World Journal of Neuroscience
Vol.3 No.4(2013), Article ID:39541,5 pages DOI:10.4236/wjns.2013.34040
Intracranial aneurysm with neck indistinguishable from surrounding artery branches by cerebral angiography
![]()
Department of Neurosurgery, Fuzhou General Hospital, Fuzhou, China
Email: liuzhengfz18@126.com, yuanbongqing@126.com
Copyright © 2013 Zheng Liu, Bangqing Yuan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 20 September 2013; revised 20 October 2013; accepted 27 October 2013
Keywords: Intracranial Aneurysm; Computed Tomographic Angiography; Digital Subtraction Angiography; Aneurysm Clipping; Embolization
ABSTRACT
The aim of this study is to examine morphology of intracranial aneurysm with neck indistinguishable from surrounding artery branches by cerebral angiography and discuss whether such aneurysms can be treated by interventional embolization. 6 patients who had not been treated by embolization due to irregular wide-necked aneurysms indistinguishable from surrounding artery branches by cerebral angiography received craniotomy for aneurysm clipping. The operations succeeded. Morphologically, neck width and location of the aneurysms were carefully observed and photographed from different directions and multi-angles during operation. The intraoperative findings were compared with the preoperative CTA and DSA images. Walls of the 6 patients’ aneurysms tightly clung to or adhered to surrounding branches and oppressed the branches into arcs, similar to the aneurysm walls in shape, and arterial branches and aneurysm walls suffered from segmental adhesion. In addition, abnormalities of communicating arteries to vary degrees were observed in 4 patients. However, after successful surgical clipping, it was revealed that the aneurysms would have been better treated by embolization since they are basically saccular aneurysms with regular sizes. Deformations in preoperative angiography may be due to anatomical variations of surrounding vessels near the aneurysms, aneurysm wall oppression or incomplete adhesion of surrounding arterial branches. Such deformations can be recognized by careful observation in preoperative angiography from different directions and multi-angles.
1. INTRODUCTION
Intracranial aneurysm is a fairly common malformation and it occurs in approximately 5% of the general population [1,2]. It is often asymptomatic until the time of rupture, causing bleeding into the brain or the space closely surrounding the brain. Subarachnoid hemorrhage (SAH) associated with aneurysmal rupture is potentially lethal with a mortality rate of 50 percent or more and many patients who survived the initial hemorrhage have shown permanent disability [3]. Due to increased knowledge of natural history and prevalence of aneurysms, and advances in imaging technologies, early detection of unruptured asymptomatic intracranial aneurysms has increased significantly in recent years [4]. An unbiased assessment of most appropriate treatment option for the malformation is crucial to achieve a favorable outcome for patients. However, the choice of open surgery verses embolization treatment option in aneurysm management, whether ruptured or unruptured, remains a challenging, and often an individualized decision making with following factors, such as age, past medical history, medical/neurological conditions and interpretation of preoperative angiography, plays an important role for both surgeons and patients as well as their family members.
Here we report that during a period from Feb 2011 to Feb 2012 at our hospital, we had performed clipping operations for 136 patients with ruptured intracranial aneurysms. Among their family members, 6 patients had strongly requested for embolization treatment; however, it was found in those patients by preoperative cerebral angiography that irregular wide-necked aneurysms were not distinguished from surrounding artery branches. Although based on the observations, 6 patients then received craniotomy for aneurysm clipping, during operation it was clearly observed that the intracranial aneurysms were regular in size and saccular in nature. They would have been better treated by embolization, instead of open surgery, suggesting that the interpretation of preoperative angiography plays an even more important role in treatment options for patients suffered from intracranial aneurysm. Deformations in the preoperative angiography can be recognized by more careful observation from different directions and angles.
2. METHODS
This study was approved by Fuzhou General Hospital Institutional Review Board and the board has waived the need for written informed consent from the participants. 6 patients, 4 males and 2 females, aged 34 - 56 years with a mean age of 44.6 years, were hospitalized, and all in emergency SAH admission. Time of onset was 1 - 10 days with an average of 3.3 days. The main symptoms included severe headache, seizure, stiff neck and vision problems. Neuroimaging examinations were performed upon admission and Computed Tomographic Angiography (CTA) examinations clearly showed SAH at cerebral base cistern in all 6 patients, 3 cases with a small amount of hematoma in the anterior longitudinal fissure cistern near prefrontal brain, and one case with hematoma near lateral fissure cistern. All patients were also examined by Digital Subtraction Angiography (DSA).
CTA examination was performed by GE 16-Detector Row CT scanner (Light speed) with scanning range from base of the skull to top. An 80 - 100 ml bolus of nonionic contrast agent (300 - 370 mgI/ml) was intravenously injected with a high-pressure syringe at a rate of 3.0 - 3.5 ml/sec at antecubital vein and the delay time was 16 - 21 sec. Data acquisition was performed with a nominal section thickness of 1.25 mm, a table feed of 13.75 mm per rotation, and a 0.8 sec rotation time (pitch, 1.375). The x-ray tube voltage setting was 120 kV, and mean tube current was 265 mA (range, 250 - 280 mA). After scan the data were transferred to AW4.2 workstation, bone or boneless 3-D images were reconstructed, respectively, and multi-directional and multi-angle images were obtained.
DSA examination (2D-DSA and 3D-DSA) was performed using GE Innova 3100 IQ and Mark V highpressure syringe. The Seldinger technique was used for conducting Femoral artery puncture and placing 5F arterial sheath at the puncture site. The 5F single curved imaging tube went through bilateral internal carotid artery, vertebral artery for conventional 2D DSA. 8 ml of nonionic contrast agent iopromide with iodine concentration at 370 mgI/ml was injected at a rate of 6 ml/sec for internal carotid artery and 6 ml of the same contrast agent was administered at a rate of 4 ml/sec for vertebral artery, respectively. If an aneurysm or a highly suspected case was found, rotational DSA 3D images (40 degree/sec, 5 ml/sec for 4 sec) were acquired. 20 ml of the contrast agent was injected at a rate of 5 ml/sec for internal carotid artery for 4 sec and 15 ml of the contrast agent was administered at a rate of 3 ml/sec for vertebral artery for 5 sec. The rotational angiography digital data were transferred to AW4.2 workstation and images were reconstructed by shaded surface display (SSD), transparency and Navigater techniques.
For those aneurysms with irregular wide-neck or neck indistinguishable from surrounding artery branches by preoperative angiography, aneurysm clipping craniotomy was performed. During surgery, aneurysm morphology, its neck width, and whether its wall was connected directly with surrounding branch lumen, were observed carefully and photographed in order to compare them with preoperative CTA and DSA images, respectively, as well as to assess whether embolization treatment would be an alternative option for the patients.
3. RESULTS
The cerebral angiography performed on the 6 patients clearly revealed intracranial aneurysms, including 5 cases of anterior communicating artery aneurysm and a case of middle cerebral artery aneurysm. It was observed by rotational 3D-DSA that all 6 cases of aneurysm were located at the artery bifurcation with a wide and fuzzy neck, and irregular body size, and the aneurysm wall “adhered” to, therefore was indistinguishable from adjacent artery branches, rendering it difficult to determine the aneurysm neck width and define clear boundary between the aneurysm wall and the surrounding branches. In addition, it was even observed that some artery branches were derived from the aneurysm wall (Figures 1(A) and (B) and Figures 2(A) and (B), arrow). Based on the observations, surgical craniotomy was chosen for aneurysm clipping for the patients, although whose family members strongly requested for embolization treatment.
From admission of the patients to performing craniotomy the average time was 7.3 h. Microsurgical operation via pterional approach was performed. The aneurysm and the surrounding artery branches were exposed and carefully separated. It was found clearly during operation that walls of the 6 patients’ aneurysms were tightly clung to or adhered to the surrounding artery branches, which were oppressed into arcs, similar to the aneurysm walls in shape. In 4 cases, the aneurysm walls were easily separated from the adjacent artery branches and the aneurysms became completely free (Figures 1(C) and (D)). However, in another 2 cases, the arterial branches and the aneurysm walls suffered from segmental adhesion (Figures 2(C) and (D)) and it was further observed that the aneurysm wall was not connected directly with the branched arteries. On both sides of the aneurysm neck enough space was created, suitable for aneurysm clipping. The aneurysms then were successfully clipped and the clipping of the aneurysm caused its
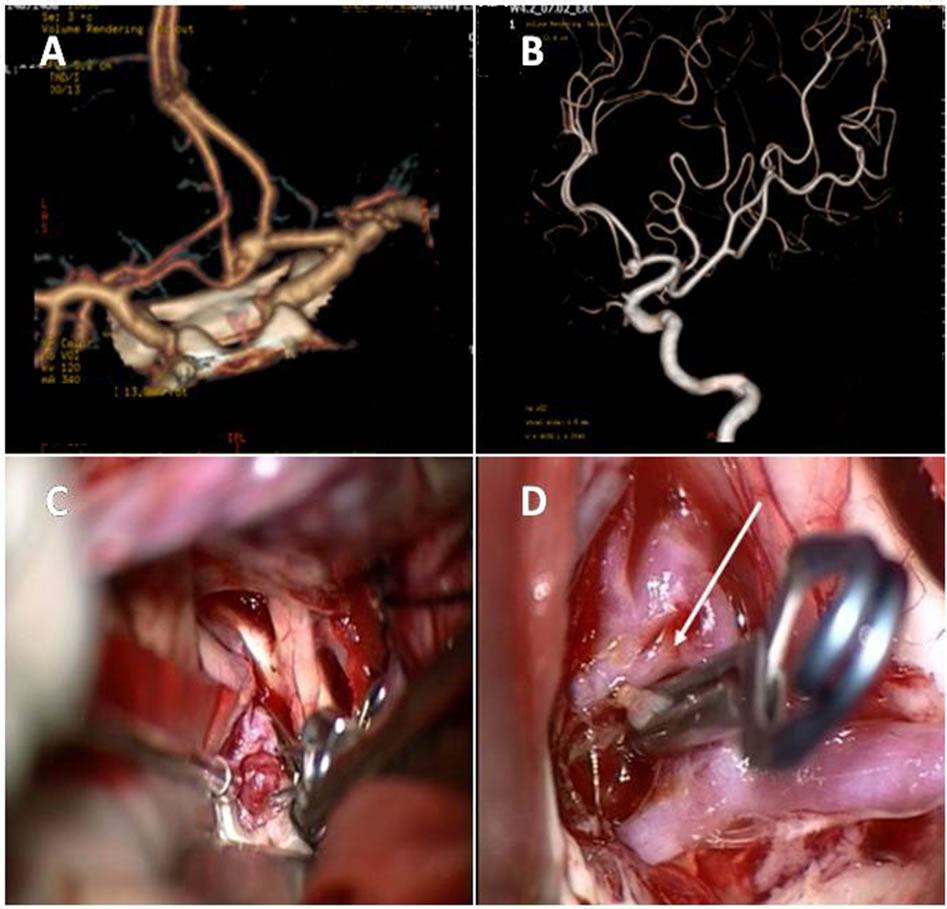
Figure 1. Preoperative CTA, DSA angiography and intraoperative findings of the anterior communicating artery aneurysms. The preoperative CTA (A) and DSA (B) revealed that the aneurysms were located at the artery bifurcation with a wide neck and irregular body size, and the aneurysm wall was indistinguishable from the adjacent artery branches, which appeared to be derived from the aneurysm wall. However, during operation, it was observed that the anterior artery aneurysm was regular cystic in shape and the aneurysm wall oppressed around the artery branches (C). The clipping of the aneurysm caused its neck retracted (D). In addition, it was shown that the left anterior communicating artery appeared abnormal with fenestration (D, arrow) and its branches were in close proximity with the aneurysm wall.
neck retracted. However, the aneurysm wall still partly adhered to the branched artery walls. In addition, in 4 cases, variations of anterior communicating artery in varying degrees, such as duplication and fenestration, were found (Figure 1(D), arrow).
After successful clipping, it was observed that the aneurysm morphology belonged to regular cystic appearance in shape with smaller neck, suitable for embolization. The 6 patients recovered and were discharged from hospital in about 3 weeks.
4. DISCUSSION
Interventional treatment has gradually become an important method of aneurysm management and virtually almost 100% of aneurysms have been occluded by embolization in recent years [5]. However, with regard to widenecked and large aneurysms, current technical limitations in embolization technology have prevented complete and durable aneurysm occlusion [6]. It is generally believed that contraindications of embolization include the following: aneurysm is too small in size (<2 mm); intubation artery is with severe hardening distortions or
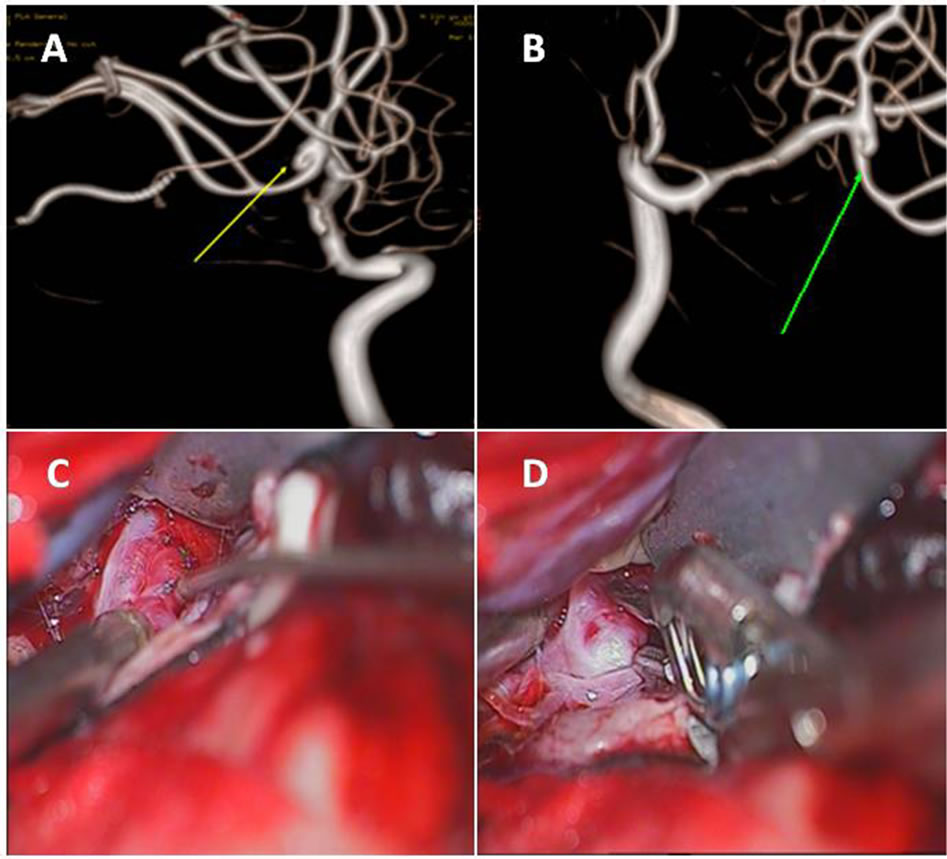
Figure 2. Preoperative CTA, DSA angiography and intraoperative findings of the right middle cerebral artery aneurysm. The preoperative CTA (A) and DSA (B) revealed that the size of the right middle cerebral artery aneurysm was irregular with a wide neck and the aneurysm wall was indistinguishable from adjacent arterial branches (arrows). During operation, it was observed that the right middle cerebral artery aneurysm was regular cystic in shape and the aneurysm wall adhered to the artery branches (C). On both sides of the aneurysm neck enough space was created because of incomplete adhesion and the aneurysm was clipped successfully (D).
severe vasospasm and micro-catheter is difficult to enter the aneurysm cavity; artery is ruptured of a serious condition and the clinical symptoms reach Hunt and Hess Scale 5 [7]; prolonged clotting time, coagulation disorders, and liver and kidney failure limit the use of the contrast agent; contrast agent allergy or there is a clear history of allergies; when performing angiography cavity and neck of aneurysm is indistinguishable from adjacent arteries and there are artery branches derived from aneurysm cavity or neck, unsuitable for embolization treatment [8,9]. The last contraindication is similar to our preoperative observations in this study and that was why the craniotomy was chosen over embolization for the 6 patients involved.
It is well known that CTA and DSA (including 2Dand 3D-DSA) are very useful detection tools for diagnosis of intracranial aneurysms in neurosurgery [1]. It is also paramount important to examine by angiography the morphology, neck width and location of the aneurysm prior to making treatment decisions. Although CTA is able to show the morphology of aneurysm and reveal its relations with adjacent blood vessels, sometimes it is with difficulty to find aneurysms in small sizes (2 - 3 mm). Therefore, DSA is still recognized as the gold standard for diagnosis of intracranial aneurysm because of its high image resolution (down to 0.3 mm in size) and high degree of sensitivity and specificity [10], which can be used to more carefully analyze preoperative images as it overcomes overlapping of vascular structures and observes aneurysm and its boundary with the surrounding artery branches from a full range of six directions and multi-angles. We herein suggest that preoperative CTA and DSA angiographic images should be reviewed independently by at least two experienced radiologists. The multiplanar reconstruction and source images should be always analyzed in conjunction with the corresponding 3D images because of misinterpretation potential. The radiologists assess whether intra-arterial CTA is superior, equal, or inferior to DSA with regard to the depiction and delineation of morphology, neck width and location of the aneurysm. The morphology indicates the aneurysm contour, which includes the number of lobes of the aneurysm. The neck width referred to the size of aneurysm neck: narrow, medium, wide, or irregular. The location refers to the relationship between the aneurysm and its surrounding artery branches, including the middle cerebral artery bifurcation, anterior and posterior communicating arteries, branches derived from the aneurysm, and duplication or fenestration [11]. The final interpretation should be obtained by consensus and k statistics are used to assess inter-observer reliability for aneurysm characteristics [12].
In this study, anterior communicating artery aneurysm, often related with the anterior communicating artery anatomy abnormities, was accounted for as much as five cases (83%). It is well known that up to 85% of patients with the anterior communicating artery aneurysm suffer A1 segment dysplasia, or even complete absence of the segment, i.e., internal carotid artery “three-pronged” deformity, which is one of the most common variations [13]. Another variation of anterior communicating artery such as duplication or fenestration, accounts for 10% - 40% of patients [14]. Normally there is only one anterior communicating artery, but sometimes it occurs with 2 - 3 duplications or fenestration, as is the case for patients involved in this study (Figure 1(D), arrow), making it more difficult to correlate the preoperative angiography with the intraoperative findings. The surgical craniotomy confirmed that due to the duplication or fenestration of the anterior communicating artery, and the aneurysm wall and the anterior communicating artery in close proximity (with or without adhesion), the aneurysm walls can oppress the anterior communicating arteries into arcs, similar to themselves in shape. Meanwhile, the aneurysm neck opening and the anterior communicating artery bifurcation starting point anatomically were on the same surface, leading to the side-by-side appearance of the aneurysm cavity and the anterior communicating artery in the preoperative angiography. Furthermore, the images of the aneurysm cavity and the surrounding branch lumens (i.e., the anterior communicating arteries) appeared superimposed, causing volume increase for the aneurysm images and resulting in doubtful images (artifacts); for example, vague irregular and wide-necked aneurysms were shown and the anterior communicating artery appeared derived from the aneurysm wall. In addition, it was found that in 2 patients the middle cerebral artery or anterior communicating artery and the aneurysm walls suffered from segmental adhesion and the aneurysm wall oppressed the surrounding artery branches into similar arc shape. However, during surgery we found that the adhesion was incomplete and often easier to separate.
For some older patients with less severe SAH bleeding, if preoperative angiography showed the aneurysm neck or wall indistinguishable from surrounding artery branches and there existed arterial anatomic variations, it may be possible that incomplete adhesion between the aneurysms and the artery branches as well as oppression from the aneurysms is involved. Neurosurgeons and radiologists should analyze preoperative image data more carefully from different directions and multi-angles as described earlier in this section, not rule out intervenetional embolization treatment option easily, and choose most appropriate treatment to achieve a favorable outcome for the patients involved.
5. CONCLUSION
Our present study is provided to raise awareness for nonoptimized correlation between preoperative angiography and intraoperative findings with regard to treatment options for patients suffered from intracranial aneurysms. Deformations in preoperative angiography may be due to anatomical variations of the surrounding artery branches near the aneurysms and aneurysm wall oppression or incomplete adhesion of the arterial branches. Such deformations can be recognized by more careful observation in preoperative angiography from different directions and multi-angles.
6. ACKNOWLEDGEMENTS
The authors acknowledge the contribution of other physicians who participated in the care and treatment of the patients involved in this study.
REFERENCES
- Seibert, B., Tummala, R.P., Chow, R., Faridar, A., Mousavi, S.A. and Divani, A.A. (2011) Intracranial aneurysms: Review of current treatment options and outcomes. Frontiers in Neurology, 2, 45. http://dx.doi.org/10.3389/fneur.2011.00045
- McCormick, W.F. and Schochet Jr., S.S. (1976) Atlas of cerebrovascular disease. WB Saunders Co., Philadelphia, 422.
- Vega, C., Kwoon, J.V. and Lavine, S.D. (2002) Intracranial aneurysms: Current evidence and clinical practice. American Family Physician, 66, 601-608.
- Wolstenholme, J., Rivero-Arias, O., Gray, A., Molyneux, A.J., Kerr, R.S., Yarnold, J.A., Sneade, M. and International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group (2008) Treatment pathways, resource use, and costs of endovascular coiling versus surgical clipping after a SAH. Stroke, 39, 111-119. http://dx.doi.org/10.1161/STROKEAHA.107.482570
- Li, H., Pan, R., Wang, H., Rong, X., Yin, Z., Milgrom, D.P., Shi, X., Tang, Y. and Peng, Y. (2013) Clipping versus coiling for ruptured intracranial aneurysms: A systematic review and meta-analysis. Stroke, 44, 29-37. http://dx.doi.org/10.1161/STROKEAHA.112.663559
- McLaughlin, N., McArthur, D.L. and Martin, N.A. (2013) Use of stent-assisted coil embolization for the treatment of wide-necked aneurysms: A systematic review. Surgical Neurology International, 4, 43. http://dx.doi.org/10.4103/2152-7806.109810
- Hunt, W.E. and Hess, R.M. (1968) Surgical risk as related to time of intervention in the repair of intracranial aneurysms. Journal of Neurosurgery, 28, 14-20. http://dx.doi.org/10.3171/jns.1968.28.1.0014
- Benitez, R.P., Silva, M.T., Klem, J., Veznedaroglu, E. and Rosenwasser, R.H. (2004) Endovascular occlusion of wide-necked aneurysms with a new intracranial microstent (Neuroform) and detachable coils. Neurosurgery, 54, 1359-1368. http://dx.doi.org/10.1227/01.NEU.0000124484.87635.CD
- Li, M. (2000) Neuro-interventional radiology. Shanghai Science and Technology Literature Press, Shanghai, 60- 70.
- Chappell, E.T., Moure, F.C. and Good, M.C. (2003) Com-parison of computed tomographic angiography with digital subtraction angiography in the diagnosis of cerebral aneurysms: A meta-analysis. Neurosurgery, 52, 624- 631. http://dx.doi.org/10.1227/01.NEU.0000047895.82857.EB
- Hirai, T., Korogi, Y., Ono, K., Murata, Y., Suginohara, K., Omori, T., Uemura, S. and Takahashi, M. (2001) Preoperative evaluation of intracranial aneurysms: Usefulness of intraarterial 3D CT angiography and conventional angiography with a combined unit-initial experience. Radiology, 220, 499-505.
- Landis, J.R. and Koch, G.G. (1977) The measurement of observer agreement for categorical data. Biometrics, 33, 159-174. http://dx.doi.org/10.2307/2529310
- Wilson, G., Riggs, H. and Rupp, C. (1954) The pathologic anatomy of ruptured cerebral aneurysms. Journal of Neurosurgery, 11, 128-134. http://dx.doi.org/10.3171/jns.1954.11.2.0128
- Serizawa, T., Saeki, N. and Yamaura, A. (1997) Microsurgical anatomy and clinical significance of the anterior communicating artery and its perforating branches. Neurosurgery, 40, 1211-1218. http://dx.doi.org/10.1097/00006123-199706000-00019

