American Journal of Analytical Chemistry
Vol. 4 No. 7A (2013) , Article ID: 34470 , 8 pages DOI:10.4236/ajac.2013.47A016
Heterogeneous Oxidation of Methylene Blue with Surface-Modified Iron-Amended Activated Carbon
Department of Molecular Bioscience and Bioengineering, University of Hawaii at Manoa, Honolulu, USA
Email: *ekan@hawaii.edu
Copyright © 2013 Jihyun R. Kim et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received May 18, 2013; revised June 19, 2013; accepted July 7, 2013
Keywords: Adsorption; Activated Carbon; Iron-Amended Activated Carbon; Heterogeneous Oxidation; Fenton Oxidation; Methylene Blue
ABSTRACT
The present study aims to develop effective adsorption and oxidation of synthetic dye in wastewater by using the newly synthesized iron-amended activated carbon. Recently synthetic dye-containing wastewater has gained more attention due to its mass discharge, high toxicity and low biodegradation. For enhancing adsorption of dye and oxidative regeneration of dye-exhausted activated carbon, the novel amendment of iron-deposited granular activated carbon (GAC) was developed. It was to amend ferrous ion onto the acid-pretreated GAC when pH of iron solution was higher than the pH at point of zero charge (pH, pzc) of the GAC. Methylene blue (MB) in water was adsorbed onto the acid-treated ironamended GAC (Fe-GAC) followed by single or multiple applications of H2O2. Batch experiments were carried out to study the adsorption isotherm and kinetics indicating adsorption of MB onto the Fe-GAC followed Langmuir isotherm and the pseudo-second order kinetics. The Fe-GACshowed the maximum adsorption capacity (qm) of 238.1 ± 0.78 mg/g which was higher than the virgin GAC with qm of 175.4 ± 13.6 mg/g at 20˚C, pH 6 and the initial concentration of 20 - 200 mg/L. The heterogeneous Fenton oxidation of MB in the Fe-GAC revealedthat increasing the H2O2 loading from 7 to 140 mmol H2O2/mmol MB led to enhancing the oxidation efficiency of MB in the GAC from 62.6% to 100% due to the increased generation of hydroxyl radicals. Further enhancement of oxidation of MB in the Fe-GAC was made by the multiple application of H2O2 while minimizing OH radical scavenging often occurring at high concentration of H2O2. Therefore, the acid-treated iron-amended GAC would provide excellent adsorption capacity for MB and high oxidation efficiency of MB in the GAC with multiple applications of H2O2 and optimum iron loading.
1. Introduction
Synthetic dyes are widely used for textile, pulp and paper, plastic, food, cosmetic and pharmaceutical industries [1]. With mass production of synthetic dyes and mass discharge of synthetic dye-containing wastewater, effective treatment of dye-containing wastewater has gained more attention for the past decades [2]. Degradation of synthetic dyes is difficult because of their complex aromatic structure and nature of the lead compounds. Besides, the intermediate and byproducts of most of dye chemicals are considered to be harmful to aquatic organisms due to their toxicity and carcinogenic activity [3]. Therefore, efficient and cost-effective treatment processes of dyecontaining wastewater need to be developed [4].
Adsorption using granular activated carbon (GAC) as one of most reliable methods has been used to remove dyes in wastewater. It is simple, effective and independent from toxicity of dye chemicals. However, regeneration of dye-exhausted activated carbon determines the overall operating costs because the dye-spent activated carbon needs to be regenerated to achieve its re-adsorptive capacity. For most of cases involving GAC regeneration, the spent GAC is thermally regenerated either on-site or transported to a thermal regeneration facility and regenerated off-site. The current thermal regeneration method often leads to significant deterioration of the carbon pore structure, specific surface area and functionality which influences on re-adsorptive capacity of contaminant-spent activated carbon [5].
Alternative to the thermal regeneration, Fenton oxidation-driven regeneration of the spent GAC is a treatment option under development to regenerate the GAC on-site and in situ. It has shown excellent performance to oxidize various contaminants including MTBE, BTEX, TCE and chlorobenzene from the activated carbon [6,7]. The Fenton oxidation-driven regeneration relies on heterogeneous Fenton oxidation which oxidizes contaminants at the GAC by using OH radicals generated by reaction between H2O2 and iron immobilized at the GAC [7]. Thus, it leads to effective oxidation of contaminants at surface of the GAC with little generation of iron sludge which is different from mass production of iron sludge by homogeneous Fenton oxidation. For heterogeneous Fenton oxidation, iron amendment onto the GAC is of prime importance to determine oxidation efficiency of contaminants in the GAC since more uniform iron amendment onto the GAC minimizes intraparticle diffusion limitation, the rate-limiting step during heterogeneous Fenton oxidation in the GAC [7].
For this study, the acid-treated iron-amended GAC (Fe-GAC) was synthesized by amending Fe2+ onto the acid-pretreated GAC at the conditions that the pH of Fe2+ solution was higher than the pH at point of zero charge (pH, pzc) of the GAC. As reported by Kan and Huling [8], the acid-treatment of the GAC was conducted to increase acidic surface groups containing oxygen and to lower pH, pzc of the GAC. When the acid-treated GAC was suspended in the Fe2+ solution at the conditions that the pH of ferrous solution was higher than the pH, pzc of the acid-treated GAC, the more negatively charged surface of the acid-treated GAC enhanced electrostatic attraction with Fe2+ for making more uniform Fe deposition into the GAC [7]. The more uniform Fe deposition into the GAC would help effective oxidation of the contaminants in the GAC with steady diffusion and reaction of H2O2 into the pores of the GAC.
Therefore, the present study characterized the adsorption capacity and the oxidation efficiency of the acidtreated Fe-amended GAC (Fe-GAC) for treating MBcontaminated water. The adsorption isotherm and kinetics using the Fe-GAC were analyzed for understanding the major mechanisms associated with adsorption of MB onto the Fe-GAC. The oxidation efficiency of MB in the Fe-GAC by heterogeneous Fenton oxidation was investigated at various iron and H2O2 loading.
2. Materials and Methods
2.1. Adsorbent and Adsorbate
The GAC (URV, 8 × 30 mesh, Calgon Carbon Corp., Pittsburgh, PA) was derived from bituminous coal and activated in a manner to minimize H2O2 reactivity (Harrision, CalgonCarbon personal communication). The GAC was rinsed with deionized (DI) water, dried in an oven at 105˚C, and stored in a desiccator until used. The surface area and pore volume of the GAC as received was 1290 m2/g and 0.64 mL/g, respectively [6]. Methylene blue (Figure 1, Fisher Scientific, Waltham MA), a cationic dye, which is difficult to be degraded in a natural environment, was chosen as the model adsorbate. A standard stock solution of 1000 mg/L was prepared and suitably diluted to the required initial concentration.
2.2. Preparation of Fe-Amended Activated Carbon
A stock solution of ferrous chloride was prepared immediately before the Fe solution was amended to the GAC by dissolving ferrous chloride (FeCl2·4H2O) into the DI water (50 mg/L as Fe2+). The acid-treated GAC was prepared by treating the 10 g virgin GAC with nitric acid at pH 3 for 4 d for increasing the acidic surface oxides and lowering its pH, pzc (pH at point of zero charge). A pH, pzc is the pH at which positive and negative surface charges are equal and GAC surface has a net charge of zero.
The acidic treatment of the GAC was to enhance the electrostatic interaction between the GAC and Fe2+ for nearly uniform iron deposition in the GAC. The 6.3 - 187.5 mL of 50 mg·L−1 Fe2+ was added to the 0.5 g the acid-treated GACs for iron amendment onto the GAC. After the iron amendment onto the acid-treated GACs, the GACs were rinsed three times with DI water to eliminate the chloride residual in the GACs and dried in an oven at 80˚C for 24 h.
2.3. Batch Adsorption Studies
The batch experiments for adsorption isotherm were conducted by suspending 25 mg of the Fe-GAC in a series of flasks containing 50 mL of methylene blue solutions at the concentration of 0.02 - 0.2 g/L. The initial pH of MB solution (pH = 6.0) was adjusted by adding 0.1 M HNO3 or 0.1 M NaOH. The initial concentrations of MB were obtained by measuring O.D. at 663 nm (λmax) using BioSpec-mini UV-Vis spectrophotometer (Shimadzu, Carlsbad CA). The Fe-GAC used for the adsorption isotherm experiments contained 6 mg Fe/g GAC. The adsorption process was carried out by agitating at the constant speed (200 rpm) at room temperature (20˚C) for 72 h to ensure equilibrium was reached. The post-sorption MB solution was sampled after equilibrium (>3 d) in replicate and analyzed. The Differences between initial and final concentrations were used to calculate the mass of MB adsorbed to the GAC. The MB uptake at equilibrium, qe (mg/g), was calculated by Equation (1):
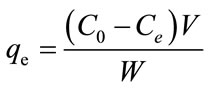 (1)
(1)
where C0 and Ce (mg/L) are the liquid-phase concentration of dye at initial and at equilibrium, respectively. V (L) is the volume of the solution, and W (g) is the mass of dry adsorbent used. All samples were centrifuged prior to analysis to minimize the interference of carbon fines with the analysis.
For understanding the adsorption mechanism and capacity of the GAC, the Langmuir and Freundlich isotherm models were used to interpret the batch isotherm data. The Langmuir equation [9] is valid for monolayer adsorption on a surface with a finite number of identical sites while Freundlich model [10] is an empirical equation based on adsorption on a heterogeneous surface. The linear form of Freundlich and Langmuir isotherms are shown in Equations (2) and (3), respectively:
Freundlich isotherm:
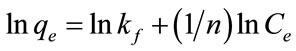 (2)
(2)
Langmuir isotherm:
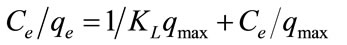 (3)
(3)
where lnkf is roughly a measure of the maximum adsorption capacity and 1/n is an indicator of adsorption effectiveness; qmax is the maximum adsorption capacity (mg/g) corresponding to complete monolayer coverage of the surface, qe is the amount of dye adsorbed per unit mass of adsorbent (mg/g), Ce is the liquid-phase concentration of dye at equilibrium and KL is the Langmuir constant (L/g) related to the sorption/desorption energy.
2.4. Effect of pH
The effect of pH on the color removal and adsorption capacity of MB was analyzed over the pH range from 3 to 11. The pH was adjusted using 0.1 M HCl and 0.1 M NaOH solutions. The dye solution (60 mL) at concentration of 1000 mg/L with 0.5 g of acid-treated Fe amended GAC was shaken for 72 h at room temperature (20˚C) on a shaker at a constant speed of 200 rpm. The samples were then centrifuged and analyzed using a UV spectrophotometer.
2.5. Adsorption Kinetics
Adsorption kinetic experiments were carried out by suspending 0.5 g of the Fe-GAC in 60 mL of each MB solution at initial concentrations of 80 mg/L to 1000 mg/L, a pH 6 and 20 for 24 h under mixing. Aliquots of 0.1 mL to 1 mL depending on the solution of concentration were withdrawn at different time intervals, centrifuged and analyzed for the MB concentration using a UV spectrophotometer. The kinetic of the adsorption were analyzed using two different kinetic models: the pseudo-first order and pseudo-second order. The pseudo-first order kinetic model is given by Lagergren [11]:
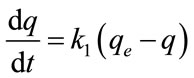 (4)
(4)
where q and qe represent the amount of dye adsorbed (mg/g) at any time t and at equilibrium time, respectively, and k1 represents the sorption rate constant (min−1). Integrating Equation (4) with respect to boundary conditions q = 0 at t = 0 and q = qt at t = t, then Equation (4) becomes:
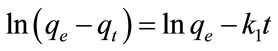 (5)
(5)
where qe and qt (mg/g) are the amounts of adsorbate adsorbed at equilibrium and at any time, t (min), respectively, and k1 (min−1) is the adsorption rate constant. Thus the rate constant k1 (min−1) and qe can be calculated from the plot of ln(qe − qt) versus time t.
The pseudo-second-order equation [12] based on equilibrium adsorption is expressed as:
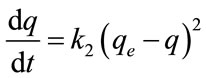 (6)
(6)
where k2 (g/mg min) is the rate constant of second-order adsorption, qe and q represent the amount of dye adsorbed (mg/g) at equilibrium and at any time t. Separating the variables in Equation (6) gives:
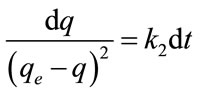 (7)
(7)
Integrating Equation (7).with respect to boundary conditions t = 0 to t = t and q = 0 and q = qe gives
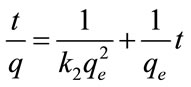 (8)
(8)
The rate constant of second-order adsorption (k2) and qe will be evaluated from the linear plot t/qt versus t.
2.6. pH, Point of Zero Charge
The pH at point of zero charge (pH, pzc) of the GAC was determined using the pH drift method [7]. The 50 mL of DI water amended with 0.01 M NaCl was placed in the 100 mL amber vials and sparged with N2 (200 mL/min; 10 - 15 min) to eliminate CO2 and to stabilize pH. The pH was adjusted from pH 2 to pH 11 in a series of vials by adding either HCl or NaOH while purging the headspace with N2. 0.15 g of the GAC was added and the vial was capped immediately. The final pH (pH, final) was measured in each of the vials after 48 h and plotted versus the initial pH (pH, initial). The pH, pzc was determined graphically at the intersection of pH, final and the line pH, final = pH, initial.
2.7. Heterogeneous Fenton Oxidation of the MB-Saturated Fe-GAC
The effect of various iron loading (0.5 to 15 mg Fe/g GAC) on the oxidation efficiency of MB in the Fe-GAC was investigated. The oxidation of the MB-saturated Fe-GAC was performed with a single injection of 3.0 mL of 30% H2O2 (52.9 mmol H2O2/g GAC; Sigma Aldrich, MO) at pH of 3.0 - 3.5. The pH of solution before adding H2O2 was between 3.0 - 3.5. Since ·OH scavenging by high concentrations of H2O2 is a probable source of overall oxidation inefficiency [13], the multiple applications of H2O2 were made to minimize the initial H2O2 concentration and ·OH scavenging. H2O2 concentrations were monitored over time under complete mixing condition (H2O2, initial of 14.3 g/L). The oxidation efficiency of MB in the GAC was evaluated by measuring the MB in the GAC after Fenton oxidation. Similarly, the effect of H2O2 concentration on oxidation of MB in the Fe-GAC was investigated when various load of H2O2 (7 mmol to 140 mmol H2O2/mmol MB) at the fixed iron content in the Fe-GAC (5 mg Fe/g GAC) for determining the optimum load of H2O2.
The residual amount of MB in the Fe-GAC after Fenton oxidation was evaluated by extracting the MB in the Fe-GAC with 10 - 20 mL methanol for 3 d. The MB desorption and diffusion rate from MB-spent GAC was evaluated using the fill and draw method. This involved post-sorption applications of the DI water (60 mL) to MB-spent GAC (0.5 g) and measuring MB in solution at a different time interval. The rates of desorption was calculated as the mass of MB desorbed from the GAC divided by the mass of GAC and time period of desorption. In all cases tested, most of MB in the GAC was not released to the aqueous phase and the concentration of MB in the aqueous phase wasfar below the equilibrium MB concentration.
2.8. Effect of Semi-Continuous Feeding of H2O2 on Oxidation of MB in the Fe-GAC
In order to investigate the effect of the semi-continuous feeding of H2O2 on increasing MB oxidation efficiency in the Fe-GAC, one fourth of H2O2 amount was added at four different points of time at every 12 h. The initial H2O2 concentration (28 mmol H2O2/mmol MB) was selected from the previous experiment with the same iron load which was 6 mg Fe/g GAC. The single point H2O2 injection from the previous section and semi-continuous feeding of H2O2 was evaluated the effectiveness of the H2O2 injections for oxidation ofthe MB in the Fe-GAC.
2.9. Analytical Methods
MB concentration in the supernatant solution after and before adsorption was determined using UV-Vis spectrophotometer at 663 nm. The filtered samples using 0.2 μm membrane filters were measured for H2O2 (n = 3) using amodified peroxytitanicacid colorimetric procedure with a detection limit of 0.1 mg/L [7]. Iron was measured in the GAC slurry solution using thePhenanthroline Method [7].
3. Results and Discussion
3.1. Adsorption of MB onto the GACs
3.1.1. Adsorption Isotherm Analysis
In order to estimate the adsorption capacity of GACs for MB and to optimize the design of adsorption system, it is important to analyze the adsorption equilibrium data. In the present study, the Freundlich and Langmuir isotherms that are the most commonly used isotherms were applied to investigate the overall adsorption efficacy and to characterize the MB adsorption process in the system using Fe-GAC. The isotherm parameters of the Freundlich and Langmuir models for the adsorption of MB on virgin GAC and the Fe-GAC obtained at temperature of 20˚C after 72 h, are summarized in Table 1. The results indicated that the maximum adsorption capacity of the Fe-GAC (qm = 238.1 m ± 0.78 mg/g) in this study was higher compared with the virgin GAC (qm = 175.4 ± 13.6 mg/g). The result indicates the maximum adsorption capacity of the activated carbon prepared in this study was comparable with the works done by other studies on adsorption of MB in GAC (qm = 240 - 299 mg/g) [14,15]. Also, the analysis of the batch isotherm data supported that Langmuir isotherm model is the appropriate model for adsorption of MB onto the GAC indicating homogeneous monolayer adsorption of MB onto the site of the GAC.
3.1.2. Effect of Initial MB Solution pH on Adsorption of Methylene Blue on Fe-GAC
The pH of solution at which adsorption occurs is found to influence the extent of adsorption. The pH of MB solution affects adsorption in that it governs the degree of ionization of the acidic and basic functional groups in methylene blue (see Figure 1 for chemical structure of MB) [16]. The effect of initial pH of the MB solution on the amount of MB adsorbed was studied by varying the initial pH with constant process parameters. The experimental results indicated that the pH of initial MB solution did not possess any significant influence on the adsorption of methylene blue. The adsorption of MB at the
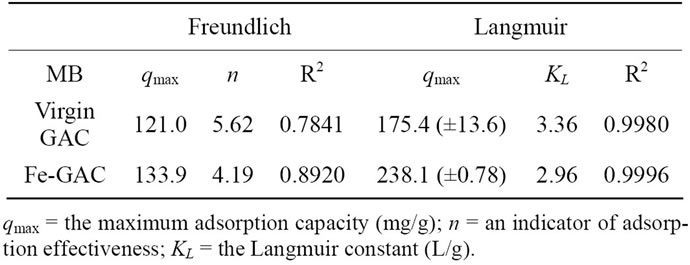
Table 1. Langmuir and Freundlich isotherm model coefficient for adsorption of MB on GACs. Conditions: Initial concentration of MB, 20 - 120 mg/L; volume of solution, 60 mL; initial solution pH, 6.0; temperature, 20˚C; contact time,72 h.
initial pH of 3 to 11 showed nearly complete uptake of methylene blue with the adsorption capacity of 123.9 to 135.1 mg MB/g GAC(data not shown). This finding is different from others’ investigation that initial pH value may enhance or depress adsorption of dye on activated carbon [17]. As reported by others, MB adsorption capacities are significantly improved at a higher solution pH [17,18]. This can be explained by the electrostatic attraction (Table 2) between the positively charged MB (solution pH > pKa, MB) and the negatively charged surface of activated carbon (solution pH > pH, pzc, Table 3). Increasing solution pH increases the number of hydroxyl groups thus, increases the number of negatively charged sites and enhances the attraction between dye and adsorbent surface [19]. However, the nearly constant adsorption capacity of Fe-GAC for MB over the pH range 3 to 11 in our study was an indication that the adsorption of MB on the Fe-GAC depends on both the electrostatic interaction and non-electrostatic such as van der Waals forces, hydrophobic interaction and hydrogen bonding in this system. It can be explained by the fact that adsorption of dye from aqueous solution on carbon is a complex interplay between non-electrostatic and electrostatic interactions [20].
3.1.3. Adsorption Kinetics
In order to investigate the mechanisms of MB adsorption on the Fe-GAC, the pseudo-first order and pseudo-second order kinetics models were used to fit the experimental data. The kinetic parameters of the pseudo-first
Figure 1. The molecular structure of methylene blue.
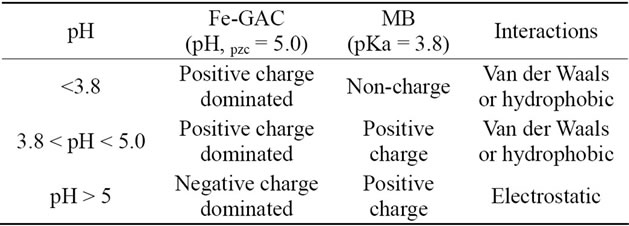
Table 2. Surface charge of the Fe-GAC and MB at various pH.
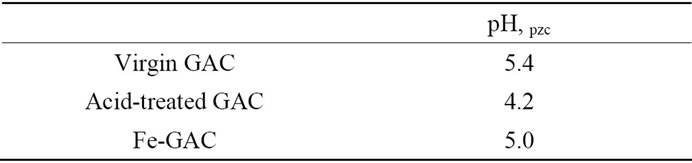
Table 3. pH, pzc of the virgin, acid-treated and acid-treated iron amended GAC.
order and the pseudo-second order models were calculated from the linearized Equations (5) and (6), respectively. MB adsorption of the pseudo-second order kinetic fitting results is shown in Figure 2. The kinetic parameters acquired from fitting the results are summarized in Table 4. The correlation coefficient values for the pseudo-first order and pseudo-second order kinetic models were found to be close to one; however, the experimental qe values for the first-order kinetic model do not agree with the calculated ones whereas the pseudo-second ordershow a good agreement between experimental and calculated qe values.
The applicability of the kinetic model to describe the adsorption process was further validated by the normalized standard deviation, 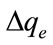 (%), which is defined as:
(%), which is defined as:
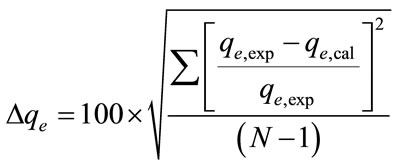 (9)
(9)
where N is the number of data points, qe,exp and qe,cal (mg/g) are the experimental and calculated equilibrium adsorption capacity value, respectively [21]. The 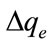 obtained for the pseudo-first-order kinetic model was
obtained for the pseudo-first-order kinetic model was
 (a)
(a)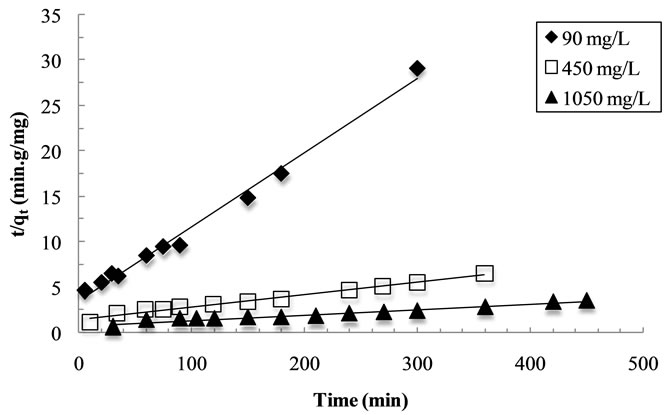 (b)
(b)
Figure 2. Adsorption kinetics of MB on the Fe-GAC. Conditions: Initial concentration of MB, 90, 450 and 1050 mg/L; volume of solution, 60 mL; initial solution pH, 6.0; temperature, 20˚C; contact time, 300 - 500 min. (a) Pseudofirst-order kinetics; (b) Pseudo-second-order kinetics.
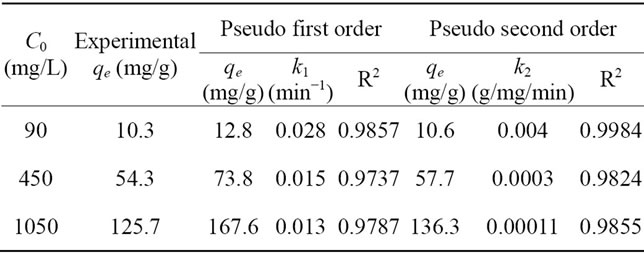
Table 4. Kinetic parameters for MB adsorption onto Feamended GAC.
38.53%, which is relatively high as compared to the 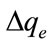 values of 7.04% obtained for the pseudo-second order kinetic model. Base on the high correlation coefficient values and the low
values of 7.04% obtained for the pseudo-second order kinetic model. Base on the high correlation coefficient values and the low 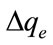 value, the pseudo second order model adequately described the experimental data of the adsorption of MB onto Fe-GAC. This suggested that the overall rate of the adsorption process was controlled by chemical sorption or chemisorption, which involved valency forces through sharing or exchange of electrons between the adsorbent and adsorbate [12]. The similar phenomena have been observed in the adsorption of MB on the bamboo-based activated carbon [22], the activated carbon prepared from rattan sawdust [23] and on the activated carbon from waste biomass by sulfuric acid activation [24].
value, the pseudo second order model adequately described the experimental data of the adsorption of MB onto Fe-GAC. This suggested that the overall rate of the adsorption process was controlled by chemical sorption or chemisorption, which involved valency forces through sharing or exchange of electrons between the adsorbent and adsorbate [12]. The similar phenomena have been observed in the adsorption of MB on the bamboo-based activated carbon [22], the activated carbon prepared from rattan sawdust [23] and on the activated carbon from waste biomass by sulfuric acid activation [24].
3.2. Heterogeneous Fenton Oxidation of the MB in the Fe-GAC
Fenton and Fenton-like reaction for the treatment of wastewater are very efficient in a pH range between 2.8 and 3 [25,26]. In the present study, adjusting the pH of the MB-saturated Fe-GAC in the solution was not needed because the pH of solution after MB saturation onto Fe-GAC was approximately 3. As the major reactions in Fenton oxidation describe in Equations (10)-(13), the excessive H2O2 and Fe2+ scavenge OH radical that is the major oxidant in Fenton oxidation used for non-specific oxidation of broad ranges of contaminants.
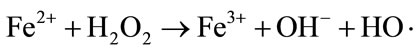 (10)
(10)
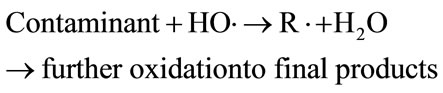 (11)
(11)
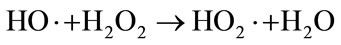 (12)
(12)
 (13)
(13)
Thus, the heterogeneous Fenton oxidation of MB in the Fe-GAC was carried out at various iron or H2O2 loading for figuring the optimum conditions for oxidation of MB in the Fe-GAC. To use a heterogeneous catalytic system in industrial practice it is important to evaluate the loss of catalyst from the support [26]. In this study, there was no leaching of iron in the solution after all oxidation process at pH 3 and 20.
3.2.1. Effect of Fe Loading on Oxidation of MB in the Fe-GAC
In order to investigate the effect of the Fe loading on the MB oxidation in the Fe-GAC, the experiments were performed at the different Fe2+ loading of 0.5 to 15 mg Fe/g GAC at the fixed concentration of H2O2 of 140 mmol H2O2/mmol MB. Figure 3 shows the effect of Fe loading on oxidation of MB in the Fe-GAC to analyze the 1st order rate constant and half-life of H2O2. It indicated enhancing the rate constant for H2O2 consumption while reducing the half-life of H2O2 at increasing iron loading in the GAC. Besides, the oxidation efficiency of MB in the Fe-GAC was close to 100% in all the conditions of Fe loading (0.5 mg/g GAC to 15 mg/g GAC) due to the excessive initial concentration of H2O2. Note that 36 moles of H2O2 are required to completely oxidize 1 mole of MB based on the balanced stoichiometric equation of oxidation of MB. Therefore, the effect of H2O2 concentration on the oxidation of MB in the Fe-GAC was investigated at the fixed loading of Fe (6 mg Fe/g GAC).
3.2.2. Effect of Single and Multiple H2O2 Injection on MB Oxidation
Two sets of experiments with the single and the multiple application of the H2O2 to the MB-saturated Fe-GAC were designed to evaluate the effectiveness of the H2O2 application [27]. The initial hydrogen peroxide concentration was varied between 7 to 140 mmol H2O2/mmol MB with 6 mg Fe/g GAC from the previous section. The effect of the single application of H2O2 on MB oxidation is shown in Figure 4. The increase of the H2O2 concentration from 7 to 140 mmol H2O2/mmol MB led to an increase in the MB removal efficiency from 62.6% to
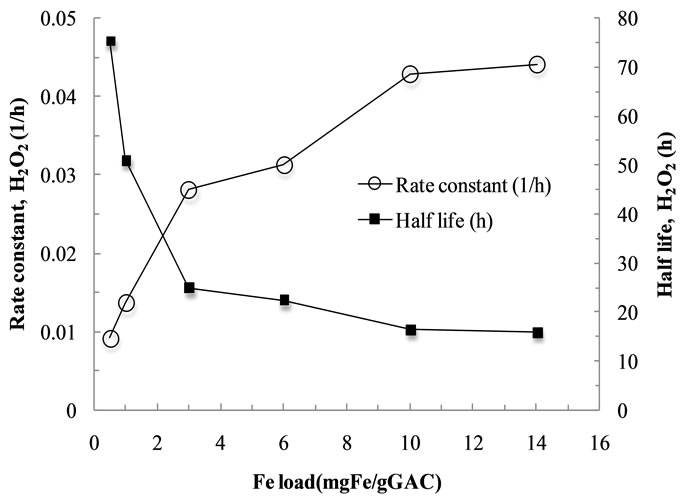
Figure 3. Effects of iron loading on oxidation of the MBspent Fe-GAC with the fixed H2O2 dose. Conditions: Initial concentration of MB, 1000 mg/L; volume of solution, 60 mL; initial solution pH, 6.0; temperature, 20˚C.
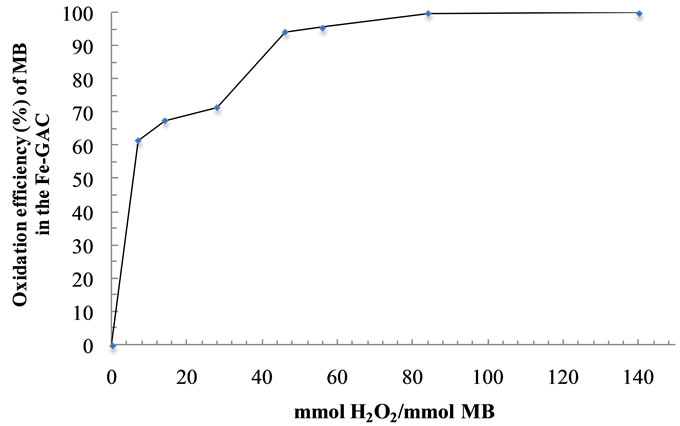
Figure 4. Effect of H2O2 on the oxidation of MB in the Fe-GAC. Conditions: Initial concentration of MB, 1000 mg/L; volume of solution, 60 mL; initial solution pH, 6.0; temperature, 20˚C.
100%, respectively. The selection of this optimum concentration is important from the commercial point of view due to the high cost of H2O2 [28].
For the multiple H2O2 application, one fourth of H2O2 amount was added at four different points of time. The initial H2O2 loadingof 28 mmol H2O2/mmol MB was selected from the previous experiment with 6 mg Fe/g GAC. It was lower than the stoichiometric demand of H2O2 for the complete mineralization of MB (36 mmol of H2O2 per mmol of MB). The results showed that the MB oxidation efficiency of multiple H2O2 application was 84.1%, which was higher than that by a single H2O2 application which was 71.6%. The multiple additions of H2O2 are more effective than the single addition of H2O2 because scavenging of OH radicals due to the excessive H2O2 could be reduced with the staged application of H2O2 [27].
4. Conclusion
The present study presents that the acid-treated iron amended activated carbon (Fe-GAC) is an effective adsorbent and catalyst for the adsorption and oxidation of methylene blue from aqueous solution. The modification of carbon surface by acid treatment has proven to play an important role for more uniform deposition of iron in the GAC. The equilibrium data were fitted to linear models of Freundlich and Langmuir model, and the equilibrium data of Fe-GAC were best described by the Langmuir isotherm model with the maximum monolayer adsorption capacity of 238.1 m ± 0.78 mg/g at 20˚C. Adsorption kinetic studies of MB demonstrated that the adsorption rate followed the pseudo-second-order kinetic model. For the heterogeneous Fenton oxidation of MB in the FeGAC, the increase of H2O2 concentration from 7 to 140 mmol H2O2/mmol MB led to an increase in MB removal efficiency from 62.6% to 100%. Besides, the multiple application of H2O2 to the MB-saturated Fe-GAC increased the oxidation efficiency of MB in the GAC up to 84.1% which was more effective than that by the single application of H2O2.
REFERENCES
- S. Khorramfar, N. M. Mahmoodi, M. Arami and K. Gharanjig, “Equilibrium and Kinetic Studies of the Cationic Dye Removal Capability of a Novel Biosorbent Tamarindus Indica from Textile Wastewater,” Coloration Technology, Vol. 126, No. 5, 2010, pp. 261-268. doi:10.1111/j.1478-4408.2010.00256.x
- S. Khorramfar, N. M. Mahmoodi, M. Arami and H. Bahrami, “Oxidation of Dyes from Colored Wastewater Using Activated Carbon/Hydrogen Peroxide,” Desalination, Vol. 279, No. 1-3, 2011, pp. 183-189. doi:10.1016/j.desal.2011.06.005
- H. J. Fan, H. Y. Shu and K. Tajima, “Decolorization of Acid Black 24 by the FeGAC/H2O2 Process,” Journal of Hazardous Materials, Vol. 128, No. 2-3, 2006, pp. 192- 200. doi:10.1016/j.jhazmat.2005.07.059
- V. P. Santos, M. F. R. Pereira, P. C. C. Faria and J. J. M. Orfao, “Decolourisation of Dye Solutions by Oxidation with H2O2 in the Presence of Modified Activated Carbons,” Journal of Hazardous Materials, Vol. 162, No. 2-3, 2009, pp. 736-742. doi:10.1016/j.jhazmat.2008.05.090
- S. G. Huling, P. K. Jones, W. P. Ela and R. G. Arnold, “Fenton-Driven Chemical Regeneration of MTBE-Spent GAC,” Water research, Vol. 39, No. 10, 2005, pp. 2145- 2153. doi:10.1016/j.watres.2005.03.027
- S. G. Huling, P. K. Jones and T. R. Lee, “Iron Optimization for Fenton-Driven Oxidation of MTBE-Spent Granular Activated Carbon,” Environmental Science and Technology, Vol. 41, No. 11, 2007, pp. 4090-4096. doi:10.1021/es062666k
- E. Kan and S. G. Huling, “Effects of Temperature and Acidic Pre-Treatment on Fenton-Driven Oxidation of MTBESpent Granular Activated Carbon,” Environmental Science and Technology, Vol. 43, No. 5, 2009, pp. 1493- 1499. doi:10.1021/es802360f
- S. G. Huling, E. Kan and C. Wingo, “Fenton-Driven Regeneration of MTBE-Spent Granular Activated CarbonEffects of Particle Size and Iron Amendment Procedures,” Applied Catalysis B-Environmental, Vol. 89, No. 3-4, 2009, pp. 651-658. doi:10.1016/j.apcatb.2009.02.002
- I. Langmuir, “The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum,” Journal of American Chemical Society, Vol. 40, No. 9, 1918, pp. 1361-1403. doi:10.1021/ja02242a004
- H. Freundlich, “Over the Adsorption in Solution,” Journal of Physical Chemistry, Vol. 57, 1906, pp. 385-470.
- S. Lagergren, “About the Theory of So-Called Adsorption of Soluble Substances,” Kungliga Svenska Vetenskapsakademiens Handlingar, Vol. 24, No. 4, 1898, pp. 1-39.
- Y. S. Ho and G. McKay, “Pseudo-Second Order Model for Sorption Processes,” Process Biochemistry, Vol. 34, No. 5, 1999, pp. 451-465. doi:10.1016/S0032-9592(98)00112-5
- T. A. Kurniawan and W. H. Lo, “Removal of Refractory Compounds from Stabilized Landfill Leachate Using an Integrated H2O2 Oxidation and Granular Activated Carbon (GAC) Adsorption Treatment,” Water Research, Vol. 43, No. 16, 2009, pp. 4079-4091. doi:10.1016/j.watres.2009.06.060
- F. Raposo, M. A. De La Rubia and R. Borja, “Methylene Blue Number as Useful Indicator to Evaluate the Adsorptive Capacity of Granular Activated Carbon in Batch Mode: Influence of Adsorbate/Adsorbent Mass Ratio and Particle Size,” Journal of Hazardous Materials, Vol. 165, No. 1-3, 2009, pp. 291-299. doi:10.1016/j.jhazmat.2008.09.106
- G. G. Stavropoulos and A. A. Zabaniotou, “Production and Characterization of Activated Carbons from OliveSeed Waste Residue,” Microporous and Mesoporous Materials, Vol. 82, No. 1-2, 2005, pp. 79-85. doi:10.1016/j.micromeso.2005.03.009
- W. J. Weber, “Physiochemical Properties for Water Quality Control,” John Wiley and Sons Inc, New York, 1972.
- E. N. El Qada, S. J. Allen and G. M. Walker, “Adsorption of Basic Dyes from Aqueous Solution onto Activated Carbons,” Chemical Engineering Journal, Vol. 135, No. 3, 2008, pp. 174-184. doi:10.1016/j.cej.2007.02.023
- S. B. Wang, Z. H. Zhu, A. Coomes, F. Haghseresht and G. Q. Lu, “The Physical and Surface Chemical Characteristics of Activated Carbons and the Adsorption of Methylene Blue from Wastewater,” Journal of Colloid and Interface Science, Vol. 284, No. 2, 2005, pp. 440-446. doi:10.1016/j.jcis.2004.10.050
- C. Lai and C. Y. Chen, “Removal of Metal Ions and Humic Acid from Water by Iron-Coated Filter Media,” Chemosphere, Vol. 44, No. 5, 2001, pp. 1177-1184. doi:10.1016/S0045-6535(00)00307-6
- C. Moreno-Castilla, “Adsorption of Organic Molecules from Aqueous Solutions on Carbon Materials,” Carbon, Vol. 42, No. 1, 2004, pp. 83-94. doi:10.1016/j.carbon.2003.09.022
- B. H. Hameed, I. A. W. Tan and A. L. Ahmad, “Adsorption Isotherm, Kinetic Modeling and Mechanism of 2,4,6- Trichlorophenol on Coconut Husk-Based Activated Carbon,” Chemical Engineering Journal, Vol. 144, No. 2, 2008, pp. 235-244. doi:10.1016/j.cej.2008.01.028
- B. H. Hameed, A. M. Din and A. Ahmad, “Adsorption of Methylene Blue onto Bamboo-Based Activated Carbon: Kinetics and Equilibrium Studies,” Journal of Hazardous Materials, Vol. 141, No. 3, 2007, pp. 819-825. doi:10.1016/j.jhazmat.2006.07.049
- B. H. Hameed, A. Ahmad and K. Latiff, “Adsorption of Basic Dye (Methylene Blue) onto Activated Carbon Prepared from Rattan Sawdust,” Dyes and Pigments, Vol. 75, No. 1, 2007, pp. 43-49. doi:10.1016/j.dyepig.2006.05.039
- S. Karagöz, T. Tay, S. Ucar and M. Erdem, “Activated Carbons from Waste Biomass by Sulfuric Acid Activation and Their Use on Methylene Blue Adsorption,” Bioresource Technology, Vol. 99, No. 14, 2008, pp. 6214- 6222. doi:10.1016/j.biortech.2007.12.019
- M. Pérez, F. Torrades, J. A. Garcı́a-Hortal, X. Domènech and J. Peral, “Removal of Organic Contaminants in Paper Pulp Treatment Effluents under Fenton and Photo-Fenton Conditions,” Applied Catalysis B: Environmental, Vol. 36, No. 1, 2002, pp. 63-74. doi:10.1016/S0926-3373(01)00281-8
- J. H. Ramirez, C. A. Costa, L. M. Madeira, G. Mata, M. A. Vicente, M. Rojas-Cervantes, et al., “Fenton-Like Oxidation of Orange II Solutions Using Heterogeneous Catalysts Based on Saponite Clay,” Applied Catalysis B: Environmental, Vol. 71, No. 1-2, 2007, pp. 44-56. doi:10.1016/j.apcatb.2006.08.012
- D. Kim, J. K. C. Chen and T. F. Yen, “Naval Derusting Wastewater Containing High Concentration of Iron, Treated in UV Photo-Fenton-Like Oxidation,” Journal of Environmental Sciences, Vol. 22, No. 7, 2010, pp. 991-997. doi:10.1016/S1001-0742(09)60209-6
- K. Dutta, S. Mukhopadhyay, S. Bhattacharjee and B. Chaudhuri, “Chemical Oxidation of Methylene Blue Using a Fenton-Like Reaction,” Journal of Hazardous Materials, Vol. 84, No. 1, 2001, pp. 57-71. doi:10.1016/S0304-3894(01)00202-3
NOTES
*Corresponding author.

