American Journal of Plant Sciences
Vol.06 No.19(2015), Article ID:61856,7 pages
10.4236/ajps.2015.619307
Isoenzyme Expression in Bean Seed Germination Treated with Thiamethoxam with and without Drought Stress
Arthur Blois Villela1, Andreia da Silva Almeida2, César Iván Suárez Castellanos2, Cristiane Deuner2, Vanessa Nogueira Soares2, Thais Ongaratto de Camargo2, Geri Eduardo Meneghello2, Francisco Amaral Villela2, Lilian Madruga de Tunes2, Paulo Dejalma Zimmer2
1Universidade Federal de Pelotas, Pelotas, Brazil
2PPG em Ciência e Tecnologia de Sementes, Universidade Federal de Pelotas, Pelotas, Brazil

Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).


Received 26 November 2015; accepted 8 December 2015; published 11 December 2015

ABSTRACT
The thiamethoxam acts as enhancer, allowing maximizing the expression of seed vigor. Isozymes are products of gene expression, highly influenced by the environment, because genes that control its expression are manifested in certain stages of development and in specific organs and tissues, or even under the action of certain stimulus. This study evaluated the isoenzyme expression in bean seedlings from seeds treated with thiamethoxam. Bean seeds were used, cultivar Pérola and IAPAR Siriri, submitted or not to drought stress. Seeds were treated at doses of thiamethoxam: 0, 1, 2, 3, 4 and 5 mL∙kg−1 of seed. Isozymes were extracted from seedlings collected five days after sowing. We evaluated the expression of isoenzymes: Glutamate Oxalacetate transaminase (GOT), acid phosphatase (FAC), peroxidase (PO), Esterase (EST), malate dehydrogenase (MDH) and glutamate dehydrogenase (GTDH). Interpretation of the results was based on visual analysis of electrophoresis gels, considering the presence/absence and the intensity of each electrophoretic band. Bean seeds treated with thiamethoxam with doses up 3 mL∙kg−1 of seed are higher germination untreated, even when submitted to drought stress. The expression of isozymes GOT, FAC, PO, EST, MDH and GTDH is not affected significantly in bean seedlings of Pérola and IPR Siriri under different doses of thiamethoxam with and without drought stress.
Keywords:
Phaseolus vulgaris, Enzymes, Germination, Bioactivator

1. Introduction
Insecticides and fungicides are usually evaluated by the efficiency of the control of pests and diseases. However, some pesticides can cause still unknown effects, including changing metabolism and plant morphology [1].
Among the many active ingredients of pesticides available in the market, the thiamethoxam molecule has demonstrated effect on the metabolism, being able to increase the expression of the vigor, increases biomass formation and the photosynthetic rate, and produces seedlings with larger roots. These effects of thiamethoxam were also observed in carrot seedlings [2] and cotton [3], originated from seeds treated with the product.
The thiamethoxam is transported within the plant through its cells, activating several physiological responses, such as expression of some proteins. These proteins interact with several stress defense mechanisms of the plant, allowing more efficiently coping with possible adverse conditions such as drought stress, low pH, high soil salinity, presence of free radicals by temperature stress, toxic effects of high aluminum levels, injuries caused by pests, wind, hail, viruses attack and nutrient deficiency. It has fitotonic effect, by providing faster development of the plant and thus allowing better express their vigor. In soybean, an increase in the vigor, productivity, leaf area and root length, plant stand with emergence more uniform and greater uniformity in the initial development were observed [4].
Used as treatment of soybean seeds, the thiamethoxam accelerates the germination, induces further development of the embryonic axis minimizing negative effects in situations of aluminum presence, salinity and drought stress. It accelerates germination, to stimulate peroxidase activity, preventing oxidative stress. Furthermore, the product reduces the time to crop establishment in the field, reducing the negative effects of weed competition or essential nutrients in the soil [5].
In the field, plants are usually exposed to various stresses factors that can reduce your ability to express and achieve the genetic potential productivity. Plants treated with thiamethoxam are more tolerant of these stress factors and, therefore, can develop more vigorously in suboptimal conditions, increasing the chances to achieve their genetic potential productivity [6].
In seed technology, a major difficulty in evaluating the physiologic quality of seeds refers to the methodology to perform the tests. Thus, new test has been developed to obtain effective results faster in an attempt to predict the quality of lots arriving at the laboratory. Electrophoresis of enzymes that allows evaluating not only to changes in physiological seed quality, but also on genetic and biochemical regulations [7].
Isozymes are products of gene expression and consequently, highly influenced by the environment, because genes that control its expression are manifested in certain stages of development and in specific organs and tissues, or under a given stimulus [8] [9].
Among the enzymes involved in metabolic pathways in plants, stand out peroxidase to act in protection against biotic and abiotic stresses. The peroxidase is an enzyme found routinely in vegetables, which have varied duties from growth, cell differentiation and development [10]. Moreover, it is a general indicator of the physiological activities of the plant, since its performance is influenced by external conditions [11].
The esterase isoenzyme system consists of a complex and heterogeneous group of enzymes reactive with a wide range of specific substrates [12]. Variants of these proteins found in plants, for example, are usually monomeric or dimeric [13], with high variability [14]. As a result, Esterase is one of the most polymorphic isozyme systems in plants [13].
Glutamate oxaloacetate transaminase (GOT), participating in the synthesis of new amino acids, is a source of energy to the embryo developing. According to [15] and [16], this enzyme participates in the process of degradation and amino acid synthesis presenting important role in seed germination.
The enzyme acid phosphatase (FAC) has been widely characterized in plants, and their activity increases in those with phosphorus deficiency.
Bean seeds treated with thiamethoxam have better physiological quality compared to untreated seeds. The aim of this study was to evaluate the isoenzyme expression in bean seedlings from seeds treated with thiamethoxam and submitted or not to drought stress.
2. Material and Methods
To develop the work, bean seeds of cultivars Pérola and IAPAR Sirirr were used. Seeds were subjected to treatment with commercial product containing 350 grams of active ingredient of thiamethoxam per liter of product. Six doses were used: 0 (untreated seeds); 1 mL∙kg−1 of seed; 2 mL∙kg−1 of seed; 3 mL∙kg−1 of seed, 4 mL∙kg−1 of seed and 5 mL∙kg−1 of seed.
The mixture (product + distilled water) was applied with a pipette in the bottom of a clear plastic bag and spread through the walls thereof to a height of 15 cm. The spray volume used was 0.6 L 100 kg−1 of seeds.
Treatments with thiamethoxam were applied to seeds submitted or not to drought stress by −0.4 MPa of hydric potential, constituting two experiments, one for each cultivar. Each experiment had two factors (five doses × two levels of drought stress). Drought stress was obtained with solutions of polyethylene glycol (PEG 6000). The calculation of the amount of solute was carried out according [17] . For the evaluation of seeds germination under drought stress was used polyethylene glycol solutions.
The physiological seed quality was determined by germination test, it was conducted with four replicates of 50 seeds in germitestTM paper rolls, per experimental unit, moistened with solutions in the ratio of 2.5 times the dry weight of the paper. The rolls were incubated in germination chamber at 25˚C. The evaluations were performed in the fifth and ninth day after sowing, computing the normal seedlings percentages [18] .
The plant material (seedlings) used for protein extraction were collected on the fifth day after sowing.
Ten seedlings randomly collected were macerated in a mortar over ice cubes. The Extraction and enzyme patterns were obtained by [12] . Gels were developed for system of glutamate oxaloacetate transaminase enzyme (GOT), acid phosphatase (FAC), peroxidase (PO) and esterase (EST as [12] and [19] . The electrophoresis gels were fixed in a solution of 5:5:1 in distilled water: methanol: acetic acid.
Interpretation of the results was based on visual analysis of electrophoresis gels, considering the presence or absence and the intensity of each of the electrophoretic bands, comparing different doses and cultivars used.
The experiment was conducted in a completely randomized design with two factors 6 × 2 (six doses of thiamethoxam with drought stress and without drought stress) for each cultivar, with four replications. Statistical analysis was performed using the statistical program Winstat [20] , and the average subjected to analysis of variance and polynomial regression for the germination variable.
3. Results and Discussion
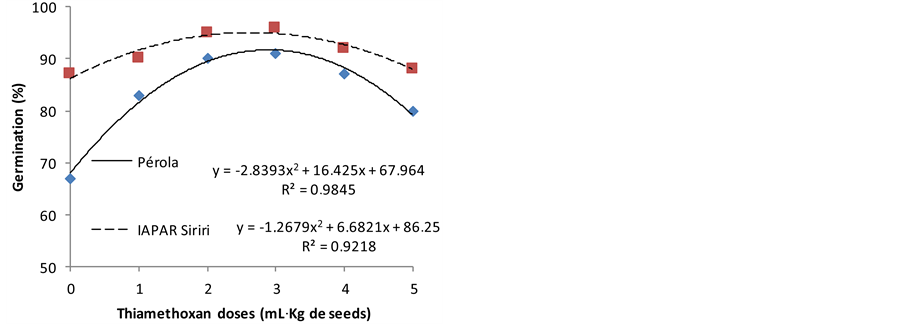
The germination of the bean seeds, cultivars Pérola and IAPAR Siriri are shown in the Figure 1. This figure shows that from the zero dose, the germination has increased quadratic trend, reaching until doses of 2 to 3 mL of product kg−1 of seed for the two cultivars. After this point, the germination decreases with the increase in dose of the product.
Note that using doses of 2 to 3 mL of product kg−1 of seed, there was an increase in the expression of germination of 16 to 27 percentage points from the zero dose in the seeds not subject to drought stress, and an increase of 10 to16 percentage points when the stress took place. In soybeans seeds, [5] also observed that thiamethoxam accelerate germination, induce further growth of the embryonic axis and that results are corroborate by [2] for carrot seeds, by [21] to rice seeds and by [3] in cotton seeds.
Drought stress reduces seed germination. It is observed that seeds of the cultivar Pérola had 80% germination, whereas the minimum required by law, and even doses 2 and 3 mL of product kg−1 of seed stimulate the germi-

Figure 1. Germination (%) of bean seeds, cultivars Pérola and IAPAR Siriri exposed to different doses of thiamethoxam without (a) and with (b) drought stress.
nation process, allowing seeds reach higher values. This effect was also observed in soybeans seeds, which were treated and submitted to germination under water stress. It accelerated the germination and reduced the time of the immersion process [5] .
In the analysis of the six enzymatic systems was possible to see that there was significant variation in the intensity of isozyme expression according to the cultivar and dose of thiamethoxam used, but there were no differences with and without drought stress.
Analyzing the electrophoretic profiles of glutamate oxaloacetate transaminase system (GOT; Figure 2) shows that the two cultivars had the enzyme expression on two alleles and the product thiamethoxam not favored the expression of the enzyme. This enzyme is responsible for the oxidation of amino acids, providing energy to the Krebs cycle or the reduction ketoglutarate for the synthesis of new amino acids as a source of power to the developing embryo. According to [15] and [16] , this enzyme participates in the process of degradation and synthesis of amino acids having an important role in the seed germination. The function of this enzyme is directly involved in the nitrogen metabolism, it is possible that changes happens in the synthesis and degradation of amino acids during the germination process. The GOT enzyme has a fundamental stake in protein metabolism, not only during germination, but also throughout the plant life cycle.
The enzyme acid phosphatase (Figure 3) also expressed in two alleles, eiin the cultivar Pérola and IAPAR Siriri without drought stress (Figure 3(a)) and there was a greater expression in zero dose (without product), 1 and 2 mL∙kg−1 of seeds. To submit the seeds to drought stress (Figure 3(b)), the enzyme expression occurred at all doses and in one allele. This enzyme participates in reactions of esters hydrolysis and can cause peroxidation of membrane of phospholipids. According to [22] that enzyme is also involved in maintaining cellular phosphate and its activity can affect the seed phosphate metabolism, like the levels of ATP and nucleotides.
The results indicate that the peroxidase (PO; Figure 4) bands formation was more intense in the zero dose, 1, 2 and 5 mL∙kg−1 of seed in the cultivars Pérola and IAPAR Siriri. The enzyme was expressed with greater intensity at doses zero, 1 and 2 mL∙kg−1 of seeds without drought stress (Figure 4(a)).
In the cultivar Pérola with drought stress (Figure 4(b)), there was bands in zero dose, 1, 2, 3 and 5 mL∙kg−1 of seed. In the cultivar IAPAR Siriri, the expression was more pronounced at doses 1 and 4 mL∙kg−1 of seeds with drought stress. The enzyme was expressed in more than one allele when the seeds were subjected to stress, indicating the direct effect that stress causes on the metabolism which enzyme is involved. The peroxidase is an enzyme involved in several reactions, polysaccharides links, healing, injury defense of pathogens, regulation and cell elongation.
In esterase enzyme (Figure 5), there was the expression of two alleles in both situations with and without drought stress (Figure 5(a) and Figure 5(b)), being possible to observe the presence of bands with intensity in
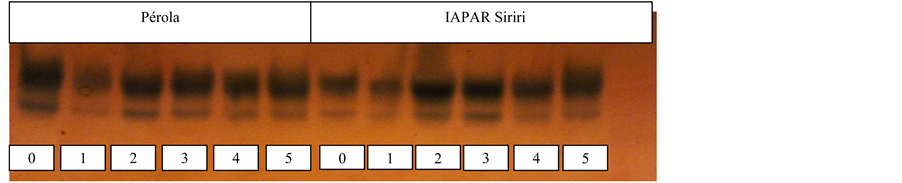

Figure 2. Electrophoretic pattern obtained with glutamate oxaloacetate transaminase isoenzyme system in seedlings of bean cultivars, (a) no stress (b) with water stress, depending on the dose of thiamethoxam.


Figure 3. Electrophoretic pattern of acid phosphatase in seedlings bean, cultivars Pérola and IAPAR Siriri exposed to different doses of thiamethoxam without (a) and with (b) drought stress. Doses in mL of thiamethoxam per kg of seeds.


Figure 4. Electrophoretic pattern of peroxidase isoenzyme system in seedlings bean, cultivars Pérola and IAPAR Siriri exposed to different doses of thiamethoxam without (a) and with (b) drought stress. Doses in mL of thiamethoxam per kg of seeds.


Figure 5. Electrophoretic pattern of Esterase isoenzyme system in seedlings bean, cultivars Pérola and IAPAR Siriri exposed to different doses of thiamethoxam without (a) and with (b) drought stress. Doses in mL of thiamethoxam per kg of seeds.
both cultivars without drought stress (Figure 5(a)). When subjected to stress, in the cultivar Pérola, the expression occurred in doses 3, 4 and 5 mL∙kg−1 of seed. In the cultivar IAPAR Siriri, more intense bands were formed in the zero dose and 5 mL∙kg−1 of seeds. This enzyme consists of a complex and heterogeneous group of enzymes reactive with a wide range of specific substrates. Padilha et al. (2001) observed an increase in intensity of bands for the most drastic stress on corn seeds and [23] and [24] in soybean and [16] in barley, found that increase of the number of bands of this enzyme. Changes in the enzyme esterase patterns indicate the occurrence of deteriorative events, which can contribute to reduction in seed germination, because this enzyme is involved in ester hydrolysis reactions, being directly linked to lipid metabolism.
The results obtained in this study indicate that, depending on the enzyme system used, there is a differentiation of proteins. As a result, the joint analysis of various enzymatic systems is recommended for allowing verify changes that occur in the seeds submitted to any treatment during its development.
4. Conclusions
Bean seeds treated with thiamethoxam with doses up to 3 mL∙kg−1 of seeds present germination superior to untreated seeds, even when subjected to drought stress.
The expression of the enzymatic systems GOT, FAC, PO and EST does not show variability in bean seedlings of cultivars Pérola and IAPAR Siriri treated with different doses of thiamethoxam and subjected or not to drought stress.
Cite this paper
Arthur BloisVillela,Andreia da SilvaAlmeida,César Iván SuárezCastellanos,CristianeDeuner,Vanessa NogueiraSoares,Thais Ongaratto deCamargo,Geri EduardoMeneghello,Francisco AmaralVillela,Lilian Madruga deTunes,Paulo DejalmaZimmer, (2015) Isoenzyme Expression in Bean Seed Germination Treated with Thiamethoxam with and without Drought Stress. American Journal of Plant Sciences,06,3157-3163. doi: 10.4236/ajps.2015.619307
References
- 1. Castro, P.R.C. and Pereira, M.A. (2008) Bioativadores na Agricultura. In: Gazzoni, D.L., Ed., Tiametoxam: Uma Revolução na Agricultura Brasileira, 118-126.
- 2. Almeida, A.S., Tillmann, M.A.A., Villela, F.A. and Pinho, M.S. (2009) Bioativador no Desempenho Fisiológico de Sementes de Cenoura. Revista Brasileira de Sementes, 31, 87-95.
http://dx.doi.org/10.1590/S0101-31222009000300010 - 3. Lauxen, L.R., Villela, F.A. and Soares, R.C. (2010) Desempenho Fisiológico de Sementes de Algodão Tratadas com Tiametoxam. Revista Brasileira de Sementes, 32, 61-68.
http://dx.doi.org/10.1590/S0101-31222010000300007 - 4. Castro, P.R.C., Pitelli, A.M.C.M., Peres, L.E.P. and Aramaki, P.H. (2007) Análise da atividade reguladora de cresci-mento vegetal de tiametoxam através de biotestes. Publicatio UEPG (Ponta Grossa), 13, 25-29.
- 5. Cataneo, A.C. (2008) Ação do Tiametoxam (Thiametoxam) sobre a germinação de sementes de soja (Glicine Max. L.): Enzimas envolvidas na mobilização de reservas e na proteção contra situação de estresse (deficiência hídrica, salinidade e presença de alumínio). In: Gazzoni, D.L., Ed., Tiametoxam: Uma revolução na agricultura brasileira, 123-192.
- 6. Tavares, S., Castro, P.R.C., Ribeiro, R.V. and Aramaki, P.H. (2008) Avaliação dos efeitos fisiológicos de tiametoxam no tratamento de sementes de soja. In: Gazzoni, D.L., Ed., Tiametoxam: Uma revolução na agricultura brasileira, 193-204.
- 7. ISTA (1992) Handbook of Variety Testing: Electrophoresis Testing. International Seed Testing Association, Zurique, Suíça, 44 p.
- 8. Ramirez, H., Calderon, A. and Rocca, W. (1991) Técnicas moleculares para evaluar y mejorar el germoplasma vegetal. In: Rocca, W. and Mroginski, L., Eds., Cultivo de Tejidos en la Agricultura: Fundamentos y aplicaciones, CIAT, Cali, 825-856.
- 9. Malone, G., Zimmer, P.D., Meneghello, G.E., da Silva de Castro, M.A. and Peske, S.T. (2007) Expressão diferencial de isoenzimas durante o processo de Germinação de sementes de arroz em grandes profundidades de semeadura. Revista Brasileira de Sementes, 29, 61-67.
http://dx.doi.org/10.1590/S0101-31222007000100009 - 10. Aouad, A., Baaziz, M. and Mergoum, M. (1998) Quantitative Aspects of Peroxidases in Some Moroccan Cereal Varieties. Actes des Premieres Journees de l’Arbre, Laboratorie de Biochimie Amélioration des Plantes, Université Cadi Ayyad, Morrocco, 7 p.
- 11. Buxton, D.R., Shibles, R., Forsberg, R.A., Blad, B.L., Assay, R.H., Paulsen, G.M. and Wilson, R.F. (1993) Crop Science Society of America, Inc. 677. International Crop Science, Madison, 757 p.
- 12. Scandálios, J.G. (1969) Genetic Control os Multiple Molecular Forms of Enzimes in Plants: A Review. Biochemical Genetics, 3, 37-79.
http://dx.doi.org/10.1007/BF00485973 - 13. Weeden, N.F. and Wendel, J.F. (1990) Genetics of Plant Isozymes. In: Soltis, D.E. and Soltis, P.S., Eds., Isozymes in Plant Biology, Chapman and Hall, London, 46-72.
- 14. Gillespie, J.H. and Langley, C.H. (1974) A General Model to Account for Enzyme Variation in Natural Populations. Genetics, 76, 837-887.
- 15. Brandão-Junior, D.S., Carvalho, M.L.M. and Vieira, M.G.C. (1999) Variações eletroforéticas de proteínas e isoen-zimas relativas à deterioração de sementes de milho envelhecidas artificialmente. Revista Brasileira de Sementes, 21, 114-120.
http://dx.doi.org/10.17801/0101-3122/rbs.v21n1p114-121 - 16. Tunes, L.M., Pedroso, D.C., Meneghello, G.E., da Silva de Castro, M.A., Barros, A.C.S.A., Badinelli, P.G. and Muniz, M.F.B. (2010) Perfil enzimático em sementes de cevada em resposta a diferentes concentrações salinas. Interciencia, 35, 369-373.
- 17. Villela, F.A., Doni-Filho, L. and Sequeira, E.L. (1991) Tabela de potencial osmótico em função da concentração de polietileno glicol 6000 e da temperatura. Pesquisa Agropecuária Brasileira, 26, 1957-1968.
- 18. ISTA (1992) Handbook of Variety Testing: Electrophoresis Testing. International Seed Testing Association, Zurique, 44 p.
- 19. Alfenas, A.C. (1998) Eletroforeses de isoenzimas e proteínas afins: Fundamentos e aplicações em plantas e micror-ganismos. UFV, Viçosa, 574 p.
- 20. Almeida, A.S., Carvalho, I., Deuner, C., Villela, F.A. and Tillmann, M.A.A. (2011) Bioativador no desempenho fisio-lógico de sementes de arroz (Oryza sativa L.). Revista Brasileira de Sementes, 33, 501-510.
http://dx.doi.org/10.1590/S0101-31222011000300013 - 21. Camargo, M.L.P., Mori, E.S., DE Mello, E.J., Oda, S. and Lima, G.P. (2000) Atividade enzimática de sementes envel-hecidas artificial e naturalmente. Ciência Florestal, 10, 113-122.
- 22. Chauhan, K.P.S. (1985) Electrophoretic Variations of Proteins and Enzymes in Relation to Seed Quality. Seed Science and Technology, 13, 629-641.
- 23. Bock, F.L. (1999) Resposta a nível molecular do envelhecimento artificial, natural e pré-condicionamento de sementes e soja. Pelotas. 27f. Dissertação (Mestrado em Ciência e Tecnologia de Sementes), Universidade Federal de Pelotas, Pelotas.
- 24. Bock, F.L. (1999) Resposta a nível molecular do envelhecimento artificial, natural e pré-condicionamento de sementes e soja. Dissertação (Mestrado em Ciência e Tecnologia de Sementes), Universidade Federal de Pelotas, Pelotas.