Paper Menu >>
Journal Menu >>
 Journal of Environmental Protection, 2010, 1, 24-29 doi:10.4236/jep.2010.11004 Published Online March 2010 (http://www.SciRP.org/journal/jep) Copyright © 2010 SciRes JEP Comparative Performance and Computational Approach of Humic Acid Removal by Clay Adsorption Chao Yu1, Jiaqian Jiang2* 1Department of Mathematics, East China Normal University, Shanghai, China; 2Division of Civil, Chemical and Environmental En- gineering, Faculty of Engineering and Physical Sciences, University of Surrey, Guildford, UK. Email: J.Jiang@surrey.ac.uk Received January 3rd, 2010; revised February 3rd, 2010; accepted February 3rd, 2010. ABSTRACT The effective removal of humic acid is an important factor influencing th e qua lity of treated waters. Adsorption is one of major techniques used for the removal of humic acid. This study demonstrated that modified clays could be used as alternatives to activated ca rbons for adsorbing humic acid. Both Al-Fe modified and Fe modified clays had high affinity to humic acid and then high removal efficien cy. Al-modified clay had less removal capacity for adsorbing humic acid. Mathematics formulas were developed to predict the adsorption performance of modified clays for the humic acid removal via the parameters of UV254 absorbance and DOC concentrations. The optimal clay dose could be predicted using the developed model. The F test was used to validate t he model developed by examining if it fells into the reject field. The reject field varied accordi ng to each F test. The res ults showed that the model devel oped was 99% confident and can be used to perform the simulation. Keywords: Adsorption, Clay, Humic Aid (HA), Mathematics Approach, Modification, Water Treatment 1. Introduction Humic acid in surface water causes a lot of problems such as colour, taste, odour and lower efficiency in water treatment process. In addition to this, in the chlorination process, humic acid reacts with chlorine and produces disinfection-by-products (DBPs) [1]. The World Health Organization (WHO) has recommended the maximum concentrations of the DBPs and these parameters have been regulated in most countries’ environmental agencies. The effective removal of humic acid is thus an important factor influencing the quality of treated waters. Among techniques used for the removal of humic acid, coagu- lation, adsorption and membrane processes are widely adapted. Clay is one of the most common earth’s minerals, which are the residue of weathering or hypothermal ac- tion. The classification and origin of clay depends on particle size, physical characteristics, chemical composi- tions and common crystal structural characteristics. Clay’s size is less than 2 micrometers with plastic prop- erties when moist. Fundamentally, clay exhibits a layered structure and itself can be subdivided into groups ac- cording to its underlying structure and layer’s charge. An ideal structure of the most rigid clays is the 2:1-layered silicates which can be seen in Figure 1. The 2:1 notation means that the host layers consist of two tetrahedral sili- cate sheets sandwiching one octahedral sheet. The two other subclasses of clays have a 1:1 layer type and a 2:1 inverted ribbon structure, respectively [2]. At the central of the tetrahedral layers are silicon or aluminium ions, while the number of A1 ions in tetrahedral sites deter- mines the net negative charge of the host layer. Those oxygens forming the tetrahedral bases border the inter- lamellar gallery and are arranged in hexagonal rings that form a kagom´e lattice. The approximate chemical formula for the vermincu- lites is (Mg3(Si3Al)O10(OH)2)(Mg0.5(H2O)y); where the first set of brackets denotes the host layer, the second set denotes the guest layer, and the hydration state is vari- able. The host layers in clays can adopt a number of in- teresting stacking arrangements to form ordered, partially ordered, or disordered three-dimensional structures. Par- ticular clays are prone to form poly-types in which dif- ferent stacking sequences are associated with lateral layer- to-layer shifts. Overall, natural mineral clays possess specific surface chemical properties, e.g., cation exchange capacity, and adsorptive affinity for some organic and inorganic com- pounds, and then have attracted research interesting to investigate the potential use of clays as adsorbents for treating heavy metals and organic pollutants, or as co- agulant aids for improving the settling performance in coagulating low particle content water. By replacing the  Comparative Performance and Computational Approach of Humic Acid Removal by Clay Adsorption Copyright © 2010 SciRes JEP 25 Oxygens ●Aluminum Hydroxyls Silicons replaced by aluminums Oxygens ●Aluminum Hydroxyls Silicons replaced by aluminums Oxygens ●Aluminum Hydroxyls Silicons replaced by aluminums Figure 1. Diagrammatic illustration of 2:1 layer lattice (af- ter [2]) natural inorganic exchange cations with alkylammonium ions, clay surfaces are converted from being primarily hydrophilic to hydrophobic, which enable them to interact strongly with organic vapours and organic compounds dissolved in water [3]. Previous studies [4,5] have dem- onstrated that the polymeric Al/Fe species are the most efficient coagulating/adsorbing chemicals for removing natural and synthetic organic impurities in potable water treatment. The combination of the natural mineral clays with polymeric Al/Fe species may produce somewhat optimal properties and enhance the adsorption of metal and organic compounds from the solution. The feasibility of this idea has been confirmed by preceding work [6– 10], where, modified clays had comparatively great af- finities for the heavy metals, and phenol and dye struc- tured pollutants. The aim of this paper is to use modified clays for humic substances removal and to develop a model to forecast the operating conditions based on the experimental results of using modified clay to adsorb humic acid. It is expected that using the developed model, the most efficient outcome of adsorption of humic acid could be predicted. 2. Materials and Methods 2.1 Modifying and Characterising Clays The raw clay used in this study, montmorillonites KSF, and the other chemicals were supplied by Sigma-Aldrich Chemicals Corporation UK. The modi- fication of clays was following an established procedure [7]. The modification involved with the mixing of the given amount of clays with polymeric metal species for four hours at 55℃ and then the mixtures were separated by filtration to obtain the solid phase of the modified clays. The resulting clays were dried using a freeze dryer (Dry Winner3, HETO Ltd., UK) operating at –0.5MPa and –52℃. The chemical composition of the modified clays were analysed using X-ray Fluorescence (XRF), and the XRF data was collected on a Philips PW1480 XRF Spectrometer. The clays used in the study were raw montmorillonites KSF (termed as raw clay), poly-aluminium modified mont- morillonites KSF (Al-clay), poly-iron modified montmo- rillonites KSF (Fe-clay) and poly-aluminium and iron modified montmorillonites KSF (Al-Fe-clay). 2.2 Procedures of Adsorption Experiments and Water Quality Measurement The model water containing humic acid (HA) was pre- pared using a commercial HA (Fisher, UK), and the HA concentration was 6.5 mg/L, giving UV254 abs of 15 1/m and dissolved organic carbon (DOC) concentration 3.2 mg/L as C. The adsorption experiments were carried out using the batch equilibration technique. For each isotherm, given amount of clay was weighed into 40 mL polypropylene centrifuge tubes, and 30 mL of the above stated HA solu- tion were added. The pH value of HA solution was pre- adjusted to 5. The suspensions were mixed on a rotary tumbler for 4 hours, which has been tested to be sufficient to reach the equilibrium status under the study conditions. After phase separation by centrifugation, the concentration of HA in the supernatant was determined by UV- absorb- ance at wavelength of 254 nm and DOC analysis. The analytical procedures were following the AWWA standard methods [11]. The adsorbed HA quantities were then de- termined using the mass balance equation: Cs m = V (C0 – Ce) where, Cs is sorbed HA concentration on clay (mg/g), m is the weight of clay used (g), V is volume of HA solu- tion (L), C0 is HA initial concentration (here expressed as DOC mg/L), and Ce is HA equilibrium concentration (DOC mg/L). Percentage removal of humic acid was calculated based on the original and treated DOC con- centrations. 2.3 Mathematical Approach The regression procedures and the least square method were used to set up a model and to analyze the data. In terms of the experimental results of humic acid removed by clays, a model was developed to forecast humic acid removal efficiency by adsorption with clays. Finnaly, the F test was used to validate the model developed. 3. Results and Discussion 3.1 Characterisation of the Modified Clays Figure 2 shows an example XRD traces for the modified montmorillonites. The peaks marked by (x) are the d001 reflections indicative of 2:1 swelling clays. The other peaks are impurities corresponding to quartz, plagioclase feldspar, illite and mica. Illite is a non-swelling 2:1 clay, mica is a non-swelling 2:1 phyllosilicate (sheet silicate) 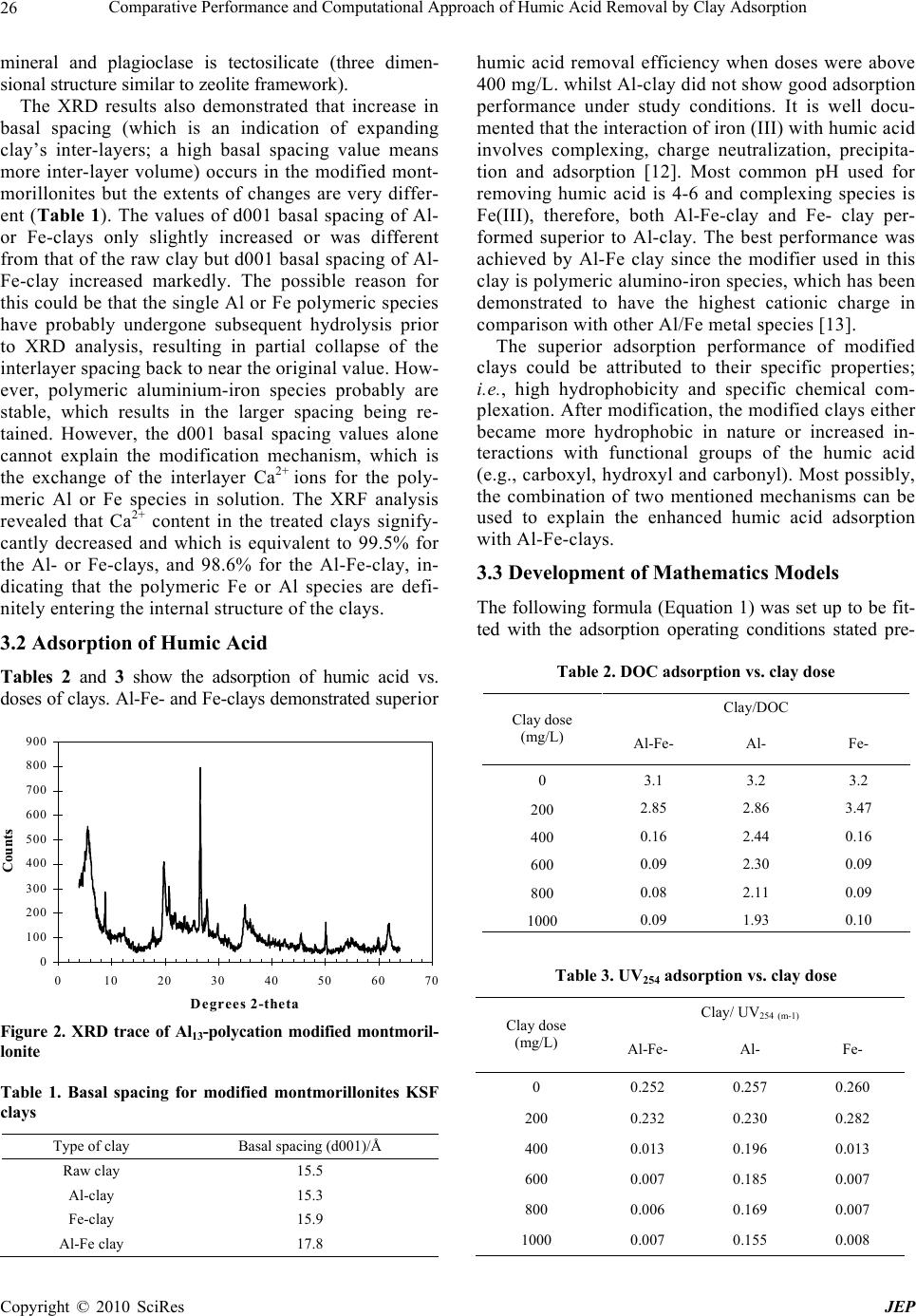 Comparative Performance and Computational Approach of Humic Acid Removal by Clay Adsorption Copyright © 2010 SciRes JEP 26 mineral and plagioclase is tectosilicate (three dimen- sional structure similar to zeolite framework). The XRD results also demonstrated that increase in basal spacing (which is an indication of expanding clay’s inter-layers; a high basal spacing value means more inter-layer volume) occurs in the modified mont- morillonites but the extents of changes are very differ- ent (Table 1). The values of d001 basal spacing of Al- or Fe-clays only slightly increased or was different from that of the raw clay but d001 basal spacing of Al- Fe-clay increased markedly. The possible reason for this could be that the single Al or Fe polymeric species have probably undergone subsequent hydrolysis prior to XRD analysis, resulting in partial collapse of the interlayer spacing back to near the original value. How- ever, polymeric aluminium-iron species probably are stable, which results in the larger spacing being re- tained. However, the d001 basal spacing values alone cannot explain the modification mechanism, which is the exchange of the interlayer Ca2+ ions for the poly- meric Al or Fe species in solution. The XRF analysis revealed that Ca2+ content in the treated clays signify- cantly decreased and which is equivalent to 99.5% for the Al- or Fe-clays, and 98.6% for the Al-Fe-clay, in- dicating that the polymeric Fe or Al species are defi- nitely entering the internal structure of the clays. 3.2 Adsorption of Humic Acid Tables 2 and 3 show the adsorption of humic acid vs. doses of clays. Al-Fe- and Fe-clays demonstrated superior 0 100 200 300 400 500 600 700 800 900 0 10203040506070 De grees 2-theta Counts Figure 2. XRD trace of Al13-polycation modified montmoril- lonite Table 1. Basal spacing for modified montmorillonites KSF clays Type of clay Basal spacing (d001)/Å Raw clay 15.5 Al-clay 15.3 Fe-clay 15.9 Al-Fe clay 17.8 humic acid removal efficiency when doses were above 400 mg/L. whilst Al-clay did not show good adsorption performance under study conditions. It is well docu- mented that the interaction of iron (III) with humic acid involves complexing, charge neutralization, precipita- tion and adsorption [12]. Most common pH used for removing humic acid is 4-6 and complexing species is Fe(III), therefore, both Al-Fe-clay and Fe- clay per- formed superior to Al-clay. The best performance was achieved by Al-Fe clay since the modifier used in this clay is polymeric alumino-iron species, which has been demonstrated to have the highest cationic charge in comparison with other Al/Fe metal species [13]. The superior adsorption performance of modified clays could be attributed to their specific properties; i.e., high hydrophobicity and specific chemical com- plexation. After modification, the modified clays either became more hydrophobic in nature or increased in- teractions with functional groups of the humic acid (e.g., carboxyl, hydroxyl and carbonyl). Most possibly, the combination of two mentioned mechanisms can be used to explain the enhanced humic acid adsorption with Al-Fe-clays. 3.3 Development of Mathematics Models The following formula (Equation 1) was set up to be fit- ted with the adsorption operating conditions stated pre- Table 2. DOC adsorption vs. clay dose Clay/DOC Clay dose (mg/L) Al-Fe- Al- Fe- 0 3.1 3.2 3.2 200 2.85 2.86 3.47 400 0.16 2.44 0.16 600 0.09 2.30 0.09 800 0.08 2.11 0.09 1000 0.09 1.93 0.10 Table 3. UV254 adsorption vs. clay dose Clay/ UV254 (m-1) Clay dose (mg/L) Al-Fe- Al- Fe- 0 0.252 0.257 0.260 200 0.232 0.230 0.282 400 0.013 0.196 0.013 600 0.007 0.185 0.007 800 0.006 0.169 0.007 1000 0.007 0.155 0.008  Comparative Performance and Computational Approach of Humic Acid Removal by Clay Adsorption Copyright © 2010 SciRes JEP 27 viously, i.e., that the Al and Fe have an equally fixed ratio towards clay when they were modified, Al, Fe and clay are independent variables, i.e. they won’t interfere with each other when performing as adsorbents. 0112233 yxxx (1) where y represents the remaining DOC or UV254 treated by Al-Fe-clay; x1, x2, and x3 represent the remaining DOC or UV254 treated by Al-, Fe- and raw clay respec- tively; i are unknown parameters (i=0,1,2,3); is the random error item which satisfy: () 0 E and 2 () Var The purpose of the above model is to predict the ad- sorption performance of either Al-Fe-clay (y) or other clays (xi). The influence of three factors were considered: Al content, Fe content and raw clay. And the least square method was used to obtain i. Take the form 0112233 iiiii yxxx,1, 2, 3...,in where, 3n. A matrix form was written as follows: 12 ( ,,...,)T n Yyy y 11 1213 123 4 1 1 nn n n xxx X xxx 0123 (,,,) T 12 ( ,,...,) T n Thus, the original equation was written as: YX Compute the parameters. According to the least square method, the estimated vector was found which should satisfy: 012 2 0120 123 123 1 2 0112233 ,, 1 (,,) () min () n iiii i n iiii i Qyxxx yxxx The parameters were then computed by using method from multivariable calculus and were written in the matrix form: ()0 T XYX Since T X X is a non-singular matrix, thus the formula was obtained to compute 1 () TT X XXY In real practice, Matlab could be used to compute the above calculation. For the DOC value, = (-0.1813, 0.0833, 0.8057, 0.1391)T, and then Equation 2 was obtained, 123 ˆ0.1813 0.08330.80750.1391 DOC yxxx (2) Similar procedures were applied to UV254-abs removal prediction, or, Equation 3: 123 ˆ0.013 0.0730.80720.1448 UV yxxx (3) Figures 3 and 4 show the remaining DOC concen- trations and UV254 abs. after clay adsorption via either the experimental and the model simulated data. Both approaches deliver significantly consistent results indicating that the developed model could be used to predict the humic acid adsorption performance by clays. 3.4 Hypothesis Test The F test was used to validate the model developed, which determines whether y and x1, x2 and x3 are linear correlated at a significant level (alpha equals to 0.01), i.e., whether it is appropriate to represent y with beta0 + 0 0.5 1 1.5 2 2.5 3 3.5 02004006008001000 1200 Dose (mg/L) Remaining DOC (mg/l) Experimental Simulated Figure 3. Model simulated and experimental remaining DOC 0 0.05 0.1 0.15 0.2 0.25 0.3 0200 400 600 80010001200 Dose(mg/L) Remaining UV 254 (m-1) Experimental Simulated Figure 4. Model simulated and experimental remaining UV254  Comparative Performance and Computational Approach of Humic Acid Removal by Clay Adsorption Copyright © 2010 SciRes JEP 28 beta1*x1 + beta2*x2 + beta3*x3 with the confidence level of 99%. The F value is examined to see if it fells into the reject field. If it is, the developed model is wrong, and has to be reconstructed. The reject field varies according to each F test. As shown in the following equations, that the F test needs three components to locate, which are 0.01, 4 and 3, respectively. The 0.01 means the confident level (equals to 99%), 4 and 3 are the degree of freedom which are set due to the data numbers. 16.69 stands for the re- ject field (obtainable from the table provided with statis- tics books). If the F value computed is less than 16.69, then the hypothesis that original model is correct would be rejected. As the F value in this case is greater than 16.69 (Equations 4 and 5), it means that the developed model was 99% in confidence (with the coefficients beta0 - beta3 are not all zero) for its accuracy, and can be used to simulate and predict the adsorption performance. 00 1 23 :0 H Versus 10123 :( , ,,) (0,0,0,0) H 22 2 111 ˆˆ ()() () nnn Tyy iiii iii RE S lyyyyyy SS 4(4,4 1) 41 R E S FFn S n ∼ 254 0.01 26322.29>(4,3) 16.69 UV FF (4) 0.01 7748.875>(4,3) 16.69 DOC FF (5) So H0, was rejected and the parameters can be fit to the model significantly at the 99% confidence. The developed models were verified, and then they can be used to predict that when the clays’ doses are about 400~600 mg l-1, the overall adsorption of UV254 and DOC could reach to the maximum. Furthermore, if the remaining UV254 or DOC could be known, the outcome of Al-Fe modified clay at the same dose level could be forecasted. 4. Conclusions This study demonstrated that modified clays could be used as alternatives to activated carbons for humic acid removal. Both Al-Fe modified and Fe modified clays have high affinity to humic acid and then high removal efficiency. Al-modified clay has less removal capacity for adsorbing humic acid. Higher d-spacing values of Al-Fe modified and Fe modified clays could explain such phenomena. Mathematics formulas were developed to predict the adsorption performance of modified clays for the humic acid removal via the parameters of UV254 absorbance and DOC concentrations. The optimal clay dose could be predicted using the developed models. The F test was used to validate the model developed by examining if it fells into the reject field. The reject field varied according to each F test. The results showed that the model developed was 99% confident and can be used to perform the simulation. 5. Acknowledgement The authors would like to thank UK Engineering Physi- cal Science Research Council (EPSRC) for funding Chao Yu’s internship at the University of Surrey under the Knowledge Transfer Award Scheme. REFERENCES [1] B. M. Chow and P. V. Robert, “Halogenated by products formation by ClO2 and Cl2,” Journal of the Environmental Engineering Division, ASCE, Vol. 107, No. 4, pp. 609– 615, 1981. [2] G. W. Brindley and G. Brown, “Crystal structures of clay minerals and their X ray identification,” Mineralogical Society, London, pp. 495, 1980. [3] H. T. Zhao and G. F. Vance, “Sorption of trichloroethyl- ene by organo-clays in the presence of humic substances,” Water Research, Vol. 32, No. 12, pp. 3710– 3716, 1998. [4] J. Q. Jiang and N. J. D. Graham, “Enhanced coagulation using Al/Fe(III) coagulants: Effect of coagulant chemis- try on the removal of colour-causing NOM,” Enviromen- tal Technology, Vol. 17, No. 9, pp. 937–950, 1996. [5] J. Q. Jiang and N. J. D. Graham, “Preparation and charac- terization of an optimal polyferric sulphate (PFS) as a coagulant for water treatment,” Journal of Chemical Tech- nology & Biotechnology, Vol. 73, pp. 351–358, 1998. [6] J. Q. Jiang, C. Cooper, and S. Ouki, “Comparison of modi- fied montmorillonite adsorbents, Part I: Preparation, charac- terization and phenol adsorption,” Chemosphere, Vol. 47, No. 7, pp. 711–716, 2002. [7] J. Q. Jiang and Z. Zeng, “Comparison of modified mont- morillonite adsorbents, Part II: The effects of the type of raw clays and modification conditions on the surface pro- perties and adsorption performance of modified clays,” Chemosphere, Vol. 53, No. 1, pp. 53–62, 2003. [8] J. Q. Jiang, Z. Q. Zeng, and P. Pearce, “Preparation and use of modified clay coagulants for wastewater treat- ment,” Water, Air, & Soil Pollution, Vol. 158, No. 1, pp. 53–65, 2004. [9] S. Richards and A. Bouazza, “Phenol adsorption in or- gano-modified basaltic clay and bentonite,” Applied Clay Science, Vol. 37, No. 1–2, pp. 133–142, 2007. [10] E. I. Unuabonah, K. O. Adebowale, and F. A. Dawodu, “Equilibrium, kinetic and sorber design studies on the adsorption of Aniline blue dye by sodium tetraborate- modified Kaolinite clay adsorbent,” Journal of Hazardous Materials, Vol. 157, No. 2–3, pp. 397–409, 2008. [11] APHA, AWWA, and WEF, “Standard Methods for the  Comparative Performance and Computational Approach of Humic Acid Removal by Clay Adsorption Copyright © 2010 SciRes JEP 29 examination of water and wastewater,” 18th Edition, American Publication Health Association, Washington, DC, 1992. [12] C. P. Huang and H. L. Shiu, “Interactions between alum and organics in coagulation,” Colloids and Surfaces A: Physicochemical and Engineering Aspects, Vol. 113, No. 1–2, pp. 155–163, 1996. [13] J. Q. Jiang, “Development of coagulation theory and pre-polymerised coagulants for water treatment,” Separa- tion and Purification Methods, Vol. 30, No. 1, pp. 127– 142, 2001. |

