement if given the opportunity 62% of the participants (n = 39) indicated in the affirmative. Participants were asked to describe their experience of using the supplement and also whether they felt the supplement had improved their health. Nine participants (one athlete and eight members of the public) provided answers indicating (without prompts) that they felt the supplement either provided them with more energy or reduced fatigue. Regarding palatability, a number of participants commented on the taste and solubility of the supplement when asked to describe their experience of using the supplement. Two participants made negative comments about the taste of the supplement, two participants were unhappy with the product’s level of solubility and another subject found the product hard to drink initially. Four participants reported that they enjoyed the taste and another found the supplement easy to drink. These responses and the relatively low number of negative responses to this question from the whole cohort were interpreted to imply that the product was well received and generally deemed palatable. In regards to the tolerance of the supplement, six members of the general public cohort and four members of the athlete group attributed gastrointestinal tract (GIT) symptoms to the supplement. Of the GIT symptoms reported, five participants complained of flatulence. The supplement contained the pre-biotic FOS which has previously been found to result in flatulence and discomfort, although at much higher doses of FOS than found in the current supplement [9]. The inclusion of FOS in the formulation was important to achieve palatability without a direct contribution to sugar loading. Fructooligosaccharide is not directly absorbed by the body, but may potentially promote the growth of beneficial gut bacteria such as lactobacilli and bifidobacteria [10]. An alternative supplement formulation containing reduced levels of FOS for those individuals likely to be GIT-sensitive to FOS may address this potential problem. From the comments provided following supplementation it appears that in general, the supplement was well tolerated and palatable.
The symptoms of fatigue were assessed using the Chalder fatigue scale [8] where lower scores reflect lower levels of fatigue in individuals. The mean total fatigue scores produced in the current study were in agreement with previous results recorded for the general community [11] as opposed to the scores associated with conditions such as chronic fatigue syndrome. Prior to supplementation, the general public group (n = 21) had a mean total fatigue score of 13.3 ± 1.0 (mean ± SEM), which was significantly higher than that obtained for the professional athletes (n = 18) at 9.6 ± 1.1 (P < 0.02) (Figure 1). Following supplementation with Fatigue Reviva™, the general public group reported a significant reduction in total fatigue with a score of 9.6 ± 0.8 (P < 0.004). This reduced the reported level of total fatigue in the general public group to the level of fatigue reported by the professional athlete group prior to supplementation. The professional athlete group also reported a reduction in total fatigue following supplementation to 8.4 ± 1.0, but this reduction was not statistically significant.
The question arises as to why a significant response was not seen in the professional athlete group when such an effect was apparent in the general public group. The athletes were likely to have completed significantly higher exercise loads, which in turn would have increased the demand for protein turnover in the recovery and repair of muscles. It was possible that the dosage of the supplement was insufficient to meet demand under these conditions. Further research is required to confirm and address this issue. Following on from this interpretation, it should be noted that the extremely poor levels of compliance in taking the Fatigue Reviva™ by the professional athlete group may have resulted in not meeting
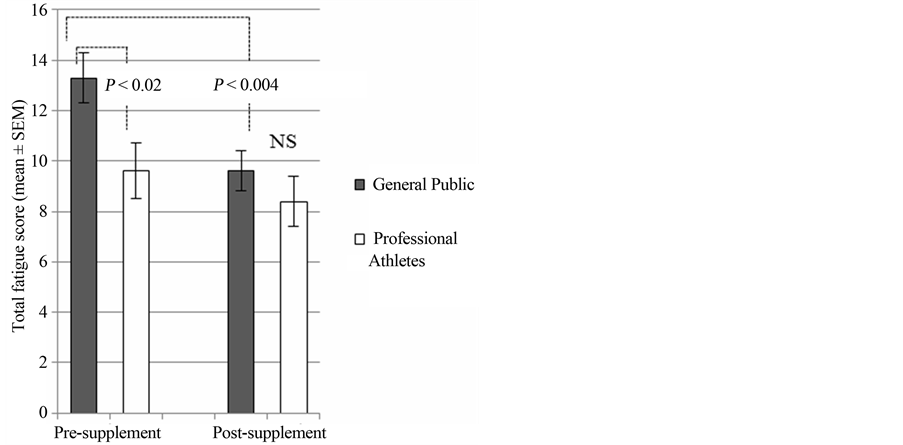
Figure 1. Chalder total fatigue scores: comparison between professional athlete group and general public group preand post-supplement including both compliant and noncompliant participants. Statistical test: Mann-Whitney U test; P < 0.05; NS = non-significant. Sample size: n = 18 and 21 for the professional athletes and general public males respectively.
their amino acid demands generated from the exercise and training regime. The results from the general public group of males were consistent with the hypothesis that Fatigue RevivaTM could reduce fatigue symptoms of sub-health in a group of otherwise healthy men.
In an endeavor to explore the performance of the supplement in individuals that were fully compliant, four members of the professional athlete group were selected on the basis that they reported full compliance regarding taking the supplement on a daily basis for 30 days. An age-matched group (n = 6) from the general public was selected with equivalent full compliance for all participants under the age of 29 years. Due to the small sample sizes, meaningful statistical evaluations were not possible. Nevertheless, comparison of preand post-supplementation responses for the total group (12.5 ± 1.3 and 10.0 ± 0.7, preand post-supplement, mean ± SEM), professional athletes (11.5 ± 1.3 and 9.5 ± 0.9) and general public group (13.2 ± 2.0 and 10.3 ± 1.1) show consistent reductions in total fatigue post-supplementation although the reductions were not statistically significant.
The professional athlete group recorded lower levels of fatigue both before and after supplementation compared with that of the general public group. The four professional athletes in the compliant group had presupplement levels of total fatigue (11.5 ± 1.3) higher than the mean for the whole group of professional athletes (9.6 ± 1.1, n = 18). These recruits may have been more motivated to comply with the supplementation regime because their higher reported baseline levels of fatigue were closer to the general public fatigue scores. Three of the four athletes and four of the six general public males indicated they would continue using the supplement if given the opportunity.
Since the amino acids were provided in a free, unbound form, there was no requirement for digestion allowing the amino acids to be quickly absorbed, transported to muscle tissue and directly utilized. In humans, amino acids have been demonstrated to provide improved muscle recovery following exercise [12]. During endurance exercise it is clear that a net loss of protein occurs and that recovery involves an elevation of protein synthesis that requires delivery of amino acids via ingestion [13]. Oral administration of essential amino acids, following resistance exercise, has been shown to result in net muscle protein synthesis in humans [14] and amino acid supplementation has been shown to reduce muscle damage following exercise [15].
Based upon amino acid profiling, our research team has previously demonstrated the existence of a range of subgroups that display differing phenotypic amino acid homeostasis [4]. It has been proposed that certain subgroups may have higher dietary requirements for particular amino acids, which may infer that these individuals are more prone to deficiencies. Susceptibility to environmental challenges could lead to sub-health if amino acid requirements cannot be fulfilled through diet, or in the case of non-essential amino acids, though de novo synthesis. In the current study the varying levels of fatigue remaining after amino acid supplementation may be explained by different individual amino acid requirements.
4. Conclusion
This preliminary study found that the Fatigue Reviva™ was well tolerated in terms of palatability and usage across 39 participants. The group of professional athletes reported significantly lower levels of fatigue compared with the general public group prior to commencement of the supplementation trial. Compliance was extremely poor amongst the professional athletes making it impossible to assess whether significant alterations in fatigue occurred over the trial period for this group. The general public cohort however, showed a significant reduction in fatigue following the use of Fatigue Reviva™ over a 30-day period. The Fatigue Reviva™ should be tested in a double-blinded placebo based cross-over trial.
Acknowledgements
This work was funded by a research contract from TOP Nutrition Pty Ltd. The product being assessed, Fatigue Reviva™, was donated by TOP Nutrition Pty Ltd. Marcus Crompton is thanked for processing and analysis of samples.
REFERENCES
- T. Roberts, “Sub-Health: The Chronic Catabolic State and the Possible Role of Chronic Infection,” Australian Universities International Alumni Convention, 2007. http://wenku.baidu.com/view/dc0ec9ef0975f46527d3e19a.html
- R. H. Dunstan, D. L. Sparkes, M. M. Macdonald, T. K. Roberts, C. Wratten, M. B. Kumar, S. Baines, J. W. Denham, S. A. Gallagher and T. Rothkirch, “Altered Amino Acid Homeostasis and the Development of Fatigue by Breast Cancer Radiotherapy Patients: A Pilot Study,” Clinical Biochemistry, Vol. 44, No. 2-3, 2011, pp. 208- 215. http://dx.doi.org/10.1016/j.clinbiochem.2010.10.002
- S. N. Niblett, K. E. King, R. H. Dunstan, P. Clifton-Bligh, L. A. Hoskin, T. K. Roberts, G. R. Fulcher, N. R. McGregor, H. L. Butt, I. Klineberg and T. B. Rothkirch, “Hematologic and Urinary Excretion Anomalies in Patients with Chronic Fatigue Syndrome,” Experimental Biology and Medicine, Vol. 232, No. 8, 2007, pp. 1041-1049. http://dx.doi.org/10.3181/0702-RM-44
- R. H. Dunstan, N. R. McGregor, H. L. Butt, T. K. Roberts, I. J. Klineberg, S. H. Niblett, T. Rothkirch and I. Buttfield, “Characterization of Differential Amino Acid Homeostasis amongst Population Subgroups: A Basis for Determining Specific Amino Acid Requirements,” Journal of Nutritional and Environmental Medicine, Vol. 10, No. 3, 2000, pp. 211-223. http://dx.doi.org/10.1080/13590840050134881
- Australian Government, “Australian New Zealand Food Standards Codes,” 2013. http://www.comlaw.gov.au/Search/Australia%20New%20Zealand%20Food%20Standards
- New South Wales Government, “Food Act 2003 No 43,” 2013. http://www.legislation.nsw.gov.au/viewtop/inforce/act+43+2003+FIRST+0+N/
- New South Wales Government, “Food Regulation 2010,” 2013. http://www.legislation.nsw.gov.au/viewtop/inforce/subordleg+250+2010+cd+0+N
- T. Chalder, G. Berelowitz, T. Pawlikowska, L. Watts, S. Wessely, D. Wright and E. P. Wallace, “Development of a Fatigue Scale,” Journal of Psychosomatic Research, Vol. 37, No. 2, 1993, pp. 147-153. http://dx.doi.org/10.1016/0022-3999(93)90081-P
- S. J. M. Ten Bruggencate, I. M. J. Bovee-Oudenhoven, M. L. G. Lettink-Wissink, M. B. Katan and R. van der Meer, “Dietary Fructooligosaccharides Affect Intestinal Barrier Function in Healthy Men,” Journal of Nutrition, Vol. 136, No. 8, 2006, pp. 70-74.
- C. C. Roy, C. L. Kien, L. Bouthillier and E. Levy, “ShortChain Fatty Acids: Ready for Prime Time?” Nutrition in Clinical Practice, Vol. 21, No. 4, 2006, pp. 351-366. http://dx.doi.org/10.1177/0115426506021004351
- M. Cella and T. Chalder, “Measuring Fatigue in Clinical and Community Settings,” Journal of Psychosomatic Research, Vol. 69, No. 1, 2010, pp. 17-22. http://dx.doi.org/10.1016/j.jpsychores.2009.10.007
- J. D. Buckley, R. L. Thomson, A. M. Coates, P. R. C. Howe, M. O. DeNichilo and M. K. Rowney, “Supplementation with a Whey Protein Hydrolysate Enhances Recovery of Muscle Force-Generating Capacity Following Eccentric Exercise,” Journal of Science and Medicine in Sport, Vol. 13, No. 1, 2010, pp. 178-181. http://dx.doi.org/10.1016/j.jsams.2008.06.007
- J. L. Bowtell, “Protein and Amino Acid Requirements for Athletes,” In: D. MacLaren, Ed., Nutrition and Sport, Elsevier, Edinburgh, 2007, pp. 93-118. http://dx.doi.org/10.1016/B978-0-443-10341-4.50008-4
- K. D. Tipton, A. A. Ferrando, S. M. Phillips, D. Doyle Jr, and R. R. Wolfe, “Postexercise Net Protein Synthesis in Human Muscle from Orally Administered Amino Acids,” American Journal of Physiology-Endocrinology and Metabolism, Vol. 276, 1999, pp. E628-E634.
- M. Ohtani, M. Sugita and K. Maruyama, “Amino Acid Mixture Improves Training Efficiency in Athletes,” Journal of Nutrition, Vol. 136, No. 2, 2006, pp. 538S-543S.


