Food and Nutrition Sciences
Vol. 3 No. 7 (2012) , Article ID: 20499 , 6 pages DOI:10.4236/fns.2012.37131
Antioxidant Activity of Pomegranate (Punica granatum L.) Fruit Peels
![]()
1Department of Food Science & Technology, Faculty of Agriculture, Sana’a University, Sana’a, Yemen; 2Department of Food & Nutrition Sciences, College of Agricultural and Food Sciences, King Faisal University, Al-Ahsa, KSA.
Email: *zoreky@yahoo.com
Received May 1st, 2012; revised June 2nd, 2012; accepted June 9th, 2012
Keywords: Punica granatum; Phenolic Compounds; DPPH Scavenging; Reducing Power
ABSTRACT
The antioxidant activity of pomegranate fruit peels was evaluated using in vitro tests. 80% methanolic extracts (ME) of peels had higher yield (45.4%) and total phenolics (27.4%) than water (WE) or ether extracts (EE). The reducing power of ME was more potent (P < 0.05) than either WE or EE. The DPPH radical scavenging activity (%) of ME was stronger than that of α-catechin. Pomegranate peels contained phenolics, exhibited DPPH scavenging activity and reducing power.
1. Introduction
There is increasing epidemiological and pharmacological evidence that plants contain biologically active components (e.g. free radical scavengers) offering health benefits and protection against degenerative diseases. In fact, oxygen radicals and lipid peroxides have been known for their alleged role in the etiology of many in vivo pathological reactions such as aging and cancer. In this regard, epidemiological studies have shown that consumption of fruits and vegetables is inversely associated with morbidity and mortality of cardioand cerebro-vascular diseases and certain types of cancers [1,2]. Unstable reactive oxygen species (ROS) react rapidly and destructively with biomolecules such as protein, lipid, DNA and RNA in the body. Uncontrolled generation of free radicals is associated with lipid and protein peroxidation, resulting in cell structural damage, tissue injury or gene mutation [3,4].
The antioxidants contained in fruits and vegetables, such as ascorbic acid, flavonoids, and tannins, are supposed to play a very important role in the prevention of these diseases [1,5]. In biochemistry and medicine, antioxidants are enzymes or other organic substances, such as vitamin E or β-carotene, that are capable of counteracting the damaging effects of oxidation in animal tissues and food [1]. It was stated that besides their endogenous defenses, the consumption of dietary antioxidants, such as phenolic compounds, play a vital role in protecting against ROS.
Plant phytochemicals (e.g. phenolics) have been associated with health benefits as a result of consumption of higher levels of fruits and vegetables. In fact, phenolic compounds from plants exhibit various physiological properties, such as anti-allergenic, anti-inflammatory, antimicrobial, antioxidant, anti-thrombotic, cardio-protective and vasodilatory effects [1,6,7]. Regarding food safety, one of the major causes of quality deterioration is lipid peroxidation. The oxidative deterioration of fats and oils in food products is responsible for rancidity and off flavors and thus leads to decrease in nutritional quality and safety due to the formation of secondary potentially toxic compounds [3,8,9].
In industrial practices, synthetic antioxidants have been used as food additives for more than fifty years to prevent peroxidation of fats and oils. Butylated-hydroxytoluene (BHT), butylated-hydroxyanisole (BHA), tertbutylhydroquinone (TBHQ) are effective and common antioxidants preventing oxidation and off-flavor development in fats and oils. However, those chemicals are now doubted for their safety and recent literature has expressed safety concerns and health risks associated with their use in food products [5,10].
Therefore, the attention is now increasingly paid to the development and utilization of more effective, natural and non-toxic biologically-active materials including antioxidants from natural sources such as plants [5,10]. Effective antioxidants with less toxicity, especially those originating from natural plants used in folk medicine and food, are attracting the attention of medical and food scientists alike. In this regard, numerous natural medicinal plants have been evaluated for their antioxidant activities and research outcomes have shown that crude extracts or purified constituents from different medicinal plants were more effective antioxidants in vitro than some synthetic antioxidants. Consequently, plants could be potential sources for natural antioxidants and therefore they could be better alternatives for the synthetic ones. Natural ingredients such as antioxidants in food products could have greater application in increasing consumer acceptability and also improve stability of products. Up until now, substantial data are available on antioxidant capabilities of polyphenols from various herbs, such as green tea and rosemary [3,5,7,11]. Additionally, waste products (e.g. fruit peels) from processing of agricultural commodities could offer practical and economic sources of active antioxidants which could replace the synthetic ones [6,7,12]. Recently, the interest in the antioxidant properties of phenolic constituents from pomegranate fruits (i.e., arils and peels) has emerged [2,7,10,13]. The pomegranate plant (Punica granatum L., Punicaceae family) is a shrub and its fruit is a rich source of bioactive phytochemicals such as tannins and other phenolics. It is a native plant to the Mediterranean region and has been used extensively in folk medicine of some countries in Asia and other parts of the world. Interestingly, it was stated that pomegranate peels have been used since antiquity in the Middle East as colorant for textiles because of their high tannin and phenolic contents [2].
Pomegranate fruit products have been used for centuries since ancient civilizations for medicinal purposes. Stomachic, inflammation, fever, bronchitis, diarrhea, dysentery, vaginitis, urinary tract infection, and, among others, malaria have been treated using various parts of pomegranate including fruit peels [2,7,10]. Moreover, increasing numbers of pomegranate supplements and products (functional foods, therapeutic formulae and cosmetics) are also available in markets [3,7,13]. The phenolic constituents, ellagic tannins and ellagic acid, are among the potent antioxidants in peels [2,3,7,10,13,14].
Therefore, the purpose of the present investigation was to evaluate the antioxidant activity of peel extracts using in vitro methods.
2. Materials and Methods
2.1. Chemicals
Gallic acid monohydrate, 2,2-diphenyl-1-picrylhydrazyl (DPPH) and (+)-Catechin monohydrate were from Sigma (USA). Rutin was from Oxford Lab. Reagent (Mumbai, India). Other chemicals were from BDH (UK) and Fisher Scientific (USA).
2.2. Preparation of Plant Extracts
Pomegranate fruits (Yemeni varieties) at the maturity stage (17.5˚ Brix) were manually peeled, washed and air dried prior to extraction with solvents of different polarities [15]. Briefly, finely powdered peels (5 g) were separately blended for 2 min (Waring blender) with 300 ml of 80% methanol, distilled water or diethyl ether. Each mixture was then left, in the dark, at room temperatures for 1 h prior to filtration (Whatman No. 1) and centrifugation (Sorvall RC-5, Dupont, USA) at 8654 g for 10 min at 5˚C. When necessary, extracts of 80% methanol (ME), water (WE) and ether (EE) were kept at –20˚C prior to analysis. Other sets of extracts (ME, WE and EE) were individually concentrated to dryness under reduced pressure at 40˚C to determine yields (%) per original materials.
2.3. Determination of Total Phenolics
Total phenolics of peel extracts (ME, WE and EE) were determined using the method of Singleton and Rossi [16]. 200 µl portions of diluted extracts were introduced into test tubes followed by addition of 1000 µl of Folin-Ciocalteu reagent (1:10). Thirty seconds later and just prior to 8 min, 800 μl of Na2CO3 (7.5%) was added to extracts in tubes. The reaction mixtures were incubated at 24˚C for 1 h prior to recording the absorbance at 765 nm against blank. Total phenolics were calculated from standard gallic acid solutions used under the same conditions, and concentrations were expressed as mg gallic acid equivalents (GAE) per g extract.
2.4. Total Flavonoids of Extracts
The amount of flavonoids in the peel extract with the highest total phenolics (ME) was determined by the AlCl3 [17]. To 1 ml of ME, 1 ml of 2% methanolic AlCl3·6H2O was added. The absorbance was measured 10 min later at 430 nm. The amount of total flavonoids was expressed as mg rutin equivalents (RE) per g ME.
2.5. Determination of Ascorbic Acid
The amount of ascorbic acid (AA) in ME was spectrophotometry determined [18] using standard solutions of AA (Sigma-Aldrich, UK).
2.6. Reducing Power of Extracts
The ferricyanide-ferric chloride method of Oyaizu [19] was adopted for evaluating the reducing power of ME, WE and EE. One ml of each extract at various concentrations (0 - 500 mg/l) was added to a test tube. One ml potassium phosphate buffer (0.2 M, pH 6.6) and freshly prepared potassium ferricyanide (1 ml, 1%) were added to extracts. The mixture was incubated in a water bath (50˚C for 20 min). One ml of trichloroacetic acid (10% TCA) was added to the mixture followed by centrifugation at 5000 g for 5 min. From the upper layer of mixture, 1 ml was taken and mixed with 1 ml distilled water followed by 100 μl of freshly prepared FeCl3 (0.1 %). The absorbance (A) of samples was measured at 700 nm against blank.
2.7. DPPH· Scavenging Activity of Extracts
The DPPH radical-scavenging activity of the most active extract (ME), as revealed by both the total phenolic content and reducing power, was determined following a previously published procedure [9]. WE was used for comparison purposes. Briefly, 100 µl sample at various concentrations (12.5 - 50 ppm in methanol) were distributed into different test tubes and then 3.9 ml of a DPPH solution (25 mg/l methanol) was added to each tube. The mixtures were kept, in the dark, for 30 min at room temperature. Meanwhile, (+) catechin solutions were used as references and under the same conditions. Methanol was used as blank and had no DPPH scavenging activity.
The decrease in DPPH absorbance (A) was measured at 517 nm. The DPPH radical-scavenging activity was calculated using the following formula:
DPPH radical-scavenging activity (%) = [(1 – A1/A0) × 100]; A0 is the absorbance of the control (DPPH solution), and A1 is the absorbance of ME or the reference.
2.8. Statistical Analysis
Data are means of triplicate experiments, each in duplicate. The analysis of variance (ANOVA, one-way) was used to separate means at a 0.05 significant level (SPSS Version 13.0 for Windows, SPSS Inc., Chicago, IL).
3. Results and Discussion
3.1. Yield and Total Phenolics of Peel Extracts
Pomegranate peels were extracted with solvents of a different polarity. Depending on the extraction solvent, % yield significantly (P < 0.05) differed among extracts. As shown in Figure 1, 80% methanol afforded the most concentrated extract (ME) with the highest yield (45.4% ± 5.3%). Based on the solvent system, the percentage yield from pomegranate peels ranged from 1.0% to 29.2% [3,10,14]. It was pointed out that from a practical view point, a suitable extracting procedure should be developed to recover as many antioxidants as possible to produce extracts rich in natural antioxidants for potential application in health-promoting supplements for the food industry [2]. Comparing to water or ether, 80% methanol also offered extracts (ME) with the highest total phenolics (Table 1). Total phenolics averaged 274 ± 17 mg GAE/g, representing about 27% of ME. Reduced amounts
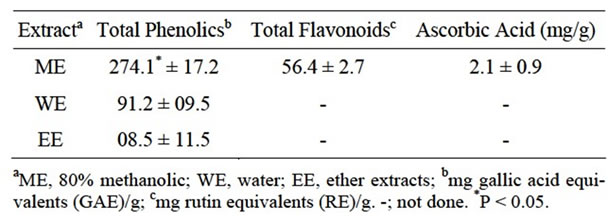
Table 1. Total phenolics, flavonoids and ascorbic acid of pomegranate peel extracts (means ± S.D.)
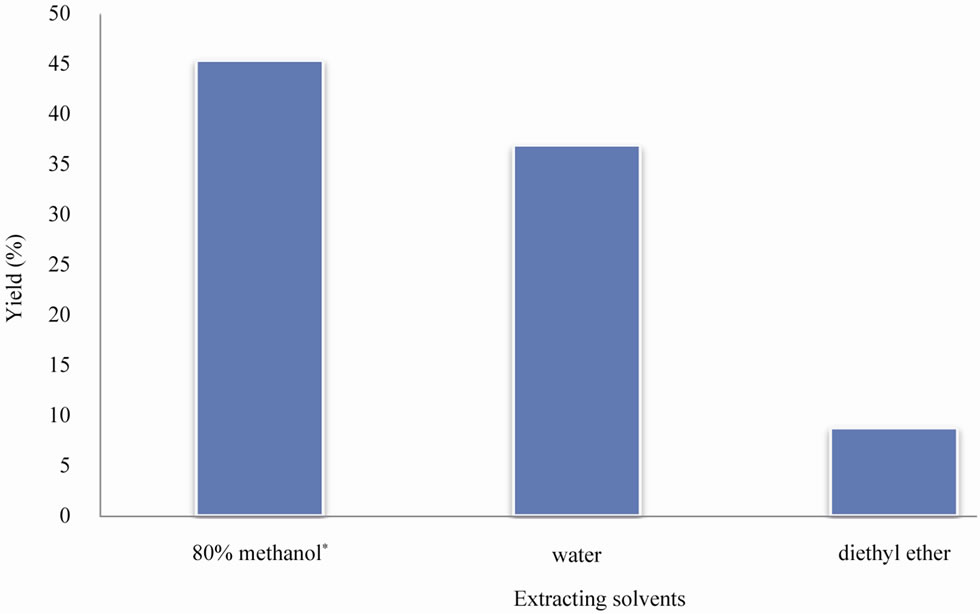
Figure 1. Extractable materials (% yield) from pomegranate fruit peels using different solvents. *P < 0.05.
of phenolics were present in WE or EE, 91 and 8 mg GAE/g, respectively (Table 1). Previous investigations reported that the phenolic concentration varied from 5 to 46% of peel extracts [2,3,14]. The variability in total phenolics among studies could be partially attributed to differences in solvents used for extracting peels, geographic sources of samples and pomegranate varieties. Polyphenols are secondary metabolites which are derivatives of the pentose phosphate, shikimate and phenylpropanoid pathways in plants [6]. They are one of the most occurring phytochemicals in plants including fruits’ pericarp. In addition to their contribution to color and sensory characteristics of fruits and vegetables, phenolics also play a very important role in providing protection against in vivo and in vitro oxidation.
Findings of the present study supported previous investigations regarding better solvents for phenolic extraction from plant materials. In this regard, methanol, water-methanol and acetone afforded better extracts (e.g. potent antioxidants) from pomegranate peels and other plants [3,10,15]. Diethyl ether, a less polar solvent, was not efficient in extracting phenolic constituents from peels (Figure 1). It is documented that phenolics are polar constituents and thus more polar solvents are better extractants of active antioxidants from plants [2,3,10,11].
Madrigal-Carballo et al. [13] mentioned that tannins were the major phenolics in pomegranate peels, which were more readily dissolved in 50% methanol. A mixture of methanol, ethanol, acetone and water was found to be a better extractant of active phenolics from pomegranate peels [2]. The antioxidant extracting efficiency, measured by the ferric reducing antioxidant power (FRAP), was higher in peels extracted with the solvent mixture.
3.2. Total Flavonoids and AA of Peel Extracts
Since the antioxidant activity of plants was well correlated (R2 > 0.87) with total phenolic content, including flavonoids [2,3,11,13], total flavonoids was also determined in ME. ME contained 56.4 mg flavonoids (RE)/g (Table 1). Concentrations of both flavonoids and AA of extracted peels were comparable to those determined by Li et al. [2]. Flavonoids are abundant phenolics in different plant materials. This group of phenolics and AA contribute largely to the antioxidant activity of different fruits and vegetables. However, AA was only present in a small amount (2 mg/g) and thus it was unlikely to substantially contribute to the antioxidant activity of ME.
3.3. Reducing Power of Extracts
Figure 2 illustrates the reducing power of various peel extracts using the ferricyanide reduction method. The increase in A at 700 nm indicated better reducing power of test materials. In a concentration-dependent manner, the reducing power (A) increased as the amount of ME doubled in concentration. While A was 0.4 at 62.5 ppm ME, it increased to 2 at 500 ppm. At the same concentrations, ME exhibited a substantial reducing power compared to WE or EE (Figure 2). Previous studies indicated the methanolic or mixture-solvent extracts of peels had better reducing power than those of water or ethyl acetate [2,3]. The antioxidant activity has been reported to be concomitant with the reducing power of plant materials. The higher reducing power indicated presence of reductones which are able to break free radical chains by donating hydrogen atoms and thus converting them to a more stable non-reactive species [1,3,9]. Since the reducing power was directly related to the phenolic content of peel extracts, WE and/or ME were further used in the remaining experiments.
3.4. DPPH· Scavenging Activity of Extracts
The DPPH· scavenging activity has been widely used to detect antiradical activity of different samples, due to its sensitivity to lower concentrations of active principles from natural sources. The stable radical, DPPH, has a maximum A at 517 nm and could readily undergo scavenging by antioxidants. Higher free radical scavenging activities of samples is indicated by lower A at 517 nm.
ME exhibited a significant scavenging activity (P < 0.05) when compared to either WE or (+)-catechin (Table 2). In fact, the DPPH scavenging activity of ME substantially elevated as the concentration increased from 12.5 to 50 ppm (Table 2). Although they were evaluated at the same concentrations, the DPPH scavenging and the above reducing power data clearly indicated that ME had superior antioxidant activity than WE. The DPPH scavenging activity as a REDOX reaction was clearly related to the total phenolics of peel extracts. The is similar to previous observations [3,20] wherein the DPPH scavenging activity of methanolic extract (rich in phenolics) of pomegranate peels was better than that of water extracts. Except at 50 ppm, the scavenging activity of WE was higher than that of the reference, (+)-catechin (Table 2). In a different assay system, the peroxyl radical-scavenging (ROO•) activity of pomegranate peels was greater than that of fruit pulp [3]. In this regard, it was men-
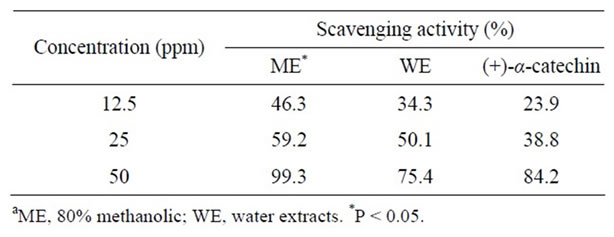
Table 2. DPPH radical scavenging activity (%) of various pomegranate fruit peel extractsa.

Figure 2. Reducing power (A at 700 nm) of 80% methanolic (ME), water (WE) and ether (EE) extracts from pomegranate fruit peels. *P < 0.05.
tioned that peels contained more phenolics than did flesh tissues. Reddy et al. [7] stated that crude pomegranate fruit total tannins and purified constituents (e.g., ellagic acid and punicalagins) possessed antioxidant activity and strongly inhibited ROS generation with IC50 of 0.33 to 11 µg/ml. Depending on the polyphenolic composition, DPPH scavenging activity varied among products from pomegranate fruits, 74 to 4485 µM/g [13]. It was emphasized that the number of phenolic residues and hydroxyl groups substantially affected the DPPH scavenging activity of extracts from grape seeds. Additionally, glycosylated flavonols were less active than the corresponding aglycones [21].
It is worthy to mention that crude extracts of pomegranate peels and purified fractions had no cytotoxicity for HL-60 cells [7].
4. Conclusion
Pomegranate fruit peels are by-products of the food industry. Added-value products could be made from those wastes. Eighty percent methanol was a better solvent for extracting active constituents from peels. Phenolics from peel extracted exhibited a potent antioxidant activity as evaluated by the DPPH scavenging activity and ferric reduction tests. Crude extracts and purified fractions from pomegranate peels could provide health benefits to humans and may be employed in food preservation and pharmaceutical purposes.
REFERENCES
- D. O. Huang and B. R. Prior, “The Chemistry behind Antioxidant Capacity Assays,” Journal of Agriculture and Food Chemistry, Vol. 53, No. 6, 2005, pp. 1841-1856. doi:10.1021/jf030723c
- Y. Li, C. Guo, J. Yang, J. Wei, J. Xu and S. Cheng, “Evaluation of Antioxidant Properties of Pomegranate Peel Extract in Comparison with Pomegranate Pulp Extract,” Food Chemistry, Vol. 96, No. 2, 2006. pp. 254-260. doi:10.1016/j.foodchem.2005.02.033
- P. Negi and J. Jayaprakasha, “Antioxidant and Antibacterial Activities of Punica granatum Peel Extracts,” Journal of Food Science, Vol. 68, No. 4, 2003, pp. 1473-1477. doi:10.1111/j.1365-2621.2003.tb09669.x
- H. Ismail, K. Chan, A. Mariod and M. Ismail, “Phenolic Content and Antioxidant Activity of Cantaloupe (Cucumis melo) Methanolic Extracts,” Food Chemistry, Vol. 119, No. 2, 2010, pp. 643-647. doi:10.1016/j.foodchem.2009.07.023
- J. Han, X. Weng and K. Bi, “Antioxidants from a Chinese Medicinal Herb—Lithospermum erythrorhizon,” Food Chemistry, Vol. 106, No. 1, 2008, pp. 2-10. doi:10.1016/j.foodchem.2007.01.031
- N. Balasundram, K. Sundram and S. Samman, “Phenolic Compounds in Plants and Agri-Industrial By-Products: Antioxidant Activity, Occurrence, and Potential Uses,” Food Chemistry, Vol. 99, No. 1, 2006, pp. 191-203. doi:10.1016/j.foodchem.2005.07.042
- M. Reddy, S. Gupta, M. Jacob, S. Khan and D. Ferreira, “Antioxidant, Antimalarial and Antimicrobial Activities of Tannin-Rich Fractions, Ellagitannins and Phenolic Acids from Punica granatum L.,” Planta Medica, Vol. 73, No. 5, 2007, pp. 461-467. doi:10.1055/s-2007-967167
- C. Jacobsen, M. Let, N. Nielsen and A. Meyer, “Antioxidant Strategies for Preventing Oxidative Flavour Deterioration of Foods Enriched with n-3 Polyunsaturated Lipids: A Comparative Evaluation,” Trends in Food Science & Technology, Vol. 19, No. 2, 2008, pp. 76-93. doi:10.1016/j.tifs.2007.08.001
- Y. Zhang, L. Yang, Y. Zu, X. Chen, F. Wang and F. Liu, “Oxidative Stability of Sunflower Oil Supplemented with Carnosic Acid Compared with Synthetic Antioxidants during Accelerated Storage,” Food Chemistry, Vol. 118, No. 3, 2010, pp. 656-662. doi:10.1016/j.foodchem.2009.05.038
- S. Iqbal, S. Haleem, M. Akhtar, M. Zia-ul-Haq and J. Akbar, “Efficiency of Pomegranate Peel Extracts in Stabilization of Sunflower Oil under Accelerated Conditions,” Food Research International, Vol. 41, No. 2, 2008, pp. 194-200. doi:10.1016/j.foodres.2007.11.005
- N. Alzoreky and K. Nakahara, “Antioxidant Activity of Some Edible Yemeni Plants Evaluated by Ferrylmyoglobin/ABTS·+ Assay,” Food Science and Technology Research, Vol. 7, No. 2, 2001, pp. 141-144. doi:10.3136/fstr.7.141
- A. Moure, J. Cruz, D. Franco, J. Domoanguez, J. Sineiro, H. Domoanguez, M. Nuana and J. Parajoa, “Natural Antioxidants from Residual Sources,” Food Chemistry, Vol. 72, No. 2, 2001, pp. 145-171. doi:10.1016/S0308-8146(00)00223-5
- S. Madrigal-Carballo, G. Rodriguez, C. Krueger, M. Dreher and J. Reed, “Pomegranate (Punica granatum) Supplements: Authenticity, Antioxidant and Polyphenols Composition,” Journal of Functional Foods, Vol. 1, No. 3, 2009, pp. 324-329. doi:10.1016/j.jff.2009.02.005
- K. C. Murthy, G. Jayaprakasha and R. Singh, “Antioxidant Activity of Pomegranate Peel Extracts in Vivo Models,” Journal of Agriculture and Food Chemistry, Vol. 50, No. 17, 2002, pp. 4791-4795. doi:10.1021/jf0255735
- N. Al-Zoreky, “Antimicrobial Activity of Pomegranate (Punica granatum L.) Fruit Peels,” International Journal of Food Micro, Vol. 134, No. 3, 2009, pp. 244-248. doi:10.1016/j.ijfoodmicro.2009.07.002
- V. Singleton and J. Rossi, “Colorimetry of Total Phenolics with Phospho-Molybdicphosphotungstic Acid Reagents,” American Journal of Enology and Viticulture, Vol. 16, 1965, pp. 144-158.
- C. Quettier-Deleu, B. Gressier, J. Vasseur, T. Dine, C. Brunet, M. Luyckx, M. Cazin, J. Cazin, B. Bailleul and F. Trotin, “Phenolic Compounds and Antioxidant Activities of Buckwheat (Fagopyrum esculentum Moench) Hulls and Flour,” Journal of Ethnopharmacology, Vol. 72, No. 1-2, 2000, pp. 35-42. doi:10.1016/S0378-8741(00)00196-3
- G. Haas and W. Dunkley, “Ascorbic Acid and Copper in Linoleate Oxidation Ascorbic Acid and Copper as Oxidation Catalysts,” Journal of Lipid Research, Vol. 10, 1969, pp. 555-567.
- M. Oyaizu, “Studies on Products of Browning Reactions: Antioxidative Activities of Product of Browning Reaction Prepared from Glucosamine,” Japanese Journal of Nutrition, Vol. 44, 1986, pp. 307-315.
- R. Singh, K. C. Murthy and J. Jayaprakasha, “Studies on the Antioxidant Activity of Pomegranate (Punica granatum) Peel and Seed Extracts Using in Vitro Models,” Journal of Agricultural Food Chemistry, Vol. 50, No. 1, 2002, pp. 81-86. doi:10.1021/jf010865b
- M. Pazos, J. Gallardo, J. Torres and I. Medina, “Activity of Grape Polyphenols as Inhibitors of the Oxidation of Fish Lipids and Frozen Fish Muscle,” Food Chemistry, Vol. 92, No. 3, 2005, pp. 547-557. doi:10.1016/j.foodchem.2004.07.036
NOTES
*Corresponding author.

