American Journal of Plant Sciences
Vol.06 No.07(2015), Article ID:55897,15 pages
10.4236/ajps.2015.67105
Review: Nitrogen Utilization Features in Cotton Crop
Nour Ali1,2
1Department of Crop Cultivation and Farmings, Huazhong Agricultural University, Wuhan, China
2Department of Field Crop Sciences, Agricultural College, Damascus University, Damascus, Syria
Email: nanaoana77@yahoo.com
Copyright © 2015 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 7 March 2015; accepted 19 April 2015; published 23 April 2015
ABSTRACT
Cotton is one of the important cash crops and a fiber crop most widely grown and the highest yielding as well. Cotton fiber is woven to be the fabrics commonly used in our daily life due to its excellent performance and great production in the world, especially in China. To access to such high quantities must ensure the requirements of materials such as nutrients for plant growth and take care of the smallest details to make the production cost less, to improve the utilization efficiency, such as nitrogen (N) fertilizer. Hence, N fertilization studies are not only about the dosages, timing and ratio, but also the uptake processes by the plant, N effect on cotton yield and its formation, as well as the movement and metabolism within the plant. As economic and ecological issues are concerned, economizing N fertilization is paid more and more attention. Many approaches have been done and suggested in order to improve NUE like combine the plant sensing techniques and precision application. Simulations and recent field trials demonstrate that site-specific nitrogen management helped reduce technological constraints to higher AE achievement, profit and more sustainable N management. Therefore, improving nitrogen use efficiency (NUE) is one of the key points to ensure cotton production development sustainable. In this review, we try to highlight the accomplishments of N effect on cotton growth and yield, NUE and factors related to NUE in cotton production based on the current knowledge, and from our viewpoint we propose some possible approaches to improve NUE through N managements in terms of application splits, rates, and timing.
Keywords:
Cotton, Growth, Yield, Nitrogen, Nitrogen Use Efficiency (NUE)

1. Introduction
Cotton (Gossypium L.) is one of the most sources of textile natural fiber in the world [1] , grown worldwide in the temperate and tropical regions in more than 50 countries [2] . There are two cotton species grown for a good quality fiber, G. hirsutum and G. barbadense, accounting for 90% and 5%, respectively, of the world cotton fiber production [3] .
N fertilization has contributed greatly to cotton production, because it plays a pivotal role to cotton growth and yield. N is always the most applied fertilizer regarding the others in cotton cultivation, used in different amounts, applied in many ways and various timing, but what is common in all those methods and dates is the high cost of the fertilizer itself [4] with rapidly high consumption. The world fertilizer consumption was increased over 2 percent yearly from 2008 to 2012, equivalent to an increment of 19.3 million fertilizer nutrient tones [5] . Therefore, it is essential to manage the N application in way that does not cause symptoms resulted from either deficiency or excessive [6] - [8] . In addition, N management faces many problems especially the cost, and the negative impacts on both environmental and economic sides (USDA, 2008), and on plant growth as well [9] - [12] due to its global rapid increase of consumption.
In conventional farming of cotton, N fertilizer was applied in three splits, pre-plant, first blooming and peak blooming application [13] , but N rates to achieve the highest yield were different based on regions [14] , soil types [15] , and cultivars.
In this concept, many studies aimed to reduce the N application splits and N amount without yield reduction, while other researches discussed N allocation, metabolic pathways and enzymes involved in plant and the changes in N forms in the soil.
2. Cotton Growth as Affected by N
2.1. Cotton Vegetative Growth and N
High nitrogen supply had obvious inhibitory effects,manifested as the decreasing of root dry weight and root length and root surface, as well nitrogen supply had no effect on average root length density [16] . Low nitrogen and high nitrogen had promoting effect on the increasing of root length and root surface area, With nitrogen supp1y increased,root surface area of whole soil layers increased. Tang et al., (2012) [17] reported that the greater efficiency of late-season vs. pre-plant fertilizer N may explained by more extensive root system, and better photosynthetic supply, and suggested that a higher ratio of N for PBA (0% PPA + 40% FBA+ 60% PBA treatment) creates a mutually-beneficial relationship between above- and below-ground growth for nutrient absorption to sustain vigorous plant leaf function and to produce more photo-products for a longer period of root absorption.
Nitrogen is an essential element for canopy area development and photosynthesis [18] . Providing the right N amount during the plant growth will provide healthy leaves with the photosynthetic capacity needed to support the growing of the reproductive components [19] - [23] . In other hand an inadequate N supply will slow or stop leaf development. N deficient supply will produce few leaves on the plant resulting in reduction in photosynthesis and formation of sugars for boll set and maturation, and plant height [22] [24] . But the primary detriment is when surplus N encourages excessive vegetative growth, resulting in poor boll set caused by vegetative shading and insect attractiveness, and lodging, late maturity and difficulty in defoliation [25] - [27] . Ramzan et al. (2013) [28] found that application of 250 kg∙ha−1 nitrogen fertilizer produced the tallest plants (84.88 cm). Hallikeri et al. (2010) [29] showed that cotton height was significantly affected by application of N levels, as taller plants were observed with N up to 120 kg∙ha−1. Kumbhar et al. (2008) [30] who found that subsequent increase in N levels from 50 to 150 kg∙ha−1 resulted in proportionate increase in the plant height in which taller plants were recorded in the treatments where 150 kg∙N∙ha−1. Soomro and Waring (1987) [31] reported significant differences in plant height with different levels of N application, as a result of inter-node elongation rather than increases in main stem node number. Ramzan et al. (2013) [28] indicated that maximum number of node (15.29) was recorded N rate of 225 kg∙ha−1 which were statistically different with 0 and 75 kg∙ha−1 nitrogen fertilizer rates, as well Clawson, et al. (2006) [32] also reported that main stem nodes∙plant−1 was significantly increased with higher N rates. In the other hand Emara and El-Gammaal (2012) [24] showed that the inter-node length average values were insignificantly affected by nitrogen fertilizer levels 0 - 60 kg/fed (0 - 140 kg∙ha−1).
Higher fertilizer rates provided the plants with more nutrients, which improved No. of monopodial/plant Kumbhar et al. (2008) [30] found and that maximum number of monopodial branches was observed in experimental units where 150 kg∙N∙ha−1 was applied. Oosterhuis et al. (1983) [33] found a distinct differences in plant dry weight and leaf area at 120 kg∙N∙ha−1 treatment, in which maximum dry matter production was 114 g∙plant−1 and leaf area index had attained a maximum of 3.7 after 115 days from sowing. Dong et al. (2012) [34] reported that high N rate increased the biological yield as 10% relative to low rate, as well increased the net photosynthetic rate (Pn) by 3 and 13%, and Bt protein content by 16.4% and 42.1% relative to moderate (225 kg∙ha−1) and high (300 kg∙ha−1) N rates.
Nevertheless Howard et al. (2001) [35] reported that higher doses of N lead to more vegetative growth and causes delay in maturity and ultimately reduction in the crop yield. Dong et al. (2012) [34] showed that increasing N rate reduced earliness. Setatou and Simonis (1996) [36] found that N fertilization caused a delay in the maturity of cotton plants, ranging from 0.2 to 2.5 days in comparison to the control in most of the experiments. However, this delay is important only when the harvesting conditions are unfavorable.
2.2. Cotton Reproductive Growth and N
At the reproductive growth, failure to ensure the right amount of N during the square development period will eventually decrease the yield [37] [38] . In the flowering period, increasing the soil-N rate has positive effect on the N content in the flower, but it does not lead to increase its size [39] . N has indirect effects on boll and seed formation. Increasing of the canopy’s size leads to increase the total photosynthesis area and form bigger source for nutrients that increase the size and weight of the bolls and seed [40] - [43] . Ramzan et al. (2013) [28] reported that the maximum number of sympodial branches plant−1 (7.51) was recorded in 225 kg∙ha−1 nitrogen fertilizer and control (0 kg∙ha−1), while Kumbhar et al. (2008) [30] reported that the highest number was observed at 150 kg∙N∙ha−1 treatment. Emara and El-Gammaal (2012) [30] found that increasing nitrogen rate up to 60 kg N/fed (140 kg∙ha−1) increased No. of sympodial/plant. This may be resulted from increasing plant growth on the expense of fruiting characters which induced at higher nitrogen rate.
The flower represents the central point of the cotton plant’s reproductive growth, it has been speculated that the effect of imbalances in plant nutrition in the flower may be the cause of lowered yield and unpredictable year-to-year yield variability. Oosterhuis et al., in experiment conducted in 2007 [44] showed that increasing soil-applied N rate (from 0 to 135 kg∙ha−1) resulted in a corresponding increase in the N content of cotton flowers, but it did not appear to be associated with flower size, and suggested more investigations in the effect of soil-N status on the N content and polyamine concentration of cotton flowers in relation to successful fertilization and seed set. Yang et al., (2011) [13] reported that N ratio (0% PPA + 60% PBA) promoted an earlier squaring and flowering but delayed the opening stage, so prolonged the boll setting and filling period. In contrast, N ratio (40% PPA + 20% PBA) delayed the squaring and flowering, but promoted an earlier opening stage, so shortened the boll setting and filling period. Zhao et al., (2012) [45] concluded that as the flowering date is delay, fiber length and strength first increased and then decreased, fiber maturity and micronaire decreased N application increase boll number per plant and slight increase boll weight by the application at various rates and split ratio, Sawan et al. 2006 [46] explained the increasing in boll weight may be due to increase in N rate and increases mineral uptake, photosynthetic assimilation and accumulation in sinks in the other hand high N rates have been linked to increased incidence of boll rot which has been attributed to additional vegetative growth that traps moisture underneath the canopy and increases infection [47] [48] . Dong et al. (2012) [34] reported that increasing the N rate to 225 or 300 kg∙ha−1 improved the boll size by 4.8% or 3.5% relative to the low N rate. Saleem et al. (2010) [43] showed that the maximum boll weight was (2.9 g) at the rate of 120 kg∙ha−1. Rashidi et al. (2011) [42] reported that 200 kg∙ha−1 N application rate resulted significant increased in the boll number (19.8), and boll weight (6.26 g) compared to low rates.
2.3. Cotton Yield and N
Nitrogen fertilization had significant impacts on plant growth, lint yields and fiber quality [49] . Maximum yield often requires application of large amounts of N fertilizers, which would increase the risk of N leaching, as well increase the production cost unless it managed in way that insure the present of nitrogen in the right amount at the right time.
N application usually split into a pre-plant application (PPA), a first bloom application (FBA), and a peak bloom application (PBA), at percentages of 30%, 40%, and 30% respectively. However, Yang et al. (2011) [13] reported that the split ratio of 0% at pre-plant, 40% at first bloom, and 60% at peak bloom harvested the highest biomass and yield when N was applied at 225 kg∙ha−1. Hallikeri et al. (2010) [29] indicated that application of N with recommended method of three splits (2267 kg∙ha−1) was similar to application seven times (2237 kg∙ha−1). The results of two-year field trial conducted by Tang et al. (2012) [17] showed that treatment of 225 kg∙ha−1 N split in to 0% PPA + 40% FBA+ 60% PBA had produced the highest yield and accumulated the most N.
Results were reported by Mullins et al. (2003) [50] and Knowles et al. (1999) [51] in which split application of fertilizer produced the same yield as a one-time application, yet with a lower cost. Yang et al. (2012) [52] reported that application of 120 kg/ha N once at first bloom produced similar cotton yield (1328 kg∙ha−1) to the conventional fertilization treatment twice (FII) at pre-plant and first bloom with 50% each, or thrice (FIII) at pre-plant (30%), first bloom (40%), and peak bloom (30%).
Many studies indicated that higher crop yield and quality were always associated with the N application. Nelson (1949) [53] and Sawan et al. (1988) [54] showed that cotton yield and cottonseed N concentration increased linearly with increasing N fertilizer rates. Saleem et al. (2010) [43] showed that the maximum seed cotton yield per plant, and seed cotton yield kg/ha were (69.3 g, 3002.4 kg) respectively recorded when nitrogen was applied at the rate of 120 kg∙ha−1. Rashidi et al. (2011) [42] reported that 200 kg∙ha−1 N application rate resulted significant increased seed cotton weight of boll (4.49 g), seed cotton yield (4363 kg∙ha−1) and lint yield (1659 kg∙ha−1). Ramzan et al. (2013) [28] found that application of 225 kg∙ha−1 nitrogen fertilizer produced the maximum yield (1731.06 kg), the highest number of bolls per plant (5.61), while the maximum boll weight observed in 150 kg∙N∙ha−1 (9.18 g).
Setatou and Simonis (1996) [36] studied the effect of time of nitrogen application on cotton yield as they applied different nitrogen rate at three different time before sowing, when the plants had 3 - 4 leaves and 20 days later, they found that the split application of N fertilizers, compared to a single pre-sowing application did not differ, however split applications increased yields in some experiments, but only at high rates of applied N fertilizer.
Cotton fiber quality is mainly influenced by genotype of the cultivars but agronomic practices and environmental conditions are the secondary factors influencing fiber quality [55] . Increases in lint length from N fertilizer treatments had been reported [56] . However, other investigators stated reductions in both fiber length and strength [57] or no effect of fertilizers upon fiber properties [58] .
Hussain et al. (2000) [59] reported that nitrogen rate had no effect on fiber uniformity. Saleem et al., 2010 [43] indicated that different fiber quality characteristics except ginning out turn remained unaffected by nitrogen application rates.
3. Nitrogen Use Efficiency (NUE)
Each year more great amounts of N fertilizer are applied to croplands and cost billion. While the estimated efficiency of applied N ranges from about 30% to about 70% [60] which means the rest is lost. So in order to increase the crop production and conserve energy as well reduce the costs and minimize the potential for adverse effect on the environment. It is critical to maximize the efficiency of plant use of the applied N [61] .
Nitrogen use efficiency (NUE) is so complicated to define, due to different terms [62] - [64] giving it different contexts. According to Moll et al. (1982) [64] there are two primary components of N use efficiency: the efficiency of absorption (uptake), and the efficiency with which the N absorbed is utilized to produce grain.
The efficiency of uptake is known as (NUpE) which expressed as total nitrogen accumulation divided by root dry weight and is equivalent to physiological efficiency (PE).
In agronomic terms, the physiological efficiency (PE) or Uptake efficiency (NUpE) used to provide a reflection of the overall efficiency of up taking the applied N and use it in producing grain yield. It can be viewed as the efficiency of crops in using N to increase the yield. It shows the plant ability to transform the N into economic yield.
For the efficient in utilization Siddiqi and Glass (1981) [65] , Gerloff and Gabelman (1983) [66] defined Nitrogen utilization efficiency (NUtE) as total plant dry weight divided by nitrogen concentration. Beside the PE, Dobermann (2007) [67] defined another concept of NUE; the agronomic efficiency (AE) is the increase of yield (kg) to each increasing of applied N (kg). AR reflects the efficiency of the crop in obtaining N-based fertilizer from the soil.
Partial factor productivity (PFP) is defined as the harvested product (kg) per kg of applied N. It is really important to integrate the use efficiency of both indigenous and applied N, is an aggregate efficiency index that includes contributions to crop yield derived from uptake of indigenous soil N, fertilizer N uptake efficiency and the efficiency of converting it into economic yield [67] . As well Xu et al. (2012) [68] defined the apparent nitrogen recovery rate (ANR) o as: the ratio of net increased total N uptake by the plant with and without N fertilization to total amount of fertilizer N, which can be used as index of N uptake capacity. Mosier et al. (2004) [10] described crop removal efficiency as removal of N (REN) in harvested crop as percentage of N applied, it is commonly used to explain NUE. In general, NUE indicates the ratio of N recovered by the crop plant to that applied to the field [26] .
However, to understand NUE and its different concepts, we should get a deep knowledge of the N forms absorbed by the plant in terms of vector transitions, pathways and enzymes participating in the processes of assimilation [69] .
3.1. N Uptake
There are many sources of the N, atmospheric N2 [70] , organic matter dissolution [71] [72] and industrial fertilizers (Table 1).
The source of soil N is derived from the atmosphere, where di-nitrogen (N2) is the predominant gas (79%). N2 is fixed as NH3 by biological process, and then to N-containing organic compounds to be available to all forms of life [73] .
Soil N is comprised of both mineral and organic N forms, in which the organic forms formed almost 80% - 90% of the total [74] . Organic N becomes available to the plant after it is mineralized [75] . Plants uptake N as nitrate (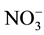 ) as main source, or ammonium (
) as main source, or ammonium ( ) such as in rice paddy fields [76] .
) such as in rice paddy fields [76] .
 and
and 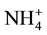 (Nitrate is the dominant form of N acquired by cotton) are both water soluble, and taken by the plant roots from different sources [77] . To be available for plant,
(Nitrate is the dominant form of N acquired by cotton) are both water soluble, and taken by the plant roots from different sources [77] . To be available for plant, 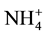 should be transformed into
should be transformed into  through “nitrification” process carried out two reactions by bacterial organisms Nitrosomonas and Nitrobacteria, respectively:
through “nitrification” process carried out two reactions by bacterial organisms Nitrosomonas and Nitrobacteria, respectively:
1) 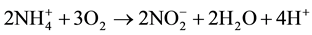 (Then)
(Then)
2) 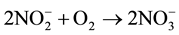
To assimilate nitrate, the plant must first take it up from the soil using transporters located in the plasma membrane of the epidermal and cortical cells of the root. Two types of nitrate transporters, NRT1 and NRT2, have been isolated from higher plants. The NRT1 transports nitrate, peptides and some amino acids, while the NRT2 transports both nitrate and nitrite [78] .
While in the plants that use 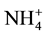 as main N resource, physiological studies showed evidences for the existence of two transport systems for
as main N resource, physiological studies showed evidences for the existence of two transport systems for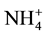 , a high affinity transport system (HATS) and low affinity transport system (LATS) [79] - [81] , those transporters are encoding by the Amt genes [76] [79] .
, a high affinity transport system (HATS) and low affinity transport system (LATS) [79] - [81] , those transporters are encoding by the Amt genes [76] [79] .
3.2. N Assimilation
Usually most of the nitrate absorbed is reduced in the shoots, while a small fraction is done in roots [82] . Nitrate is reduced into ammonia and incorporated into cells by series of assimilatory enzymes, while ammonium is incorporated into amino acids via the glutamine synthetase-glutamate synthase (GS-GOGAT) pathway, nearly all the ammonium incorporated into amino acids at the root itself [83] .
The activity of N assimilation is affected by different factors and can be regulated at different levels, the synthesis of mRNA and protein, and the activity of the enzyme (Table 2).
From Nitrate to Nitrite: After the nitrate transported from the root to the shoot, in the cytoplasm of the young leaves and by the means of NR the converting of 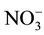 to
to 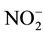 begins, where the NR transfer two electrons from NADPH to the
begins, where the NR transfer two electrons from NADPH to the 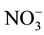 ion and form
ion and form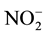 .
.
NR activity is controlled by many factors such as the availability of nitrate, the availability of biosynthetic precursors for N assimilation, and the plant’s need for the products of N assimilation, also the expression of the NR is regulated by nitrate abundance, light levels, circadian cycles, and the phytohormone cytokinins [84] .
From Nitrite to Ammonium: then 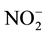 will turn to
will turn to  by NiR which is nuclear encoded enzyme that transport to the stoma of chloroplasts of the green tissues and the plastids of the roots, where it transfer six electrons from reduced ferredoxin (Fd) to
by NiR which is nuclear encoded enzyme that transport to the stoma of chloroplasts of the green tissues and the plastids of the roots, where it transfer six electrons from reduced ferredoxin (Fd) to 

From Ammonium to Amino acids: 


Table 1. Biological and non-biological N sources.
Source: Blackwell Scientific Publications 1998, Food and Agriculture Organization.
Table 2. The N assimilatory enzymes.
Source: (Zheng, 2004; Céline et al., 2010; McAllister et al., 2012).
duced from nitrate [86] . Those two enzymes lead the glutamine synthesis by incorporate 
3.3. N Mobility and Storage

The xylem is the principal pathway for long range transport of N from roots to the leaves and bolls. This physiological tendency of loading NO3-into the xylem and petioles facilitates the petiole 
Plants store N as both a short-term buffer against imbalances between demand and uptake, and a longer-term reserve (in storage organs such as seeds, roots, and tubers, for example) to provide amino acids for biosynthesis when new growth occurs [88] .
N is stored in short term as nitrate in the vacuole, and can accumulated to high concentrations when in plentiful supply, and exported to the cytosol for metabolism when circumstances change. N is also stored in the short to medium term in the form of amino acids, commonly asparagine, which has high N content like glutamine, and moves in the xylem and phloem.
Symptoms of N deficiencies occur initially in the older leaves, due to N translocation from old leaves to new ones. N deficiency most often results in stunted growth, slow growth, and chlorosis, also exhibit a purple appearance on the stems, petioles and underside of leaves from an accumulation of anthocyanin pigments (Figure 1).
3.4. N Losses
The crop use less than half of the fertilizer N applied [89] , while the other half is lost from the system through either denitrification or leaching or ammonia volatilization.
Figure 1. Schematic routes of N uptake from the rhizosphere including the source of fertilizer N to be acquired, mainly in the form of ammonium and nitrate by roots, transportation and assimilation, and remobilization inside the plant. The thicknesses of the arrows schematically represent the relative amounts of N and sugar inside the plant. Abbreviations: AMT, ammonium transporter; AS, asparagine synthetase; Asn, asparagine; Asp, aspartate; GDH, glutamate dehydrogenase; Gln, glutamine; Glu, glutamate; GOGAT, glutamine-2-oxoglutarate aminotransferase; GS, glutamine synthetase; NAC-TF, certain transcription factors belonging to the NAC family; NiR, nitrite reductase; NR, nitrate reductase; NRT, nitrate transporter. Source: Plant N Assimilation and Use Efficiency, Annu. Rev. Plant Biol. 2012.
Denitrification is a biological process of 

As for leaching, 

Winter is the most likely time for leaching to occur due to the heavy rain. Soil characteristics play role in the leaching rate, as soils differ greatly in the extent and manner in which they transmit water [90] . There are also other factors that increase the leaching including weather conditions [91] , irrigation [92] , and fertilizers adding methods [93] .
Ammonia volatilization (


4. Cotton NUE
NUE can help in predicting the quantities of fertilizer to be added to any agricultural system in any area and thus maintain the N balance between inputs and outputs without any losses at either the economic or environmental levels [97] . Crops differ in the value of the NUE, which may be a return of genetic traits [98] - [100] , and the agronomic and environmental conditions.
Due to the escalating N fertilizers costs and the environmental impacts [101] , there were many researches had been done to improve the NUE and understand the factors affect it the most [102] [103] .
4.1. Temperature
It has indirect effects on the NUE. It affects the losing rate of the N through the nitrification process in the soil, which is faster as the soil temperature is more than 4.44˚C depend on the crop type [104] - [108] .
4.2. Soil Characteristics
The soil type has effects on the N present in the soil and the losing rate [109] . The higher cation exchange capacity comes with higher soil buffering capacity and for that the ammonium can be absorbed more and the lose rate will be lower. In the other hand, lower anion capacity can lead to losses in the negative charged molecules like nitrate [110] [111] . The activity of the bacteria which lead the mineralization process depends on the soil pH. The optimum pH to save the N from leaching, nitrification and to be available to the plant is around 7 [112] .
4.3. Humidity and Irrigation Systems
The field capacity and wilting point have effects on NUE, through affecting the leaching of
4.4. Cropping Systems
It is important to use agricultural system that achieves a balance between the input and output of N by a greater uptake and a lower lose from soil [118] .
In many cropping systems, the size of the organic and inorganic N pools has reached steady-state or it is changing very slowly. If the applied N is not taken up by the crop or immobilized in soil organic N pools, it’s vulnerable to be lost through volatilization, denitrification, or leaching.
In systems that hasn’t reach the steady-state, adoption of new management practices can affect the soil carbon (C) balance and the N balance [119] [120] because the soil organic matter is relatively constant. In such cropping systems, the overall NUE of the cropping system must include changes in the size of soil organic and inorganic N pools in addition to the nitrogen recovery efficiency (REN).
4.5. The Carbon-Nitrogen Balance
The link between C and N is critical, unless there is sufficient C available, improving the plants’ ability to take up and utilize N may be compromised, as well, N levels can significantly affect C fixation [120] [121] .
Large quantities of N are stored in photosynthetic proteins such as Rubisco and phosphoenolpyruvate carboxylase (PEPc), so N uptake, assimilation and remobilization is in partly regulated and controlled by photosynthetic rates. Thus to achieve higher NUE, the photosynthetic rates should be higher [119] [120] .
4.6. N Fertilizer Type
There are different types of N fertilizers that can be used to secure the needs of the plant during growth [122] , but it is important to select the appropriate type. Appropriate type reduces the losses arising from volatilization [122] - [124] or denitrification, and as well reduces the financial costs associated with the need of compensate. The common N fertilizers are anhydrous ammonia (82% N), urea (45% - 46% N), ammonium sulfate (21% N) and ammonium nitrate (34% N).
Anhydrous ammonia converts to nitrate N slowly, with the least chance of N loss due to leaching or denitrification; while urea converts to nitrate N fairly fast. Denitrification can be serious on wet or compacted soils; leaching or volatilization can be a problem in coarse soils or no-till situations if the urea is not placed in contact with the soil and it is dry for several days after spreading [125] .
As for ammonium sulfate (21%) is source with little or no surface volatilization loss when applied to most soils. While ammonium nitrate (34%) is 50% ammonium N and 50% nitrate N, when it is added the ammonium N quickly converts to nitrate N. For soil subjected to leaching or denitrification, ammonium nitrate would not be preferred. But for ammonium nitrate, surface application would be a good choice where volatilization of urea is expected.
4.7. N Applied Amounts
Cotton need to accumulate approximately 250 - 300 kg N/ha to achieve maximum yield potential, and it uses less than half of the N applied during that season, obtaining most of their N from soil N rather than applied N, as an average 33% of applied N is recovered [68] , 25% remains in the soil at crop maturity and the remainder (approximately 42%) is lost from the system [17] .
Studies have showed that both N deficiency and excessive use had negative effects on plant growth and the final yield. N deficiency reduces the leaf area and the total biomass, bolls and low fiber quality [126] - [128] , and conversely the excessive use of N leads to excess vegetative growth by creating bigger leaf structures with larger surface areas for the photosynthesizing pigment, and so the energy for reproductive growth is redirected to vegetative proliferation. So plants may not even produce their necessary reproductive organs during the growing season [129] .
Plants cannot absorb the entire excess N in the soil; those extra N levels slowly leach out of the soil through water runoff. As a result, groundwater and drinking water become contaminated from the nitrate levels.
4.8. N Rates
Selecting optimum N rates for cotton production varies by soil type; production, climate, and various other soil and crop management factors [130] [131] . However, the N rate for maximum economic yield [132] depends on the fertilizer N cost and the market price received from the crop [133] .
Due to the chemical changes that influence the N as its mobility, leaching, denitrification and volatilization, it is hard to accurately predict the amount of N, whereas soil should be tested each year for 
4.9. N Timing
Timing of N applications has important effects on NUE. Usually N fertilization added in three different times correspond to the phases of plant growth, pre-plant application, first bloom application and peak bloom application.
The first application added before planting to provide the sufficient time for N to turn into absorbable forms, but this application is subject to higher risks of N losses especially if the temperature is getting lower or due to the heavy rain in the end of autumn [134] , through this period the young seedlings don’t require too much N, and the applied N remains exposed to leaching rains for more than 60 days before demand begins to peak. Heavy applications early in the season can lead to excessive vegetative growth and delayed fruiting.
Second application is about 45 - 50 days after emergence (at the first bloom), nutrient uptake increases rapidly until it reaches a prolonged peak about two weeks after first bloom, when the processes of flower production, boll filling, and boll maturation create a heavy demand for nutrients.
The last application is after two weeks of the first bloom, and N should be provided till the maturity, but too much N late in the season may cause excessive vegetative growth.
Also the soil and petiole N test as well as the other application managements such as N rate, timing, and fertilizer types must all be at concerned to improve the NUE and decreases the cost of production.
5. Approaches for Improving NUE
Increasing NUE and protecting environmental quality are two challenges facing cotton plant nutritionists, and as described earlier, NUE is explained by different complex interdependent parameters that need close monitoring in order to improve it.
NUE is a multi-genic trait, in addition, signaling targets and regulatory elements have recently emerged as prospective candidates for biotechnological interventions, which create various transgenic ways to improve NUE by manipulating those genes and regulatory elements.
Many approaches have been done and suggested in order to improve NUE like combine the plant sensing techniques and precision application. Simulations and recent field trials demonstrate that site-specific nitrogen management helped reduce technological constraints to higher AE achievement, profit and more sustainable N management.
The agronomic managements of the field should be considered as main factors affecting the NUE. In general, it could be said that with increasing applied N lead to decreasing in AE and PE while REN increases, as well the plant density could have effects through the competition between the plant in N up taking and utilization.
These agronomic managements should come along with researches to identify genes that improve the NUE of crop plants, with candidate NUE genes existing in pathways relating to N uptake, assimilation, amino acid biosynthesis, C/N storage and metabolism, signaling and regulation of N metabolism and translocation, remobilization and senescence.
Yield will continue to be the major farm commodity, and N fertilizers will be essential, NUE improvements will be widely heralded, particularly if the price of N fertilizers rises as might be expected in an energy-short future.
Acknowledgements
I would like to thank Prof. Yang for his time to review this paper and enrich it with more valuable information, and his assistance in the English language corrections.
References
- Brubaker, C.L., Bourland, F.M. and Wendel, J.E. (1999) The Origin and Domestication of Cotton. In: Smith, C.W. and Cothren, J.T., Eds., Cotton: Origin, History, Technology, and Production, John Wiley and Sons, Inc., New York, 3-31.
- Smith, W.C. (1999) Production Statistics. In: Smith, W.C. and Cothren, J.T., Eds., Cotton: Origin, History, Technology, and Production, John Wiley and Sons, Inc., New York, 435-449.
- Wu, Z., Soliman, K.M., Zipf, A., Saha, S., Sharma, G.C. and Jenkins, J.N. (2005) Isolation and Characterization of Genes Differentially Expresses in Fiber of Gossypium barbadense L. The Journal of Cotton Science, 9, 166-174.
- Chaudhry, R. (2007) Update on Costs of Producing Cotton in the World. International Cotton Advisory Committee.
- FAO (2011) Current World Fertilizer Trends and Outlook to 2015. Food and Agriculture Organization of The United Nations Rome.
- Cisneros, J.J. and Godfret, L.D. (2003) Midseason Pest Status of the Cotton Aphid (Homoptera: Aphididae) in California Cotton: Is Nitrogen a Key Factor? Environmental Entomology, 95, 501-510.
- Fritschi, F.B., Roberts, B.A., Travis, R.L., Rains, D.W. and Hutmacher, R.B. (2004) Seasonal Nitrogen Concentration, Uptake, and Partitioning Pattern of Irrigated Acala and Pima Cotton as Influenced by Nitrogen Fertility Level. Crop Science, 44, 516-527. http://dx.doi.org/10.2135/cropsci2004.5160
- Reddy, K.R., Koti, S., Davidonis, G.H. and Reddy, V.R. (2004) Interactive Effects of Carbon Dioxide and Nitrogen Nutrition on Cotton Growth, Development, Yield, and Fiber Quality. Agronomy Journal, 96, 1148-1157. http://dx.doi.org/10.2134/agronj2004.1148
- Cassman, K.G., Dobermann, A., Walters, D.T. and Yang, H. (2003) Meeting Cereal Demand While Protecting Natural Resources and Improving Environmental Quality. Annual Review of Environment and Resources, 28, 315-358. http://dx.doi.org/10.1146/annurev.energy.28.040202.122858
- Mosier, A.R., Syers, J.K. and Freney, J.R. (2004) Agriculture and the Nitrogen Cycle. Assessing the Impacts of Fertilizer Use on Food Production and the Environment. Scope-65. Island Press, London.
- Ownsend, A.R., Howarth, R.W., Bazzaz, F.A., Booth, M.S., Cleveland, C.C., Collinge, S.K., et al. (2003) Human Health Effects of a Changing Global Nitrogen Cycle. Frontiers in Ecology and the Environment, 1, 240-246. http://dx.doi.org/10.1890/1540-9295(2003)001[0240:HHEOAC]2.0.CO;2
- Robertson, G.P. and Swinton, S.M. (2005) Reconciling Agricultural Productivity and Environmental Integrity: A Grand Challenge for Agriculture. Frontiers in Ecology and the Environment, 3, 38-46. http://dx.doi.org/10.1890/1540-9295(2005)003[0038:RAPAEI]2.0.CO;2
- Yang, G.Z., Tang, H.Y., Nie, Y.C., Zhang, X.L. (2011) Responses of Cotton Growth, Yield, and Biomass to Nitrogen Split Application Ratio. European Journal of Agronomy, 35, 164-170. http://dx.doi.org/10.1016/j.eja.2011.06.001
- Boquet, D.J. and Breitenbeck, G.A. (2000) Nitrogen Rate Effect on Partitioning of Nitrogen and Dry Matter by Cotton. Crop Science, 40, 1685-1693. http://dx.doi.org/10.2135/cropsci2000.4061685x
- Fritschi, F.B., Roberts, B.A., Travis, R.L., Rains, D.W. and Hutmacher, R.B. (2003) Response of Irrigated Acala and Pima Cotton to Nitrogen Fertilization: Growth, Dry Matter Partitioning, and Yield. Agronomy Journal, 95, 133-146. http://dx.doi.org/10.2134/agronj2003.0133
- Xie, Z.-L., Tian, C.-Y. and Bian, W.-G. (2009) Effects of Water and Nitrogen on Cotton Root Architecture under Film Drip Irrigation. Cotton Science, 21, 508-514. (Abstract in English)
- Tang, H.-Y., Yang, G.-Z., Zhang, X.-L. and Siddique, K. (2012) Improvement of Fertilizer N Recovery by Allocating More N for Later Application in Cotton (Gossypium hirsutum L.). International Journal of Basic & Applied Sciences, 12, 32-37.
- Wadleigh, C.H. (1944) Growth Status of the Cotton Plant as Influenced by the Supply of Nitrogen. Ark. Agr. Expt. Sta. Bul., 446, 138.
- Bondada, B.R. and Oosterhuis, D.M. (2001) Canopy Photosynthesis, Specific Leaf Weight, and Yield Components of Cotton under Varying Nitrogen Supply. Journal of Plant Nutrition, 24, 469-477. http://dx.doi.org/10.1081/PLN-100104973
- Gardner, B.R. and Tucker, T.C. (1967) Nitrogen Effects on Cotton. I. Vegetative and Fruiting Characteristics. II. Soil and Petiole Analysis. Soil Science Society of America Journal, 31, 780-785. http://dx.doi.org/10.2136/sssaj1967.03615995003100060022x
- Wood, C.W., Tracy, P.W., Reeves, D.W. and Edmisten, K.L. (1992) Determination of Cotton Nitrogen Status with a Hand-Held Chlorophyll Meter. Journal of Plant Nutrition, 15, 1435-1448. http://dx.doi.org/10.1080/01904169209364409
- Wullschleger, S.D. and Oosterhuis, D.M. (1990) Canopy Development and Photosynthesis of Cotton as Influenced by Nitrogen Nutrition. Journal of Plant Nutrition, 13, 1141-1154.
- Zhang, W., Wang, Z., Yu, S., Li, S., Cao, L. and Wang, D. (2002) Effect of Nitrogen on Canopy Photosynthesis and Yield Formation in High-Yielding Cotton of Xinjiang. Acta Agronomica Sinica, 28, 789-796. (In Chinese with English Abstract)
- Emara, M.A. and El-Gammaal, A.A. (2012) Effect of Plant Distribution and Nitrogen Fertilizer Levels on New Promising Hybrid Cotton (Giza 89 × Giza 86). Journal of Agricultural Research, Kafrelsheikh University, 38, 54-70.
- Galloway, J.N., Aber, J.D., Erisman, J.W., Seitzinger, S.P., Howarth, R.W., Cowling, E.B. and Cosby, B.J. (2003) The Nitrogen Cascade. Bioscience, 53, 341-356. http://dx.doi.org/10.1641/0006-3568(2003)053[0341:TNC]2.0.CO;2
- Koochaki, A., Rashedmohasel, M.H., Nasiri, M. and Sdrabady, R. (1994) Essential of Crop Physiology of Growth. Astan-e Qods Razavi Publications, Mashhad, 440.
- Walker, R.L, Burns, I.G. and Moorby, J. (2001) Responses of Plant Growth Rate to Nitrogen Supply: A Comparison of Relative Addition and N Interruption Treatments. Journal of Experimental Botany, 52, 309-317. http://dx.doi.org/10.1093/jexbot/52.355.309
- Alitabar, R.A., Salimbeck, R., Alishah, O. and Andarkhor, S.A.A. (2013) The Effects of Nitrogen and Row Spacing on Growth and Yield of Cotton Varieties. International Journal of Agriculture: Research and Review, 3, 120-125.
- Hallikeri, S.S., Halemani, H.L., Patil, V.C., Palled, Y.B., Patil, B.C. and Katageri, I.S. (2010) Effect of Nitrogen Levels, Split Application of Nitrogen and Detopping on Seed Cotton Yield and Fibre Quality in Bt-Cotton. Karnataka Journal of Agricultural Sciences, 23, 418-422.
- Kumbhar, A.M., Buriro, U.A., Junejo, S., Oad, F.C., Jamro, G.H., Kumbhar, B.A. and Kumbhar, S.A. (2008) Impact of Different Nitrogen Levels on Cotton Growth, Yield and N-Uptake Planted in Legume Rotation. Pakistan Journal of Botany, 40, 767-778.
- Soomro, A.W. and Waring, S.A. (1987) Effect of Temporary Flooding on Cotton Growth and Nitrogen Nutrition in Soils with Different Organic Matter Levels. Australian Journal of Agricultural Research, 38, 91-99. http://dx.doi.org/10.1071/AR9870091
- Clawson, E.L., Cothren, J.T. and Blouin, D.C. (2006) Nitrogen Fertilization and Yield of Cotton in Ultra-Narrow and Conventional Row Spacings. Agronomy Journal, 98, 72-79. http://dx.doi.org/10.2134/agronj2005.0033
- Oosterhuis, D.M. and Bate, G.C. (1983) Nitrogen Uptake of Field-Grown Cotton. II. Nitrate Reductase Activity and Petiole Nitrate Concentration as Indicators of Plant Nitrogen Status. Experimental Agriculture, 19, 103-109. http://dx.doi.org/10.1017/S0014479700010565
- Dong, H.Z., Li, W.J., Eneji, A.E. and Zhang, D.M. (2012) Nitrogen Rate and Plant Density Effects on Yield and Late-Season Leaf Senescence of Cotton Raised on a Saline Field. Field Crops Research, 126, 137-144. http://dx.doi.org/10.1016/j.fcr.2011.10.005
- Howard, D.D., Gwathmey, C.O., Essington, M.E., Roberts, R.K. and Mullen, M.D. (2001) Nitrogen Fertilization of No-Till Cotton on Loess-Derived Soils. Agronomy Journal, 93, 157-163. https://www.agronomy.org/publications/aj/abstracts/93/1/157
- Setatou, H.B. and Simonis, A.D. (1996) Effect of Time and Rate of Nitrogen Application on Cotton. Fertilizer Research, 43, 49-53. http://dx.doi.org/10.1007/BF00747682
- Moore, S.H. (1998) Nitrogen Effects on the Fate of Cotton Bolls. Journal of Plant Nutrition, 21, 1145-1152.
- Xue, X., Wang, J., Guo, W., Chen, B., You, J. and Zhou, Z. (2006) Effect of Nitrogen Applied Levels on the Dynamics of Biomass, Nitrogen Accumulation and Nitrogen Fertilization Recovery Rate of Cotton after Initial Flowering. Acta Ecologica Sinica, 26, 3632-3640. (In Chinese with English Abstract)
- Guo, Y., Ma, X., Yang, T., Niu, X., Wang, B., Xu, Y. and Liu, N. (2010) Effect of Using Top Nitrogen Fertilizer on Abscission Rate of Cotton Buds and Bolls. Xinjiang Agricultural Sciences, 47, 180-183. (In Chinese with English Abstract)
- Bondada, B.R., Oosterhuis, D.M., Norman, R.J. and Baker, W.H. (1996) Canopy Photosynthesis, Growth, Yield, and Boll 15N Accumulation under Nitrogen Stress in Cotton. Crop Science, 36, 127-133. http://dx.doi.org/10.2135/cropsci1996.0011183X003600010023x
- Boquet, D.J., Moser, E.B. and Breitenbeck, G.A. (1994) Boll Weight and within Plant Yield Distribution in Field Grown Given Different Levels. Agronomy Journal, 86, 20-26.
- Rashidi, M. and Gholami, M. (2011) Response of Yield and Yield Components of Cotton to Different Rates of Nitrogen Fertilizer. Academic Journal of Plant Sciences, 4, 22-25.
- Saleem, M.F., Bilal, M.F., Awais, M., Shahid, M.Q. and Anjum, S.A. (2010) Effect of Nitrogen on Seed Cotton Yield and Fiber Qualities of Cotton (Gossypium hirsutum L.) Cultivars. The Journal of Animal & Plant Sciences, 20, 23-27.
- Oosterhuis, D.M., Okuba, M.A. and Mozaffari, M. (2007) Effect of Soil-Applied Nitrogen Fertilizer Rate on the Nitrogen Content of Cotton Flowers. AAES Research Series, 558, 43-45.
- Zhao, W.Q., Wang, Y.H., Zhou, Z.G., Meng, Y.L., Chen, B.L. and Oosterhuis, D.M. (2012) Effect of Nitrogen Rates and Flowering Dates on Fiber Quality of Cotton (Gossypium hirsutum L.). American Journal of Experimental Agriculture, 2, 133-159. http://dx.doi.org/10.9734/AJEA/2012/954
- Sawan, Z.M., Mahmoud, M.H. and El-Guibali, A.H. (2006) Response of Yield, Yield Components, and Fiber Properties of Egyptian Cotton (Gossypium barbadense L.) to Nitrogen Fertilization and Foliar-Applied Potassium and Mepiquat Chloride. Journal of Cotton Science, 10, 224-234.
- Ridgway, R.L., Bell, A.A., Veech, J.A. and Chandler, J.M. (1984) Cotton Protection Practices in the USA and the World, pp. 265-365. In: Kohel, R.J. and Lewis, C.F., Eds., Cotton, American Society of Agronomy/Crop Science Society of America/Soil Science Society of America, Madison, 226-365.
- Sanders, D.E. and Snow, J.P. (1978) Dispersal of Airborne Spores of Boll-Rotting Fungi and the Incidence of Cotton Boll Rot. Phytopathology, 68, 1438-1441. http://dx.doi.org/10.1094/Phyto-68-1438
- Boquet, D.J., Moser, E.B. and Breitenbeck, G.A. (1993) Nitrogen Effects on Boll Production of Field-Grown Cotton. Agronomy Journal, 85, 34-39. http://dx.doi.org/10.2134/agronj1993.00021962008500010007x
- Mullins, G.L., Monks, C.D. and Delaney, D. (2003) Cotton Response to Source and Timing of Nitrogen Fertilization on a Sandy Coastal Plain Soil. Journal of Plant Nutrition, 26, 1345-1353. http://dx.doi.org/10.1081/PLN-120021046
- Knowles, T.C., Watson, J. and Wakimoto, V. (1999) Late Season Nitrogen Fertilizer for Cotton. Arizona Cotton Report, The University of Arizona College of Agriculture, Tucson.
- Yang, G.Z., Tang, H.Y., Tong, J., Nie, Y.C. and Zhang, X.L. (2012) Effect of Fertilization Frequency on Cotton Yield and Biomass Accumulation. Field Crops Research, 125, 161-166. http://dx.doi.org/10.1016/j.fcr.2011.08.008
- Nelson, W.L. (1949) The Effect of Nitrogen, Phosphorus, and Potash on Certain Lint and Seed Properties of Cotton. Agronomy Journal, 41, 289-293. http://dx.doi.org/10.2134/agronj1949.00021962004100070003x
- Sawan, Z.M., El-Farra, A. and El-Latif, S.A. (1988) Cottonseed, Protein and Oil Yields and Oil Properties as Affected by Nitrogen and Phosphorus Fertilization and Growth Regulators. Journal of the American Oil Chemists’ Society, 65, 948-951. http://dx.doi.org/10.1007/BF02544517
- Subhan, M., Khan, H.U. and Ahmed, R.O. (2001) Population Analysis of Some Agronomic and Technological Characteristics of Upland Cotton (Gossypium hirsutum L.). Journal of Biological Sciences, 1, 120-123. http://dx.doi.org/10.3923/jbs.2001.120.123
- Gardner, B.R. and Tucker, T.C. (1960) Nitrogen Effects on Cotton: I. Vegetative and Fruiting Characteristics. Proceedings of the Soil Science Society of America, 31, 780-785.
- Jackson, E.B. and Tilt, P.A. (1968) Effects of Irrigation Intensity and Nitrogen Level on the Performance of Eight Varieties of Upland Cotton (Gossypium hirsutum L.). Agronomy Journal, 60, 13-17. http://dx.doi.org/10.2134/agronj1968.00021962006000010005x
- Hardy, G.W. and Garrett, J.D. (1965) Nitrogen Sources, Levels and Timing for Cotton on Clay Soils in N Arkansas. University of Arkansas, Agricultural Experiment Station Bulletin, 140.
- Hussain, S.Z., Faird, S., Anwar, M., Gill, M.I. and Baugh, M.D. (2000) Effect of Plant Density and Nitrogen on the Yield of Seed Cotton-Variety CIM-443. Sarhad Journal of Agriculture, 16, 143-147.
- Pilbeam, C. (1998) The Recovery of N Fertilizer by Cereal Cropsin Different Geographical Locations. In: Vermoesen, A., Ed., Proceedings of the 11th World Fertilizer Congress, International Centre of Fertilizers, Ghent, 472-479.
- John, D. (2007) Nitrogen Efficiency and Management, Nutrient Management. Technical Note No. 6, September 2007.
- Gourley, C.J.P., Allan, D.L. and Russelle, M.P. (1994) Plant Nutrient Efficiency: A Comparison and Suggested Improvement. Plant and Soil, 158, 29-37. http://dx.doi.org/10.1007/BF00007914
- Graham, R.D. (1984) Breeding Characteristics in Cereals. In: Tinker, P.B. and Lauchli, A., Eds., Advances in Plant Nutrition, Volume 1, Praeger, New York, 57-90.
- Moll, R.H., Kamprath, E.J. and Jackson, W.A. (1982) Analysis and Interpretation of Factors Which Contribute to Efficiency of Nitrogen Utilization. Agronomy Journal, 74, 262-264. http://dx.doi.org/10.2134/agronj1982.00021962007400030037x
- Siddiqi, M.Y. and Glass, A.D. (1981) Utilization Index: A Modified Phosphorus Nutrition of Eight Forms of Two Clover Species, Trifolium ambiguum and T. repens. Journal of Plant Nutrition, 4, 289-302. http://dx.doi.org/10.1080/01904168109362919
- Gerloff, G.C. and Gabelman, W.H. (1983) Genetic Basis of Inorganic Plant Nutrition. In: Läuchli, A. and Bieleski, R.L., Eds., Encyclopaedia of Plant Physiology New Series, Volume 15B, Springer-Verlag, New York, 453-480.
- Dobermann, A. (2007) Nitrogen Use Efficiency: Measurement and Management. In: Krauss, A., Isherwood, K. and Heffer, P., Eds, Fertilizer Best Management Practices, IFA, Paris, 1-28.
- Xu, G.H., Fan, X.R. and Miller, A.J. (2012) Plant Nitrogen Assimilation and Use Efficiency. Annual Review of Plant Biology, 63, 153-182. http://dx.doi.org/10.1146/annurev-arplant-042811-105532
- Fixen, P.E. (2005) Understanding and Improving Nutrient Use Efficiency as an Application of Information Technology. Proceedings of the Symposium on Information Technology in Soil Fertility and Fertilizer Management, a Satellite Symposium at the XV International Plant Nutrient Colloquium, Beijing, 14-16 September 2005.
- Smith, D. (1982) Nitrogen Fixation. In: Burns, R.G. and Slater, J.H., Eds., Experimental Microbial Ecology, Blackwell, London, 212-220.
- Havlin, J.L., Beaton, J.D., Tisdale, S.L. and Nelson, W.L. (2005) Soil Fertility and Fertilizers: An Introduction to Nutrient Management. Pearson Education, Inc., Upper Saddle River.
- Camberato, J.J. (2001) Nitrogen in Soil and Fertilizers. South Carolina Turfgrass Foundation News, 8, 6-10.
- Mengel, K. (1996) Turnover of Organic Nitrogen in Soils and Its Availability to Crops. Plant and Soil, 181, 83-93. http://dx.doi.org/10.1007/BF00011295
- Kelley, K.R. and Stevenson, J.F. (1995) Forms and Nature of Organic N in Soil. Fertilizer Research, 42, 1-11. http://dx.doi.org/10.1007/BF00750495
- Chie, M., Ketterings, Q., Cherney, J., Kilcer, T. and Fixation, N. (2008) Agronomy Fact Sheet Series, Fact Sheet 39. Cornell University, College of Agriculture and Life Sciences, Department of Crop and Soil Sciences, Ithaca, NY.
- Wang, M.Y., Siddeqi, M.Y., Ruth, T.J. and Glass, A.D.M. (1993) Ammonium Uptake by Rice Roots. I. Kinetics of 13NH4+ Influx across the Plasmalemma. Plant Physiology, 103, 1259-1267.
- Glass, A.D.M. (1989) Physiological Mechanisms Involved with Genotypic Differences in Ion Adsorption and Utilization. Horticultural Science, 24, 559-564.
- Segonzac, C., Boyer, J.-C., Ipotesi, E., Szponarski, W., Tillard, P., Touraine, B., Sommerer, N., Rossignol, M. and Gibrat, R. (2007) Nitrate Efflux at the Root Plasma Membrane: Identification of an Arabidopsis Excretion Transporter. The Plant Cell, 19, 3760-3777. http://dx.doi.org/10.1105/tpc.106.048173
- Glass, A.D., Britto, D.T., Kaiser, B.N., Kinghorn, J.R., Kronzucker, H.J., Kumar, A., Okamoto, M., Rawat, S., Siddiqi, M.Y., Unkles, S.E. and Vidmar, J.J. (2002) The Regulation of Nitrate and Ammonium Transport Systems in Plants. Journal of Experimental Botany, 53, 855-864.
- Kronzucker, H.J., Siddiqi, M.Y. and Glass, A.M.D. (1996) Kinetics of NH4+ Influx in Spruce. Plant Physiology, 110, 773-779.
- Wagner, C.A., Devuyst, O., Belge, H., Bourgeois, S. and Houillier, P. (2010) The Rhesus Protein RhCG: A New Perspective in Ammonium Transport and Distal Urinary Acidification. Kidney International, 79, 154-161. http://dx.doi.org/10.1038/ki.2010.386
- Earl, T., Kwanyen, P. and Scheffler, J. (2010) Nitrogen Metabolism in Cotton Stems and Roots during Reproductive Development. The Journal of Cotton Science, 14, 107-112.
- Mokhele, B., Zhan, X.J., Yang, G.Z. and Zhang, X.L. (2012) Review: Nitrogen Assimilation in Crop Plants and Its Affecting Factors. Canadian Journal of Plant Science, 92, 399-405.
- Cleemput, O.V. and Samate, A.H. (1996) Nitrite in Soils: Accumulation and Role in the Formation of Gaseous N Compounds. Fertilizer Research, 45, 81-89. http://dx.doi.org/10.1007/BF00749884
- Lillo, C., Meyer, C., Lea, U.S., Provan, F. and Oltedal, S. (2004) Mechanism and Importance of Post-Translational Regulation of Nitrate Reductase. Journal of Experimental Botany, 55, 1275-1282. http://dx.doi.org/10.1093/jxb/erh132
- Blevins, R.L., Thomas, G.W., Smith, M.S., Frye, W.W. and Cornelius, P.L. (1983) Changes in Soil Properties after 10 Years of Continuous Non-Tilled and Conventionally-Tilled Corn. Soil and Tillage Research, 3, 135-146. http://dx.doi.org/10.1016/0167-1987(83)90004-1
- Slardini, A.A., Sparrow, L.A. and Holloway, R.J. (1992) The Mobility and Transformation of Soil Nitrogen and the Relationships between Soil and Plant Nitrogen and Yield at Different Times Following Application of Various Nitrogen Fertilizers to Sweet Corn. Australian Journal of Agricultural Research, 43, 1643-1652. http://dx.doi.org/10.1071/AR9921643
- Dobermann, A., Cassman, K.G., Waters, D.T. and Witt, C. (2005) Balancing Short- and Long-Term Goals in Nutrient Management. Proceedings of the XV International Plant Nutrient Colloquium, Beijing, 14-16 September 2005.
- CRC, Cotton Catchment Communities (2008) Nitrogen Losses in Cotton Production. Narrabri.
- Aronsson, H., Torstensson, G. and Bergström, L. (2007) Leaching and Crop Uptake of N, P and K from a Clay Soil with Organic and Conventional Cropping Systems. Soil Use and Management, 23, 71-81. http://dx.doi.org/10.1111/j.1475-2743.2006.00067.x
- Van Es, H.M., Klausner, S.D. and Reid, W.S. (1991) Nitrogen and the Environment. Cornell Coop. Ser. Bull. 218, Cornell University, Ithaca, NY.
- Riley, W.J., Ortiz-Monasterio, I. and Matson, P.A. (2001) Nitrogen Leaching and Soil Nitrate, Nitrite, and Ammonium Levels under Irrigated Wheat in Northern Mexico. Nutrient Cycling in Agroecosystems, 61, 223-236. http://dx.doi.org/10.1023/A:1013758116346
- Follett, R.F. (2001) Nitrogen Transformation and Transport Processes. In: Follett, R.F. and Hatfield, J., Eds., Nitrogen in the Environment: Sources, Problems, and Solutions, Elsevier Science Publishers, Amsterdam, 17-44.
- Bock, B.R. and Kissel, D.E. (1988) Ammonia Volatilization from Urea Fertilizers. Bulletin Y-206. National Fertilizer Development Center, Tennessee Valley Authority, Muscle Shoals, AL.
- Jones, C. (2006) Ammonia Volatilization: Process Ammonia Volatilization: Process, Amounts, and Yield Effects. MABA/MGEA 2006 Convention.
- Ellington, A. (1986) Ammonia Volatilization Losses from Fertilizers Applied to Acid Soil in the Field. Fertilizer Research, 8, 283-296. http://dx.doi.org/10.1007/BF01048631
- Mcallister, C.H., Beatty, P.H. and Good, A.G. (2012) Engineering Nitrogen Use Efficient Crop Plants: The Current Status. Plant Biotechnology Journal, 10, 1011-1025. http://dx.doi.org/10.1111/j.1467-7652.2012.00700.x
- Hirel, B., Tétu, T., Lea, P.J. and Dubois F. (2011) Improving Nitrogen Use Efficiency in Crops for Sustainable Agriculture. Sustainability, 3, 1452-1485.
- Hirsch, R.E. and Sussman, M.R. (1999) Improving Nutrient Capture from Soil by Genetic Manipulation of Crop Plants. Trends in Biotechnology, 17, 356-361. http://dx.doi.org/10.1016/S0167-7799(99)01332-3
- Sauerbeck, D.R. and Helal, H.M. (1990) Factors Affecting the Nutrient Efficiency of Plants. In: El Bassam, N., Dambroth, M. and Loughman, B.C., Eds., Genetic Aspects of Plant Mineral Nutrition, Kluwer Academic Publishers, Dordrecht, 11-16.
- Tilman, D., Cassman, K.G., Matson, P.A., Naylor, R. and Polasky, S. (2002) Agricultural Sustainability and Intensive Production Practices. Nature, 418, 671-677. http://dx.doi.org/10.1038/nature01014
- Larry, G. (2006) How Can We Improve Nitrogen Use Efficiency? Proceedings of the 2006 Wisconsin Fertilizer, Aglime and Pest Management Conference, Madison, 17-19 January 2006.
- Roberts, T.L. (2008) Improving Nutrient Use Efficiency. Turkish Journal of Agriculture and Forestry, 32, 177-182.
- Engel, R., Jones, C. and Wallander, R. (2011) Ammonia Volatilization from Urea and Mitigation by NBPT Following Surface Application to Cold Soils. Soil Science Society of America Journal, 75, 2348-2357. http://dx.doi.org/10.2136/sssaj2011.0229
- Grundmann, G.L., Renault, P., Rosso, L. and Bardin, R. (1995) Differential Effects of Soil Water Content and Temperature on Nitrification and Aeration. Soil Science Society of America Journal, 59, 1342-1349. http://dx.doi.org/10.2136/sssaj1995.03615995005900050021x
- Linn, D.M. and Doran, J.W. (1984) Effect of Water-Filled Pore Space on Carbon Dioxide and Nitrous Oxide Production in Tilled and Non-Tilled Soils. Soil Science Society of America Journal, 48, 1267-1272. http://dx.doi.org/10.2136/sssaj1984.03615995004800060013x
- Parton, W.J., Holland, E.A., Del Grosso, S.J., Hartman, M.D., Martin, R.E., Mosier, A.R., Ojima, D.S. and Schimel, D.S. (2001) Generalized Model for NOx and N2O Emissions from Soils. Journal of Geophysical Research, 106, 17,403-17,419. http://dx.doi.org/10.1029/2001JD900101
- Paul, E.A. and Clark, F.E., Eds. (1989) Soil Microbiology and Biochemistry, Academic Press, San Diego. Prosser, J.I. (1989) Autotrophic Nitrification in Bacteria. Advances in Microbial Physiology, 30, 125-181.
- Hou, Z.N., Wu, L.S., Liang, Y.C., Wei, C.Z. and Chen, W.P. (2010) Effects of Salinity and Nitrogen on Cotton Growth in Arid Environment. Plant and Soil, 326, 61-73.
- Fenn, L.B. and Kissel, D.E. (1976) The Influence of Cation Exchange Capacity and Depth of Incorporation on Ammonia Volatilization from Ammonium Compounds Applied to Calcareous Soils. Soil Science Society of America Journal, 40, 394-398. http://dx.doi.org/10.2136/sssaj1976.03615995004000030026x
- Ferguson, R.B., Kissel, D.E., Koelliker, J.K. and Basel, W. (1984) Ammonia Volatilization from Surface-Applied Urea: Effect of Hydrogen Ion Buffering Capacity. Soil Science Society of America Journal, 48, 578-582. http://dx.doi.org/10.2136/sssaj1984.03615995004800030022x
- Wullstein, L.H. (1969) Reduction of Nitrite Deficits by Alkaline Metal Carbonates. Soil Science, 108, 222-226. http://dx.doi.org/10.1097/00010694-196909000-00012
- Darwish, T.M., Atallah, T.W., Hajhasan, S. and Haidar, A. (2006) Nitrogen and Water Use Efficiency of Fertigated Processing Potato. Agricultural Water Management, 85, 95-104. http://dx.doi.org/10.1016/j.agwat.2006.03.012
- Bronson, K.F. (2008) Nitrogen Use Efficiency of Cotton Varies with Irrigation System. Better Crops/Volume 92, No. 4.
- Fixen, P.E. (2006) Turning Challenges into Opportunities. Proceedings of the Fluid Forum, Fluids: Balancing Fertility and Economics, Scottsdale, 12-14 February 2006.
- Holcomb III, J.C., Sullivan, D.M., Horneck, D.A. and Clough, G.H. (2011) Effect of Irrigation Rate on Ammonia Volatilization. Soil Science Society of America Journal, 75, 2341-2347. http://dx.doi.org/10.2136/sssaj2010.0446
- Susan, M. (2001) Managing Nitrogen in Irrigated Cotton Guidelines Focus on Reduced Rates. Agricultural Experiment Station Research Report.
- Herridge, D.F., Peoples, M.B. and Boddey, R.M. (2008) Global Inputs of Biological Nitrogen Fixation in Agricultural Systems. Plant and Soil, 311, 1-18. http://dx.doi.org/10.1007/s11104-008-9668-3
- Lawlor, D.W. (2002) Carbon and Nitrogen Assimilation in Relation to Yield: Mechanisms Are the Key to Understanding Production Systems. Journal of Experimental Botany, 53, 773-787. http://dx.doi.org/10.1093/jexbot/53.370.773
- Raun, W.R., Johnson, G.V., Phillips, S.B. and Westerman, R.L. (1998) Effect of Long-Term N Fertilization on Soil Organic C and Total N in Continuous Wheat under Conventional Tillage in Oklahoma. Soil & Tillage Research, 47, 323-330.
- Castro, M.S., Peterjohn, W.T., Melillo, J.M., Steudler, P.A., Gholz, H.L. and Lewis, D.H. (1994) Effects of Nitrogen Fertilization on the Fluxes of N2O, CH4, and CO2 from Soils in a Florida Slash Pine Plantation. Canadian Journal of Forest Research, 24, 9-13. http://dx.doi.org/10.1139/x94-002
- Spicer, S. (2002) Fertilizers, Manure, or Biosolids? Water Environment & Technology Publication, 14, 32-35.
- Ernst, J.W. and Massey, H.F. (1960) The Effects of Several Factors on Volatilization of Ammonia Formed from Urea in the Soil. Soil Science Society of America Journal, 24, 87-90. http://dx.doi.org/10.2136/sssaj1960.03615995002400020007x
- Wetselaar, R., Passioura, J.B. and Singh, B.R. (1972) Consequences of Banding Nitrogen Fertiliser in Soil. 1. Effects on Nitrification. Plant and Soil, 36, 159-175. http://dx.doi.org/10.1007/BF01373466
- Nielsen, R.L. (2006) N Loss Mechanisms and Nitrogen Use Efficiency. 2006 Purdue Nitrogen Management Workshops, 1-5.
- Hu, M., Tian, C., Lu, Z., Liu, H. and Chen, T. (2006) Effects of N Rate on Cotton Yield and Nitrate N Concentration in Plant Tissue and Soil. Journal of Northwest Sci-Tech University of Agriculture and Forestry (Natural Sciences Education), 34, 63-68. (In Chinese with English Abstract)
- Mohsen, S. and Rashidi, M. (2011) Effect of Different Application Rates of Nitrogen on Yield and Quality of Cotton (Gossypium hirsutum). American-Eurasian Journal of Agricultural Environmental Sciences, 10, 366-370.
- Zhang, S. and Zhang, L. (2010) Above-Ground Dry Matter Accumulation of Cotton Genetics at Different Nitrogen Applications. Journal of Cotton Science, 22, 77-82. (In Chinese with English Abstract)
- Mozaffari, M., McConnell, J.S., Hattenhauer, K., Slaton, N.A., Evans, E.E., Woody, N.M., Bourland, F. and Kennedy, C. (2004) Cotton Yield and Petiole Nitrogen Content as Affected by Nitrogen Fertilizer Application. AAES Research Series 533, 89-94.
- Boquet, D.J. and Breitenbeck, G.A. (2000) Nitrogen Rate Effect on Partitioning of Nitrogen and Dry Matter by Cotton. Crop Science, 40, 1685-1693. http://dx.doi.org/10.2135/cropsci2000.4061685x
- Boquet, D.J. (2005) Cotton in Ultra-Narrow Row Spacing: Plant Density and Nitrogen Fertilizer Rates. Agronomy Journal, 97, 279-287. http://dx.doi.org/10.2134/agronj2005.0279
- Wajid, A., Ghaffar, A., Maqsood, M., Hussain, K. and Nasim, W. (2007) Yield Response of Maize Hybrids to Varying Nitrogen Rates. Pakistan Journal of Agricultural Sciences, 44, 217-220.
- Baraich, A.H.K., Jamali, L.A. and Salarzi, A.U. (2012) Effect of Nitrogen Application Rates on Growth and Yield of Cotton Varieties. Pakistan Journal of Agriculture, Agricultural Engineering and Veterinary Sciences, 28, 115-123.
- Hallikeri, B.A. and Gershenzon, J. (2006) Biology and Biochemistry of Glucosinolates. Annual Review of Plant Biology, 57, 303-333. http://dx.doi.org/10.1146/annurev.arplant.57.032905.105228



