Journal of Modern Physics
Vol.3 No.7(2012), Article ID:21092,4 pages DOI:10.4236/jmp.2012.37084
Particles Spontaneous Concentrating
1College of Chemistry and Molecular Engineering, Qingdao University of Science and Technology, Qingdao, China
2Medical College, Qingdao University, Qingdao, China
Email: mzh62@qust.edu.cn
Received April 10, 2012; revised May 8, 2012; accepted June 1, 2012
Keywords: Second law; Thermodynamic; Entropy
ABSTRACT
A novel experiment was designed. Experimental results gave a surprising phenomenon. Homogeneous particles in a suspended solution spontaneously concentrated.
1. Introduction
The Second Law of Thermodynamics (Principle of EverIncreasing Entropy) was found in the year about 1850. R. J. F. Clausius, N. L. S. Carnot, J. Kelvin were the main three representative scientists. The law was accepted all over the world. It was regarded as a universally correct principle, which controlled the nature successfully, with no contrary events being found [1]. However, occasionally in the history, it appears someone who proposed a strange model or device, which argues against the second law of thermodynamics. Every time, it was proved wrong, and the law still stands firmly there.
The more truth is debated, the clearer it becomes. Debate is just one those things in science. However, there were no more doubts than the second law. Maybe there is really something therein.
Carefully examining, we realized that, as we know, the principle of ever-increasing entropy comes from the studies of heat. Heat is the irregular kinetic motion of a mass of atoms or molecules, and the irregular unrestricted motion becomes the main characters of heat. It led to the resultant principle of ever-increasing entropy in an isolated system. Alternatively, it is to say that the irregular unrestricted characters become the precondition of the second law of thermodynamics. Otherwise, in case of a motion, which the movements of particles were restricted, the principle of ever-increasing entropy would not always be true. Based on these analyses, a novel experiment was designed in this paper, and it resulted in a curious phenomenon.
2. Experimental Section
2.1. Design
A transparent vessel is prepared. It was divided into two parts by a thin medium. The medium possesses large numbers of small meshes in an appropriate size. Especially, the meshes are not simply a normal one; it has a tiny mechanical device in it, that allows one side particles can easily passing through, and the opposite particles cannot. After above fine making, add some turbid liquid into the two divided parts of the vessel equally at room temperature. Then just wait and observe for what happens.
2.2. Device Making
Two waste test paper boxes were used as the designed vessel. The transparent boxes are made up of plastic and have a cubic shape. Connected the two boxes in a side, and felt with glue. Drill a large hole on the osculant side with a porous medium adhered tightly on the hole. Normal cloth can be used as the porous medium.
The cloth has many small meshes in it, and there are many hairlines around the meshes in two-sided surface. Most of the hairlines on the side protrude toward the same side; it will block the particles passing through from one side to another severely but the opposite side’s particles slightly. Because both sides of the cloth have the same hairlike hemps, the effect of the blocks from of the two sides will be the same. Now then, cut off one side’s hairlines by a glance of fire carefully leaving another side’s hairlines behind. The medium of the cloth will act as a switch that let one side particles can easily pass through, and the opposite particles hardly. Particles passing through the meshes will no more be freely random but restricted. Two types of clothes were selected. One is made up of cotton, another of Terylene. Their mesh’s have the diameter of about 170 µm and 35 µm, thickness of about 350 µm and 200 µm, and their hairlines have the diameter of about 25 µm and 20 µm respectively. The amplified picture of cotton cloth was showed in Figure 1. The hairline of cotton cloth can be seen clearly from side face, which was showed in Figure 2(a). The picture of another side of the cloth, which hairlines were mostly cleared away was showed in Figure 2(b).
Turbid liquid was made by adding little edible oil in a approximately saturated water solution of surfactant (CH3(CH2)11C6H4SO3Na). Mixing around with a brush for about half a minute, the edible oil particles with the surfactant molecules around spread equably in the water
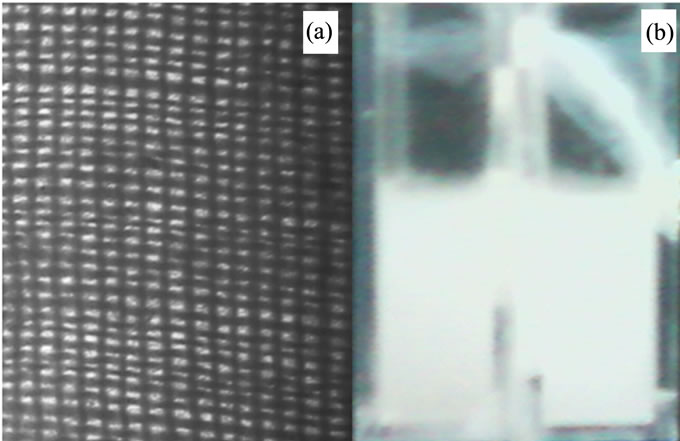
Figure 1. (a) Micrograph of cotton cloth amplified by 1 × 10; (b) The system at concentrating-releasing dynamic equilibrium state.
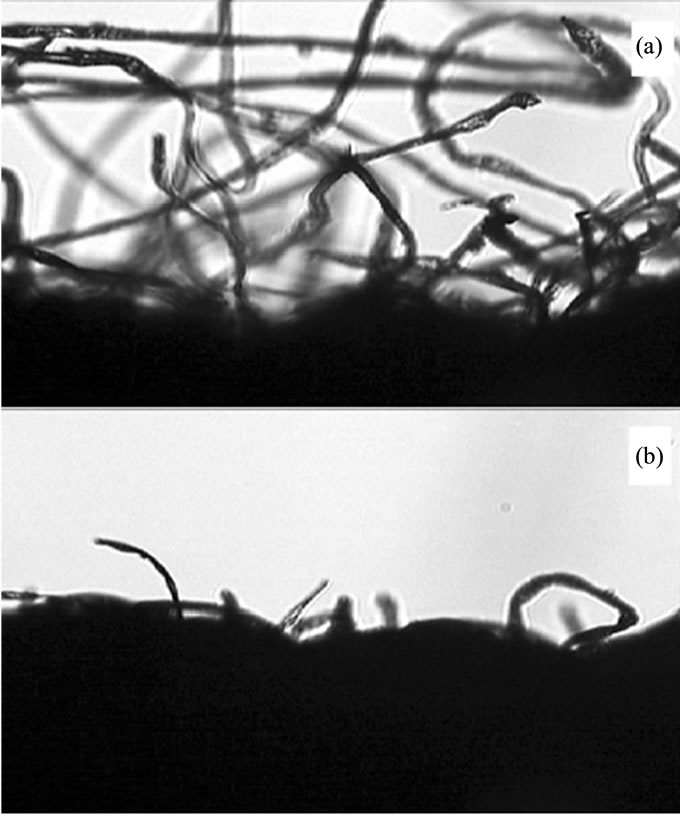
Figure 2. (a) Micrograph of side face of cotton cloth amplified by 4 × 10; (b) Micrograph of the clean out side face of cotton cloth amplified by 4 × 10.
solution, forming a suspended turbid liquid A(a nearly but not colloid solution).
3. Results and Discussion
Above prepared device was used with a dividing medium of cotton cloth. The channels with the size of about 170 µm i.d. were in the medium. Added some prepared turbid liquid A into the two dividing vessels equally, keeping the liquid height not exceeded the medium of the vessels. Then with closing down of the cover, the equipment was done. The picture was showed in Figure 3(a). Put the system silently in a closed metal box in dark room at room temperature. Ten days later, took out the system and observed; a novel phenomenon appeared. The two rooms of the system had obviously different concentration of particles showed in Figure 3(b). The larger concentration was in the side as expected that the medium have hairlines in. The absorbance of turbid liquid in the two rooms was determined to be 0.198 and 0.501 respectively. As a contrast, their initial absorbance was determined prior to be 0.340. It told us that, the suspended particles could spontaneously pass through the medium from its homogeneous state to a different concentration state without using of energy. The experiment has worked time after time, but the phenomena remained the same.
The key point is that, the energy of concentration difference can be released through a bypass (without any blocks of medium). The accumulating and releasing of energy went a circle process through the switching bypass. It continued more times as it be carried out.
A direct proof is apparently that, let the bypass open all the time with no switch actions on, the energy accumulating and releasing would continue in the system. The experiment was performed. A dynamic equilibrium (accumulating and releasing) was established after 10 days later. A slight concentration difference cannot be
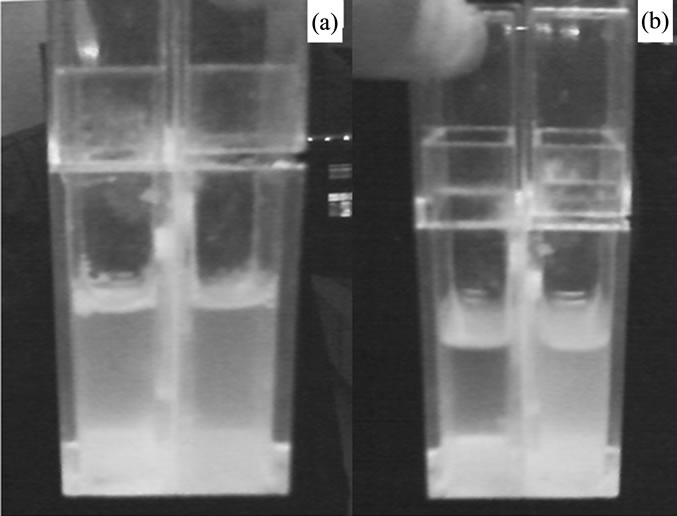
Figure 3. (a) Picture of the system at its initial state; (b) Picture of the system 10 days later.
observed between the two separated rooms, showed in Figure 1(b). The particles continuing gone cycles in macro size with no net variation of entropy in the system. As circumstantial evidence, further experiments were performed next.
Retaining the device and conditions as the same of Figure 3, but replaced the medium with terylene cloth. The experiment gave the same result of absorbance for 0.054 and 0.167 of the two rooms respectively (the initial concentration in absorbance was 0.111). It indicated that the phenomenon is independent with the kind of cloth.
Changing the direction 180˚ horizontally based on the same experiment, the experiment resulted in a same phenomenon. It stated its independence with direction, i.e. the environment is equably in direction to the experiment.
Suspected that may the excessive hairlines in one side that attracting the suspended particles in turbid liquid. An experiment was performed with the adding piece of cloth in the opposite room for proving. The result remained as before; it showed us the useless of the adding piece of cloth (see Figure 4(a)). The concentration in absorbance was to be 0.982 and 1.271 of the two rooms respectively with initial concentration in absorbance of 1.155. This experiment on the one hand proved that the appearance of concentration difference was not arosed from the attraction or absorption of medium.
The appearance of concentration difference is depended on the size of suspended particles compared with the size of meshes in medium. If the size of particles bigger than that of meshes, nothing would appear. Moreover, if the size of particles is too less than that of meshes, nothing could be observed either. The experiment was
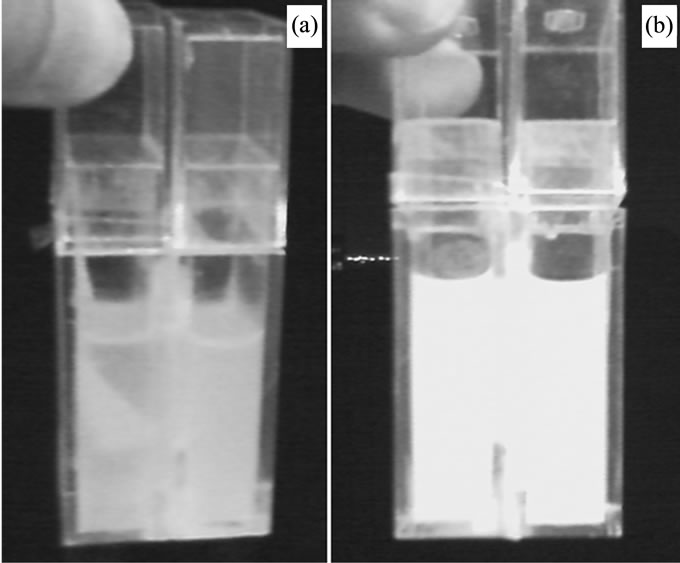
Figure 4. (a) Picture of the system with an adding piece of cloth in another side, and retaining for 10 days; (b) Picture of the system with the replacement of turbid liquid A for B, and retaining for 10 days.
done by replacing the suspended turbid liquid A with suspended turbid liquid B. The suspended turbid liquid B was prepared by adding little sodium stearate (C17H35- COONa) into hard water and mixing to a homogeneous state. In which the size of particles is far more less than the meshes’. The mechanical device (the hairlines) acts with no use. The hairlines cannot block the far more less particles. In other words that, the far more tiny particles (compared with its meshes) can pass freely through the meshes. The experimental result was presented in Figure 4(b). No concentration difference was observed. The experiment showed us again that it is not the attraction of medium that causing the concentration difference.
The source of energy, which pushes the suspended particles moving about, is the heat of the system. The famous Brownian movement proved this. The tinier the particle size is the higher frequency and more rapidly, it moves. To a system in this paper, the tinier the size of particles, meshes, and thickness of the cloth are, the more efficiently the system would be (The particles in turbid liquid that used were not made as little as that in Brownian movement (0.1 - 10 µm). Because it is difficult to find or make a medium that have meshes in so little size and enchasing with the fine one-side hairlines, which matched).
Suspended particles absorbed the heat energy passing through from one side to another. If the system were to be a closed one, the temperature of the system and surroundings would be the same. The energy supple of the system will come from the heat of surroundings. Then, the variation of entropy of surroundings would be negative. The variation of entropy of the system will equal to that of the forming of concentration difference; and it will certainly produce a negative one. If the system were to be an isolated one, the temperature of the system would decrease accordingly; this factor would cause a variation of entropy to be negative. The forming of concentration difference would also produce a negative variation of entropy as expressed above. No other factors acted on, the total variation of entropy of the isolated system will be the sum of the two parts of negative values. Ok, it is clear that the results are the same in the considered isolated and closed system.
It led us to the conclusion that the entropy in isolated system goes spontaneously decrease in our consulted restricted case. Naseer Iqbal concluded a similar result in his study in the field of gravitational universe [2]. Many others studies the entropy of the universe [3].
In addition, the hairlines in the system act like a switch (or Maxwell’s demon [4]). However, there was no net variation of entropy in this part. Because it is just a mechanical device, no outside energy was used as it working; and no change of its states was found after it working. If you like to add the concept of distinguishing or information consumption etc. something like that, we would say that no existence or meanings here of their all.
4. Tags
There are many kinds of energy forms existing in space of the surroundings even in a dark room. One may contest that it must be the system, which received some forms of high-energy (compared with heat at normal temperature) from its surroundings. Then the answer is that since the Brownian movement already exists, why not be the Brownian movement that pushing the suspended particles passed through the medium. If you still insist on, then, how can you explain the normal diffusion phenomena of particles in a liquid?
On the other hand, to our experiment result or paraphrases, many those would just simply say no, because it contrary against the second law of thermodynamics. To these reverent truth guarders, we have nothing to say.
Despite all of that, to strictly speaking, we cannot state that we proved something. It just wants to receive a more reasonable explanation of the reported phenomenon from the world. Moreover, we do not know whether it indicates that more detailed research work need to be done in the field unceasingly.
REFERENCES
- W. J. Moore, “Physical Chemistry,” 5th Edition, PrenticeHall Press, Englewood Cliffs, 1999.
- N. Iqbal, M. S. Khan and T. Masood, “Entropy Changes in the Clustering of Galaxies in an Expanding Universe,” Nature Science, Vol. 3, 2011, pp. 65-68.
- A. W. Beckwith, “Energy Content of Gravitation as a Way to Quantify Both Entropy and Information Generation in the Early Universe,” Journal of Modern Physics, Vol. 2, No. 2, 2011, pp. 58-61. doi:10.4236/jmp.2011.22010
- V. Serreli, C.-F. Lee, E. R. Kay and D. A. Leigh, “A Molecular Information Ratchet,” Nature, Vol. 445, 2007, pp. 523-527.

