American Journal of Plant Sciences
Vol.4 No.3(2013), Article ID:28999,6 pages DOI:10.4236/ajps.2013.43076
Unravelling Effects of Temperature and Soil Moisture Stress Response on Development of Dry Root Rot [Rhizoctonia bataticola (Taub.)] Butler in Chickpea
![]()
International Crops Research Institute for the Semi-Arid Tropics, Patancheru, India.
Email: *s.pande@cgiar.org
Received January 6th, 2013; revised February 8th, 2013; accepted February 28th, 2013
Keywords: Chickpea; Climate Change; Drought; Dry Root Rot; Soil Moisture; Temperature
ABSTRACT
Erratic rainfalls and rise in temperature have become more frequent under the changing scenario of climate particularly in semi-arid tropics. As a consequence of it, a drastic shift of chickpea diseases have been recorded throughout the major chickpea growing regions in India and elsewhere. Dry root rot (DRR) caused by Rhizoctonia bataticola (Taub.) Butler [Pycnidial stage: Macrophomina phaseolina (Tassi) Goid] was found as a potentially emerging constraint to chickpea production than wilt (Fusarium oxysporum f. sp. ciceris). Increasing incidence of DRR indicate strong influence of climate change variables such as temperature and moisture on the development of disease. The present study therefore was conducted to quantify the role of temperature and soil moisture associated with infection, colonization and development of DRR under controlled environment. The DRR incidence was significantly affected by high temperature and soil moisture deficit. Out of five temperature regimes (15˚C, 20˚C, 25˚C, 30˚C and 35˚C) and four moisture levels (40%, 60%, 80% and 100%), a combination of high temperature (35˚C) and soil moisture content (60%) predisposes chickpea to DRR. The study clearly demonstrates that high temperature coupled with soil moisture deficit is the climate change variables predisposing chickpea to R. bataticola infection, colonization and development.
1. Introduction
Chickpea (Cicer arietinum L.) is a major food legume grown by the poor and subsistence farmers in semi-arid tropics (SAT) of Asia and Africa. It is grown in over 50 countries of Asia, Africa, America, and Oceania in rainfed environments. The annual production of chickpea is 10.9 million tons from 11.98 million hectare [1] worldwide. South Asia is the largest producer of chickpea (76%) and India is the largest chickpea growing country with an annual production of 7.06 million tons from 7.54 million hectares [1]. In India, since last two decades, shift in the cultivation of chickpea from north to the central and southern regions has been reported and as a result shift in the disease pattern has been found. The production of chickpea is largely constrained so far by Fusarium wilt, a soil borne disease caused by Fusarium oxysporum f. sp. ciceris. Fusarium wilt is effectively managed by host plant resistance and several wilt resistant cultivars have been developed and released worldwide [2]. However, recently wilt resistant chickpea cultivars were found succumbing to dry root rot (DRR) in major chickpea growing regions [3,4].
The DRR is caused by a necrotrophic fungus Rhizoctonia bataticola (Taub.) Butler (Pycnidial stage: Macrophomina phaseolina (Tassi) Goid) and is an important component of the disease complex that causes root rots and seedling blight in many grain legumes when they are weakened by other stress factors [5]. In the absence of the host crop, it survives in soil as a competitive saprophyte on available dead organic matter. A critical analysis of the weather data (2000-2010) of the major chickpea growing areas in India indicated higher incidence of DRR in years when temperature exceeds 33˚C [4]. Increasing incidence of DRR at various locations over years suggest a strong influence of climate change on disease infection. Savary et al. (2011) [6] categorise DRR as an acute-emerging disease that occurs irregularly, both temporally and spatially, may cause massive disruptions in system performances and whose range is expanding to new areas. In chickpea, drying of the plants appears suddenly in the field where DRR affected plants are scattered. Affected plants are usually straw coloured, in some cases the lower leaves and stems show a brown colour. The tap root is black, rotten and devoid of most of the lateral and fine roots. The dead root is quite brittle and shows shredding of the bark. Dark minute sclerotial bodies can be seen on the roots exposed or inside the wood [7]. When the dry stem of the collar region is split vertically, sparse mycelium or minute sclerotia can be seen in the pith. Sclerotial survivability is greatly reduced in wet soils than the dry soils at low moisture levels.
Vary little is known about the epidemiology of the disease in relation to predisposition of chickpea plants by climate change variables (temperature and soil moisture). Limited literature on the effect of climatic factors indicates that hot, dry weather promotes infection and development of dry root rot [8]. The aim of the present study was to determine and quantify the effect of climate change variables (temperature and soil moisture) on DRR infection, colonization and expression under controlled environment. The study was initiated to develop a classification system to assess the disease potential of fields, so that growers could make management decisions in selecting control strategies such as cultural controls, resistant varieties and chemical seed treatments.
2. Materials and Methods
2.1. Fungal Isolate
A pathogenic isolate of R. bataticola, isolated from naturally infected chickpea plant at ICRISAT, Patancheru was used throughout the experiments. Isolate was purified using mono-sclerotia and maintained on PDA slants at 5˚C in refrigerator.
2.2. Effect of Temperature
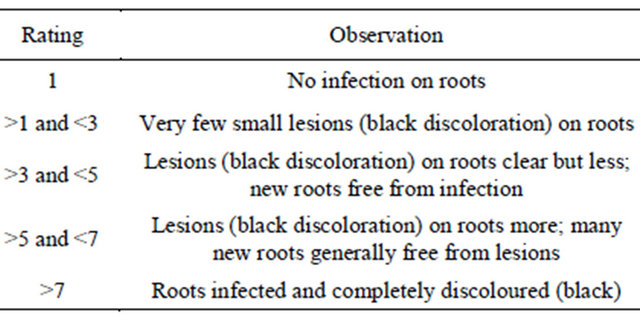
Effect of five temperature regimes(20˚C, 25˚C, 30˚C, 35˚C, 40˚C) was studied on DRR susceptible cultivar BG 212 under in vitro conditions using paper towel technique [9]. Seven-day-old seedlings of BG 212 grown in sterilized sand was uprooted and root inoculated by dipping in the inoculum for 1 min. Inoculum was mass multiplied on potato dextrose broth medium (A 7 mm agar disc of actively growing R. bataticola culture was put into each 250 ml conical flask containing 100 ml of sterilized potato dextrose broth and incubated for 5 days at 28˚C in a stationary condition. Fungal mat from two flasks macerated in 100 ml of SDW was used as inoculum). Inoculated seedlings were placed in folded, moist blotting paper with the shoots left outside, and then incubated at 35˚C with a 12 h photoperiod. The experiment was conducted in completely randomized block design (CRBD) with three replications (each replication consisted of 10 plants) and repeated once. Total 30 plants per treatment were scored for disease severity. Equal number of seedlings inoculated with only sterile water served as control. Disease severity was recorded six days after inoculation on a 1 - 9 rating scale (1 = no infection on roots and 9 = roots infected and completely discoloured (Table 1).
2.3. Effect of Soil Moisture
Effect of four soil moisture regimes, i.e. 40%, 60%, 80% and 100% was studied on the development of DRR under controlled environment. The experiment was conducted on susceptible cultivar BG 212 at an optimum temperature of 35˚C for DRR identified from previous experiment. Each treatment was replicated four times and each replication consisted of four pots (five plants/pot). Equal number of replicated pots was kept for uprooting the plants and recording disease severity. Uninoculated control was maintained for each soil moisture level. The relative humidity and air temperature were monitored with hygrothermograph. Deionized water was used for maintaining the soil moisture content (SMC) in each treatment.
The SMC was determined using the gravimetric method on an oven-dry basis. The method includes saturation of soil sample followed by removal of available soil moisture by oven drying (100˚C - 110˚C) until the weight remains constant.After removing from oven, samples were cooled slowly to room temperature and weighed again. The difference in weight was amount of moisture in the soil. The available SMC in the soil was calculated by the following formula:

The four levels of SMC (40%, 60%, 80% & 100%) was adjusted by maintaining the constant weight by regular weighing and replacing the moisture deficit in each pot by watering [10].
Sterilized soil was infested with R. bataticola (at 50 g/kg soil) multiplied on sand-maize meal medium. The pathogen infested soil was filled in the 6 inch pots (2.0 kg/pot). For each treatment, control pots were maintained by adding the uninoculated sandmaize medium
Table 1. Disease rating scale on 1 - 9 scale for dry root rot of chickpea.
to the sterilized soil. Sowing in the pots was done four days after pathogen infestation. Five surface sterilized seeds (soaked in 0.1% sodium hypochlorite for 1 minute followed by 2 - 3 times washing with sterilized water) of the chickpea DRR susceptible cultivar BG 212 were planted in each sick pot. The seedlings were allowed to grow for 10 days under normal conditions. Different levels of SMC were maintained 10 days after sowing. The experiment was repeated once with equal number of treatments and replications.
2.4. Data Collection
Data on DRR incidence was collected periodically at 15, 25, 35, 45, 55 and 65 days after sowing. For scoring the disease incidence, diseased and total plants were counted from each replication and percentage of plants infected in each treatment was calculated. Plants were also scored for root disease severity (1 - 9 scale) at 15, 25, 35, 45, 55 and 65 days after sowing.
The population of R. bataticola from the rhizosphere soil from each treatment was also assessed. To determine fungal propgules, soil samples (5 g/pot) were collected from each treatment, air dried for 48 hr and thoroughly mixed. A sub-sample of 5 g was taken from this, ground with pestle and mortar and passed through a 60 mm sieve. From the sieved fine soil, 0.01 g was taken and sprinkled on selective media (Difco Potato dextrose agar medium) followed by incubation for 3 - 4 days at 28˚C in incubator. Colonies of R. bataticola were counted four days after incubation and identified based on characteristic colony and morphological characters.
2.5. Data Analysis
The data on temperature effect on DRR was analyzed through GENSTAT statistical package (version 14.0; Rothamsted Experiment Station, herpenden, Herts AL52JQ, UK). Data on per cent DRR incidence was subjected to arcsine transformation [11]. Transformed data was analyzed through SAS software version 9.2 for Windows (SAS Institute Inc. 2008) and the significant levels and interaction effects were evaluated. The data obtained on cfu was log transformed and then analysis of variance (ANOVA) performed. Linear regression was calculated (SAS ProcLogist) and used to represent the R. bataticola cfu present in soil at different moisture levels. Correlation between soil temperature, SMC and DRR incidence was also determined.
3. Results
3.1. Effect of Temperature
The effect of range of temperature on the DRR severity was statistically significantly (P ≤ 0.0001).The optimum temperature for DRR severity was 35˚C with maximum disease severity of 9 on 1 - 9 scale. This was followed by 30˚C, 25˚C, 20˚C and 15˚C (Figure 1). Incubation period (time taken for the first symptom appearance) was delayed by 3 - 4 days at 15˚C and 20˚C as compared to 30˚C and 35˚C respectively. The disease severity was very low (2 - 3 rating on 1 - 9 scale) at 20 and 25˚C. The control plants did not show any symptoms.
3.2. Effect of Soil Moisture
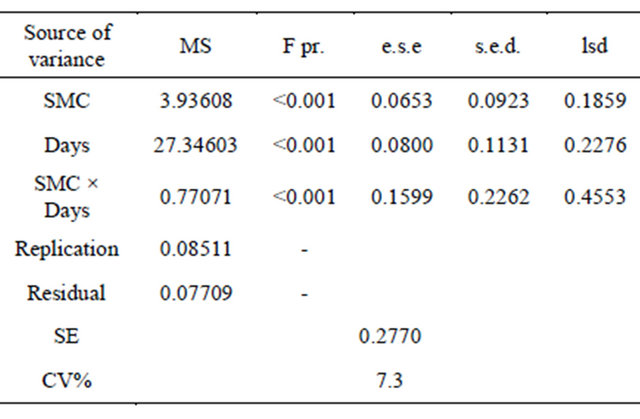
Statistically significant (P < 0.001) variation was found in the DRR incidence at different SMC. Differences in the DRR incidence were mainly contributed by SMC as indicated by the higher mean sum of squares (Table 2). The plants exposed to 40% moisture stress showed higher mortality as compared to 60%, 80% and 100% (Figure 2). It was observed that 40% SMC was insufficient for the normal growth of the plants as the plants grown in control (pathogen free soil) showed thephysiological stress (wilting of the plants due to lack of moisture). At 60% SMC, no physiological stress was found in
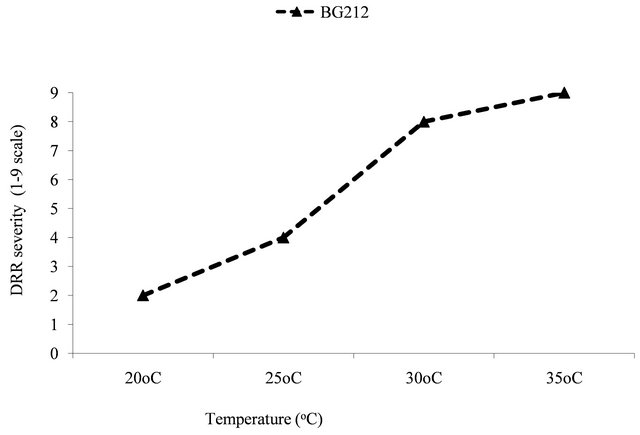
Figure 1. Effect of temperature on dry root rot severity (1 - 9 scale) under in vitro.
Table 2. Analysis of variance (ANOVA) for DRR incidence at different soil moisture levels.
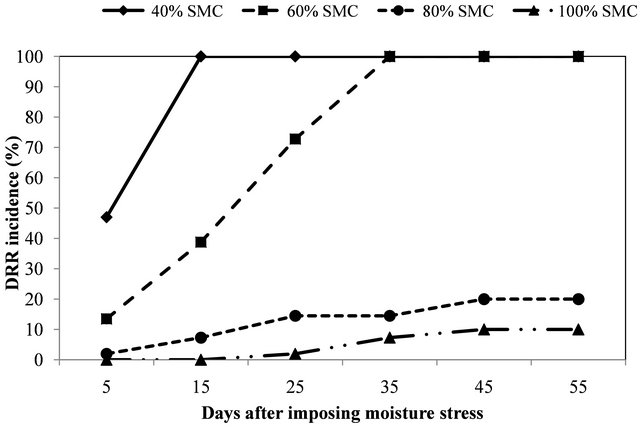
Figure 2. Effect of soil moisture contents on the incidence of dry root rot on cultivar BG 212.
control plants and DRR incidence was 100%, 35 days after imposing moisture stress. Disease progressed slowly at 80% and 100% SMC and even 55 days of stress, incidence was 20% and 10% respectively. No symptoms were recorded in un-inoculated control plants. Additionally it was noted that plants grown in pathogen free soil were significantly taller and had greater root and shoot biomass at higher moisture content as compared to lower moisture content.
Symptoms of DRR on aerial plant parts were found to be directly related with the disease severity on roots. Blackening of the roots initiated 5 days after maintaining the moisture stress at 40% and 60% SMC; however roots were apparently free from infection at 80% and 100%. Root disease severity increased rapidly at 60% and reached maximum 9 (on 1 - 9 scale) 25 days after imposing moisture stress. However, DRR severity progressed slowly at higher moisture contents and a only a small black lesion (rating 2 on 1 - 9 scale) was observedat the collar region even 55 days after imposing stress (Figure 3). Significantly high positive correlation was found between DRR incidence, soil temperature and SMC at 40% (r = 0.8) and 60% (0.4); however correlation was negative at 80 (r = −0.7) and 100% (r = −0.2) (Figure 4).
Statistically significant differences were found in the R. bataticola propagules at different SMC’s when compared with the regression lines. The R. bataticola propagules in infested soil increased with the decrease in the SMC (Figure 5). Analysis of log10 transformed data showed significant effect of SMC, days after sowing and their interaction (P < 0.001) on DRR incidence (Table 3). An exponential decrease in cfu was observed over a period of time and it was adversely affected at higher soil moisture (80% and 100%).
4. Discussion
Chickpea is largely grown in rainfed environments worldwide. The temperature and rainfall variability with in the rainfed ecologies is very high, leading to varying intensities of moisture deficit. In the Indian sub-continent, chickpea is grown during the post-rainy season on receding soil moisture, leading to terminal drought stress. Savary et al. (2011) [6] also reported that R. bataticola is becoming more intense in typically tropical-humid areas.
In the present study, optimum temperature identified for the growth of R. bataticola on the susceptible cultivar was 35˚C. This corroborates with the findings based on

Figure 3. Effect of soil moisture contents on the severity (1 - 9 scale) of dry root rot on cultivar BG 212.
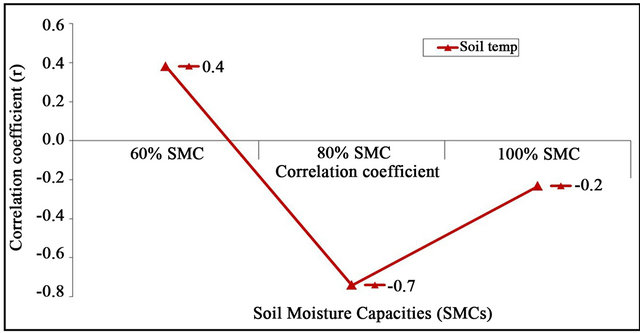
Figure 4. Correlation between soil moisture content, soil temperature and dry root rot incidence.
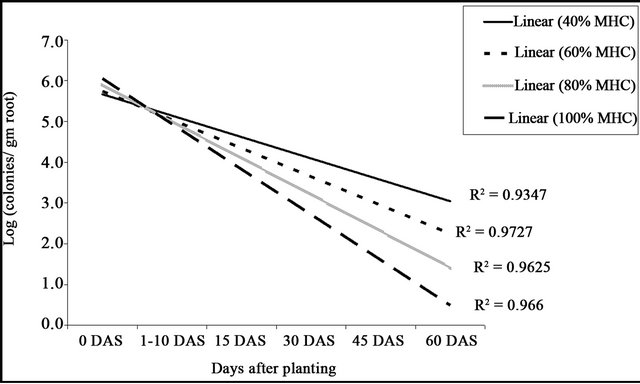
Figure 5. Linear regression of Rhizoctonia bataticola population in soil at different soil moisture contents.
Table 3. Analysis of variance (ANOVA) for Rhizoctonia bataticola population in soil at different soil moisture levels.
analysis of the weather data (2000-2010) of the major chickpea growing areas in India indicating higher incidence of DRR in years when temperature exceeds 33˚C [4]. Patel and Patel (1990) [12] reported 35˚C to be optimum temperature for growth and sclerotial formation of M. phaseolina. Singh and Mehrotra (1982) [13] suggested that if the soil temperature is reduced, the incidence of the DRR can be reduced. DRR Incidence and severity was more in plants subjected to moisture stress (40% and 60% SMC) as compared to non-stress (80% and 100% SMC). Deficit soil moisture (40% SMC) was detrimental to plant growth regardless of whether pathogen was present or not. This can be attributed to the significant effect of water stress on hormonal balance, cell wall and protein synthesis and photosynthesis [14]. Few studies on effect of environmental and soil factors on disease development indicated that plants grown in moist soil at 75% - 100% soil moisture grew vigorously with little infection, whereas the plants under low soil moisture (25%) were stunted and showed physiological stress [15,16].
The disease incidence and severity were directly proportional to each other. Apparent root infections were seen 5 days after the onset of stress, indicating that moisture stress is predisposing factor for initial infection of host tissue. On the other hand roots were free from infection at 80% and 100% till 35 days after imposing stress. The cfu was adversely affected at higher soil moisture. The adverse effect of high soil moisture on survival of sclerotia of R. bataticola in black soil was also reported by [17]. Olayaand Abawi (1996) [18] reported that microsclerotial survivability was greatly reduced in wet soils than the dry soils. This indicates that the pathogen was sensitive to high soil moisture content and cannot survive for a longer period under anaerobic conditions [19].
The study clearly demonstrates that temperature and soil moisture are the two important climate variables influencing the DRR infection, colonization and development in chickpea. This study will substantially accelerate the on-going efforts to understand the host × pathogen × environment interactions in chickpea under the changing scenario of climate. Also, better understanding of the role of temperature and soil moisture will help in standardization of DRR resistance screening techniques and will assist breeders in optimization breeding strategy for DRR that will enable long-term resistance over broader geographical areas.
REFERENCES
- FAOSTAT, “Food and Agriculture Organization of the United Nations,” Rome, 2010. http://faostat.fao.org
- C. L. L. Gowda and P. M. Gaur, “Global Scenario of Chickpea Research-Present Status and Future Thrusts,’’ In: A. Masood, B. B. Singh, S. Kumar and V. Dar. Eds., Pulses in New Perspective, Proceedings of the National Symposium on Crop Diversification and Natural Resource Management, Kanpur, 2004, pp. 1-22.
- S. Pande, S. Desai and M. Sharma, “Impact of Climate Change on Rainfed Crop Diseases: Current Status and Future Research Needs,” National Symposium on Climate Change and Rainfed Agriculture, Indian Society of Dryland Agriculture, Central Research Institute for Dryland Agriculture, Hyderabad, 18-20 February 2010, pp. 55-59.
- M. Sharma, U. N. Mangala, M. Krishnamurthy, V. Vadez and S. Pande, “Drought and Dry Root of Chickpea (Abstract),” 5th International Food Legumes Research Conference (IFLRC V), 2010 & 7th European Conference on Grain Legumes (AEP VII) 26-30 April 2010, Antlya, Akdeniz University & Ministry of Agriculture and Rural Affairs under Auspices of International Steering Committee of IFLRC & The European Association for Grain Legume Research (AEP).
- S. F. Hawang, B. D. Gossen, K. F. Chang, G. D. Turnbull, R. J. Howard and S. F. Blade, “Etiology, Impact and Control of Rhizoctonia Seedling Blight and Root Rot of Chickpea on the Canadian Prairies,” Canadian Journal of Plant Science, Vol. 83, No. 4, 2003, pp. 959-967. doi:10.4141/P02-165
- S. Savary, A. Nelson, H. Adam, Sparks, L. Willocquet, E. Duveiller and G. Mahuku, “International Agricultural Research Tackling the Effects of Global and Climate Changes on Plantdiseases in the Developing World,” Plant Disease, Vol. 95, No. 10, 2011, pp. 1204-1216. doi:10.1094/PDIS-04-11-0316
- Y. L. Nene, M. V. Reddy, M. P. Haware, A. M. Ghanekar and K. S. Amin, “Field Diagnosis of Chickpea Diseases and Their Control,” ICRISAT Information Bulletin No. 28, 1991, p. 52.
- M. A. Bhatti and J. M. Kraft, “Influence of Soil Moisture on Root Rot and Wilt of Chickpea,” Plant Disease, Vol. 76, 1997, pp. 1259-1262. doi:10.1094/PD-76-1259
- S. Pande, G. K. Kishore, H. D. Upadhyaya and J. N. Rao, “Identification of Sources of Multiple Disease Resistance in Mini-Core Collection of Chickpea,” Plant Disease, Vol. 90, No. 9, 2006, pp. 1214-1218. doi:10.1094/PD-90-1214
- M. Suriachandraselvan and K. Seetharaman, “Factors Influencing Susceptibility of Sunflower to Charcoal Rot Disease Caused by Macrophominaphaseolina,” Journal of Mycololgy and Plant Pathology, Vol. 33, No. 2, 2003, pp. 252-256.
- K. A. Gomez and A. A. Gomez, “Statistical Procedure for Agricultural Research,” 2nd Edition, Wiley, New York, 1984.
- K. K. Patel and A. J. Patel, “Meterological Correlation of Charcoal Rot of Sesamum,” Indian Journal of Mycology and Plant Pathology, Vol. 20, No. 1, 1990, pp. 64-65.
- P. J. Singh and R. S. Mehrotra, “Influence of Soil Moisture and Temperature on Rhizoctonia bataticola Infection of Gram,” Indian Phytopathology, Vol. 35, No. 2, 1982, pp. 327-329.
- J. Levitt, “Responses of Plants to Environmental Stresses: Water, Radiation, Salt, and Other Stresses,” Academic Press, New York, 1980.
- R. S. Taya, N. N. Tripathi and M. S. Panwar, “Influence of Soil Type, Soil Moisture and Fertilizers on the Severity of Chickpea Dry Root Rot Caused by Rhizoctonia bataticola (Taub.) Butler,” Indian Journal of Mycologyand Plant Pathology, Vol. 18, 1988, pp. 133-136.
- G. Singh and Y. R. Sharma, “Fungal Diseases of Pulses,” In: V. K. Gupta and Y. S. Paul, Eds., Diseases of Field Crops, Indus Publishimg, New Delhi, 2002, pp. 155-192.
- C. Umamaheswari, G. Ramakrishnan and P. Nallathambi, “Role of Inoculum Level on Disease Incidence of Dry Root Rot Caused by Macrophominaphaseolina in Groundnut,” Madras Agriculture Journal, Vol. 87, No. 1-3, 2000, pp. 71-73.
- G. Olaya and G. S. Abawi, “Effect of Water Potential on Mycelial Growth and on Production and Germination of Sclerotia of Macrophominaphaseolina,” Plant Disease, Vol. 80, No. 12, 1996, pp. 1351-1354. doi:10.1094/PD-80-1351
- K. M. Satischandra, R. V. Hiremath and R. K. Hegde, “Factors Affecting the Survival of Rhizoctonia bataticola in Black Soil,” Plant and Soil, Vol. 54, No. 2, 1980, pp. 307-312. doi:10.1007/BF02181856
NOTES
*Corresponding author.

