Food and Nutrition Sciences
Vol.4 No.9(2013), Article ID:36451,7 pages DOI:10.4236/fns.2013.49128
Nutritional and Clinical Rehabilitation of Severely Malnourished Children with Moringa oleifera Lam. Leaf Powder in Ouagadougou (Burkina Faso)
![]()
1Department of Biochemistry-Microbiology, University of Ouagadougou, Ouagadougou, Burkina Faso; 2Department of Gastroenterology, Clinique de L’amitié, Ouagadougou, Ouagadougou, Burkina Faso.
Email: *e.gaagvander@zgt.nl
Copyright © 2013 Urbain Zongo et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received June 17th, 2013; revised July17th, 2013; accepted July 24th, 2013
Keywords: Malnutrition; Anthropometric Parameters; Hemoglobin; Side Effects; Moringa Leaves
ABSTRACT
Malnutrition in all its forms remains one of the most serious and neglected health problem. This longitudinal study referred pragmatic was carried out, which tested or aimed to assess the impact of Moringa leaf powder on the nutritional status of malnourished children. It was a pilot study in Ouagadougou severely malnourished children using Moringa as a nutritional supplement. We investigated the correlations and the impact of Moringa leaf powder on the nutritional status. A sample of 110 children aged 6 - 59 months was recruited and randomly selected and assigned to two treatments Group I and Group II. They received the CREN’s standard nutritional care diet but the Group I received more dose of 10 g of Moringa leaf powder per day. At the end, a significant improvement in the key parameters was recorded in both groups. However, the group receiving the Moringa supplement recorded a higher average weight gain (8.9 ± 4.3 g/kg/day, against 5.7 ± 2.72 g/kg/day in Group II) and a quicker recovery rate, with an average stay of 36 ± 16.54 days, against 57 ± 19.20 days amongst those not receiving the Moringa supplement. There is no significant improvement in hemoglobin rate in either group (p = 0.060 Group I, p = 0.063 Group II). Tolerability was considered to be good, as there were no recorded cases of medical admittance, no any occurrence of digestive disorders. The supplementation of Moringa leaf powder appears to be effective in improving the nutritional recovery of severely malnourished children.
1. Introduction
Undernourishment and malnutrition in all their forms have been recurring problems in Burkina Faso for a long time. At the 12th ECOWAS (Economic Community of West African States) forum in 2010, it became apparent that malnutrition is one of the most serious and neglected health problems throughout the world [1]. Indeed, in 2010, the rates of acute and chronic malnutrition were 11.10% and 35.00% respectively [2]. This situation occurs and develops mainly during the complementary feeding period, between the ages of 6 and 24 months. In Burkina Faso, malnutrition and micronutrients deficiencies are two of the biggest constraints to achieve the millennium development goals. These two problems are widespread and constitute a serious public health problem [3]. Diets rich in fruits and vegetables, which bring good micronutrients and phytochimic compound for health, can be beneficial in the fight against malnutrition and obesity [4]. Every year, 2.7 million deaths are attributed to an insufficient consumption of fruits and vegetables, placing it among the top 10 mortality risk factors [5].
Moringa is an important food source for people, especially in rural areas [6], and it is consumed in various African countries (Ghana, Senegal, Malawi), Latin America (Nicaragua, Bolivia) and even New Zealand. During the recovery of moderate or severe and particularly clinical cases of malnutrition, it is important to improve the nutritional quality (energy density conditions, macronutrients and micronutrients) of complementary food with low-cost, locally available ingredients consistent with local cultural food habits, and which take into account safe handling and ease of availability [7,8]. Studies from other countries indicate that the leaves have immense nutritional value such as vitamins, minerals and amino acids [9]. This study situates itself within the context of “nutria-prevention” and the challenge to overcome the problems of the inadequate diet (low protein and micronutrients intake). Both macros as well as micronutrient deficiencies hinder the national economic development as well as the development of individual human potential. Children are frequently the victims of micronutrient deficiencies and fail to overcome micronutrient malnutrition in a sustainable fashion, which jeopardizes a nation’s future.
The overall objective of this study is to demonstrate the effectiveness of Moringa leaf powder as a dietary supplement to assist the recovery and possible improvement of the nutritional status of severely malnourished children.
2. Material and Methods
2.1. Testing of the Nutritional Rehabilitation of Children Used in the Study
Study area and subjects: The study was conducted in the rehabilitation unit of the CMA Paul VI (Centre Médical Paul VI) located at Sector 22 in the city of Ouagadougou and frequented by people of suburbs, from July 2011 to December 2011. The severely malnourished children (WHZ ≤ −3) aged between 6 and 59 months were selected for the study. Biomedical analyses were conducted at the Centre Medical Paul VI and biochemical analyses of the Moringa powder and data analyses were conducted at the Research Centre for Biological, Food and Nutritional Sciences (CRSBAN) at the University of Ouagadougou.
A sample of 110 children was recruited and randomly [10], and assigned to the Group I or Group II, one of which was subjected to the usual porridge. The second group received the exact same diet but with Moringa leaf powder as a supplement. The daily dose of 10 g of powdered Moringa dry leaves was decided upon, as it was the quantity recommended [11]. The purpose of the study was explained in detail to the parents or guardians of the malnourished children at the first working session and their written informed consent was thus obtained. Upon patient enrollment, information about the history of illness, family demographics, and socioeconomic status was acquired in an interview with the children’s mother and the inpatient hospital chart in order to determine their nutritional and illness status (presence of oedema, diarrhea···).
The protocol was approved by the Ethical Committee for Health Research of Burkina Faso.
The monitoring of the children was carried out during the day and in the afternoon they went home with their parent/guardian.
It was a longitudinal study with a pragmatic outlook, which lasted for about 6 months and aimed to evaluate the impact of Moringa on the nutritional status of malnourished children aged 6 - 59 months.
2.2. Anthropometric Parameters
Weight was measured with a 2356S model SALTER scale, with a 25 kg suspended spring in 100 g divisions. The value was read directly from the scale and rounded to the nearest 100 grams. Height was measured barefooted and, for infants under two years old, recumbent length was measured using a wooden measuring board, to the nearest 0.1 cm. Child age was determined based on the birth certificate or health record; in the absence of these, a calendar of local events was used to determine the birth date of the child as accurately as possible, and Mid-Upper Arm Circumference (MUAC) was measured with a ribbon as per the method described by Shakir and Morley [12].
2.3. Clinical and Hemoglobin Analyses
Clinical signs of malnutrition (edema, marasmus, kwashiorkor) and associated diseases (vomiting, diarrhea, Bloating, rash) were diagnosed by both a nurse and the research team. Only the hemoglobin rate was measured in order to be able appreciate anaemia in the children.
Hemoglobin rate: The hemoglobin rate was measured at the biomedical laboratory of Paul VI CMA using a Hb 201 + type HemoCue device.
All the parameters studied were measured in triplicate.
Statistical analysis: The amassed data were treated using Microsoft Excel 2007, ENA for SMART software and the software SPSS.17 for further statistical analyses. Descriptive statistics were used to explore the data and describe central tendencies. Pearson correlation was used to explore association between continuous variables, while for categorical variables Student t-test was used. A probability of p < 0.05 was considered to be statistically significant.
3. Results
The clinical criteria which were considered were essentially: signs or clinical forms of malnutrition e.g. marasme, kwashiorkor, the mixed form (marasmic-kwashiorkor), and any other associated symptoms such as diarrhea, vomiting, rashes and other adverse effects.
3.1. Improving the Nutritional Status of Children
In assessing the recovery of children in both groups, their nutritional status was evaluated according to WHO standards [13], and reported in Table 1.
Wasting represents a state of acute malnutrition, marked by an insufficient weight in relation to size. This deficit is observed in cases of inadequate food intake and/or diseases, in the space of a few days or from one to two months [14].
The variation of the weight/height and weight/age index at the start and end of the study showed a quicker recovery amongst children in Group I, e.g. those receiving the Moringa leaf powder as a food supplement.
At the end of the study a quicker recovery was observed among children in group I with a z-score of −1.00 ± 0.69, compared to those of Group II (−1.78 ± 0.87). The children are considered fed well whose WHZ index is included between −1 and −2 z-score [13]. Otherwise the digestibility of amino acids and proteins is close to 60%; this allows a good absorption of nutrients brought by the Moringa oleifera leaf powder [4]. Moringa is reported to have high quality protein which is easily digested and that is influenced by the quality of its amino acids [15]. Moringa is reported to be rich in vitamin C which increases iron absorption in the animal’s body [9]. Vitamin A and other element are necessary for many functions including vision, bone growth, immunity and maintenance of epithelial tissue. This explains partially the sharpest recovery in children of Group I, especially given that wasting is sensitive to rapid changes in the food supply [16]. According to WHO (World Health Organization), recovered children are those whose z-score is greater than or equal to −2.
Moreover, the recovery was greater when the assessment was based on the underweight factor. Children receiving the Moringa-supplemented diet recovered with a z-score of −1.99 ± 0.87, compared to −2.47 ± 1.08 amongst those not receiving the Moringa supplement.
3.2. Comparative Variation of the Nutritional Status of Children in the Study
3.2.1. The Average Daily Weight Gain
The daily weight gain (g/kg/day) is an indicator of the variation in the nutritional status of children subjected to nutritional care, and is an integral part of the management of care centers. The Figure 1 shows the average daily weight gain in children in Group I and Group II during the study.
The average daily weight gain recorded in children receiving the Moringa leaf powder was 8.9 ± 4.30 g/kg/day against 5.7 ± 2.72 g/kg/day in children not receiving the supplement. The comparison of weight gain between these two groups was statistically significant (p = 0.002) (Figure 1). However, only the weight gain obtained in children receiving the Moringa supplement (Group I) is anywhere close to the recommended gain of 10 to 20 g/kg/day [17]. This may indicate the added nutritional value of Moringa, as having contributed to a variation in the nutritional rehabilitation during the study, especially given that wasting is sensitive to rapid changes in the food supply [16]. These results support the conclusion of Lockett et al., [18] who claimed that the consumption of Moringa leaf powder can increase weight gain among those receiving it.
3.2.2. Average Duration of Stay
The average duration of stay is an indicator which helps to measure the effectiveness of the management of malnourished children. Figure 2 shows the average length of care at the CREN (rehabilitation unit) during the study.
In this study, the average duration of treatment was 36 ± 16.5 days for the children on the Moringa leaf powder diet (group I), and 57 ± 19.2 days for those not receiving the supplement (group II). Both these average durations are greater that acceptable value [19] of up to four (04) weeks, or twenty-eight (28) days. However, the average duration of stay recorded among children in Group I is closer to the acceptable value. The shorter stays, and so the more efficient recovery, observed amongst children from group I may be attributed to Moringa. Diets rich in amino acids help to boost the immune system against gastro intestinal parasites infestations [20], which is undoubtedly conducive to weight gain so fast recovery.
However, the long period of recovery in general can be explained the low contribution of bioavailable iron during nutritional recovery due to synthesis of cells and red cells [21].
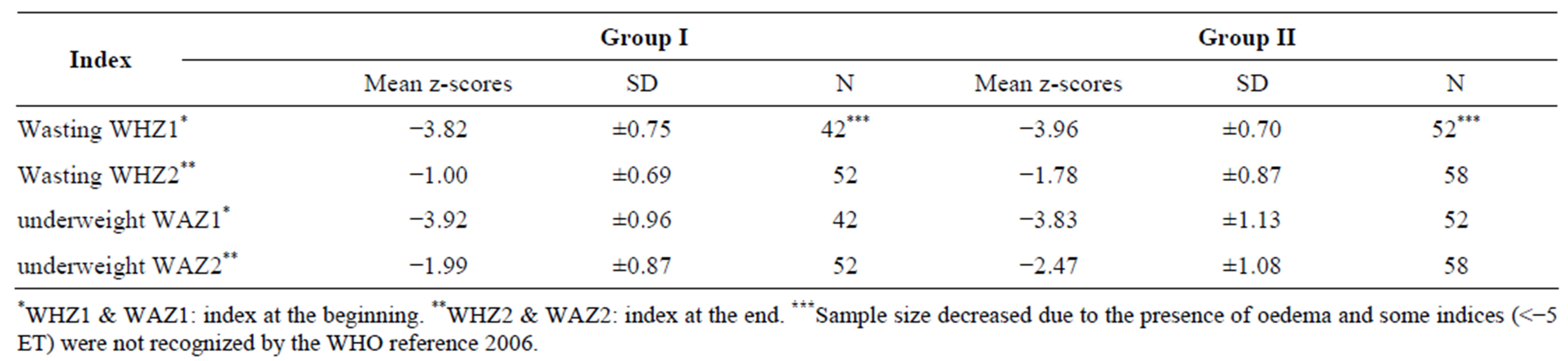
Table 1. Mean z-scores of children at the beginning and end of the study.
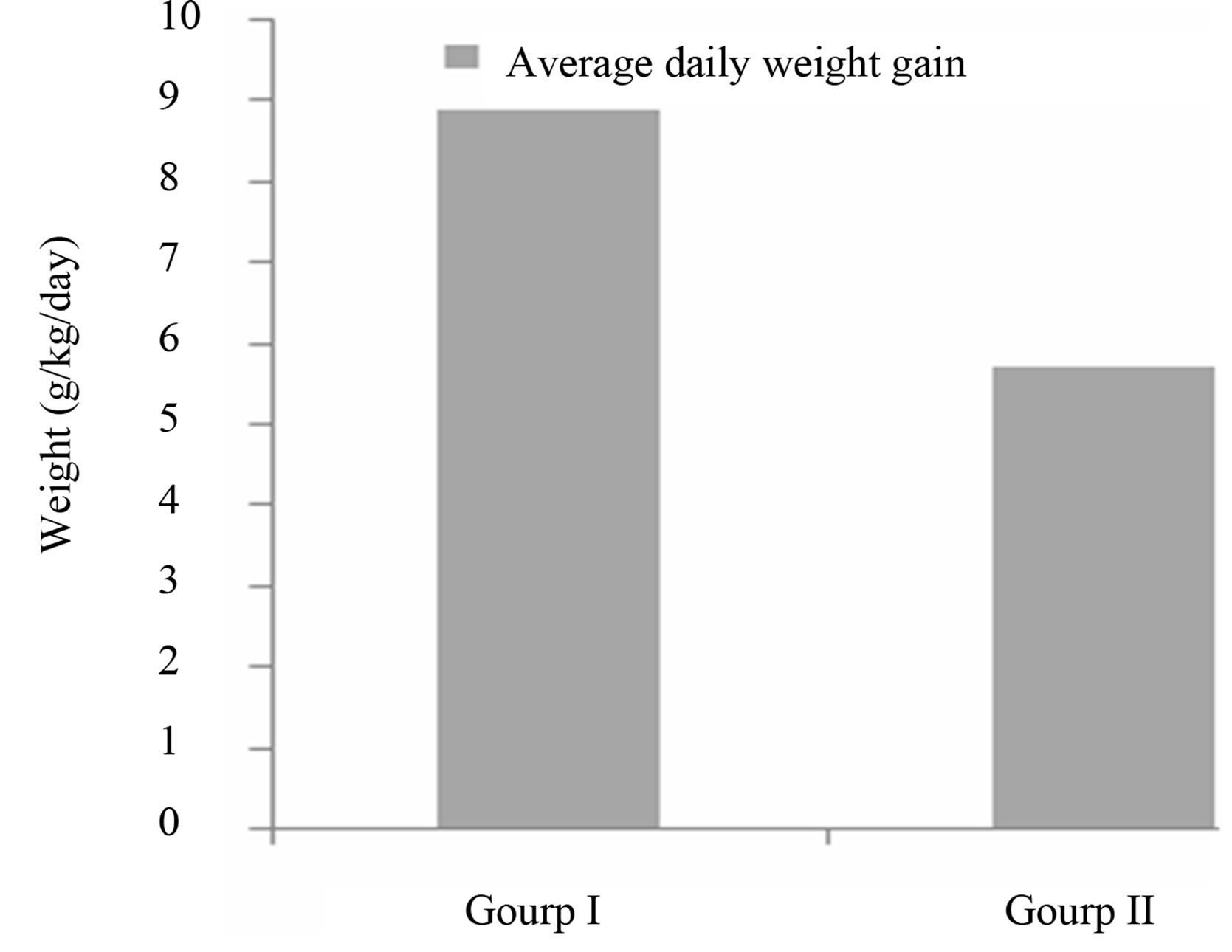
Figure 1. Average daily weight gain of children receiving Moringa (Group I) and those not receiving Moringa (group II).
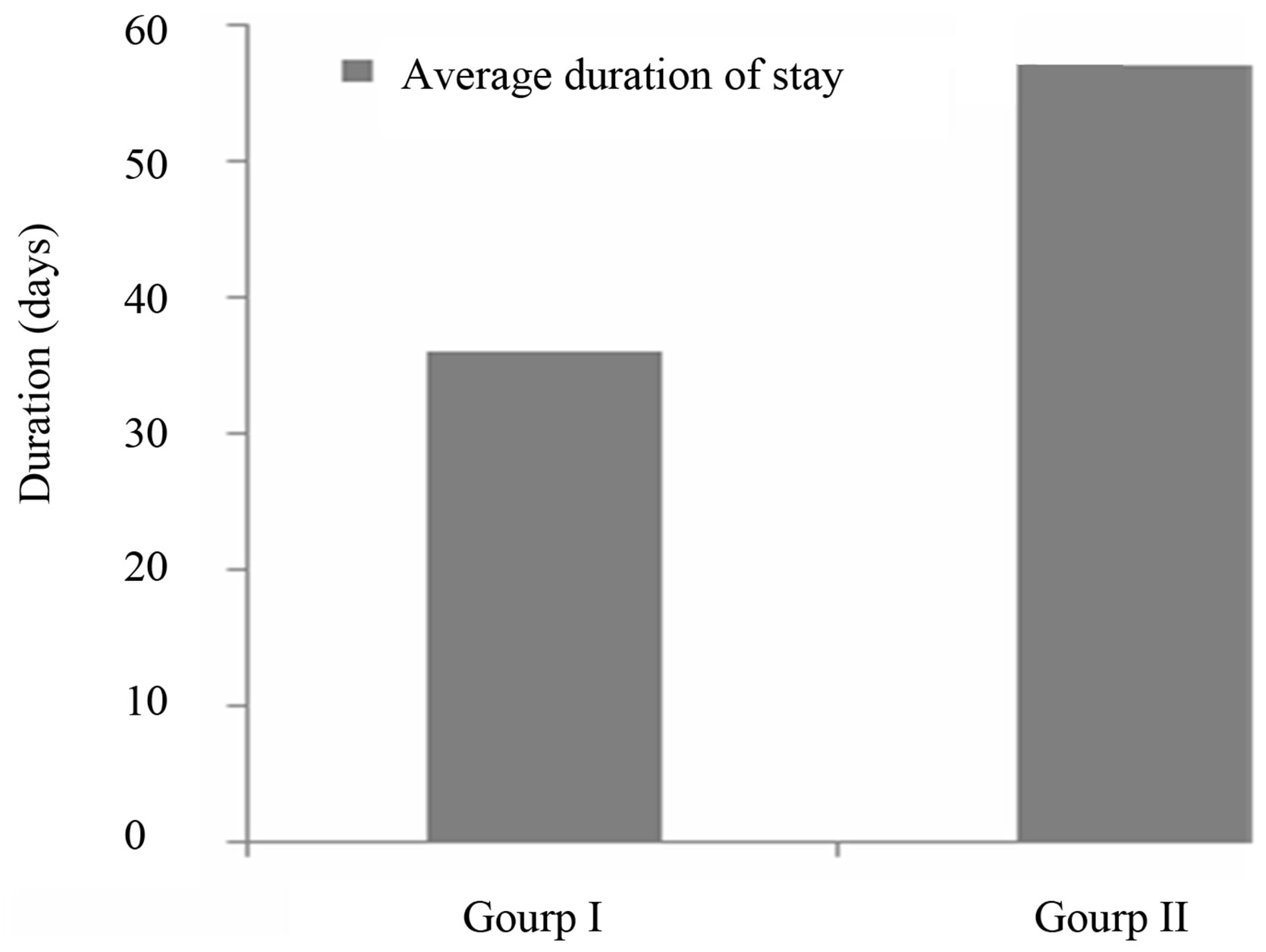
Figure 2. Average duration of stay of children during the study.
3.2.3. Variation in Anthropometric Parameters
In addition to weight, other parameters were assessed in order to appreciate the overall improvement in nutritional status. Those parameters were the MUAC and height, and the results are shown in Figures 3 and 4.
Evolution of height: Height increase during the nutritional recovery was similar between the two groups, and the difference was not statistically significant (Figure 3). The notion that diets which are entirely plant based may lack components needed for optimal growth is supported by work done in Kenya [22]. Height varies little during the nutritional care of children, and so is not a discriminative parameter in the monitoring of malnourished children during short-term hospitalization [23].
MUAC: The results of the variation of MUAC size are shown in Figure 4. A significant difference (p < 0.001) was recorded in both groups (Group I (r = 0.73; p < 0.000), Group II (r = 0.39; p < 0.001), but was greater in group I. This could be explained in part by the fact that MUAC is a sensitive parameter to the sudden change in food intake, but also to the nutrient and energy-rich porridge con-
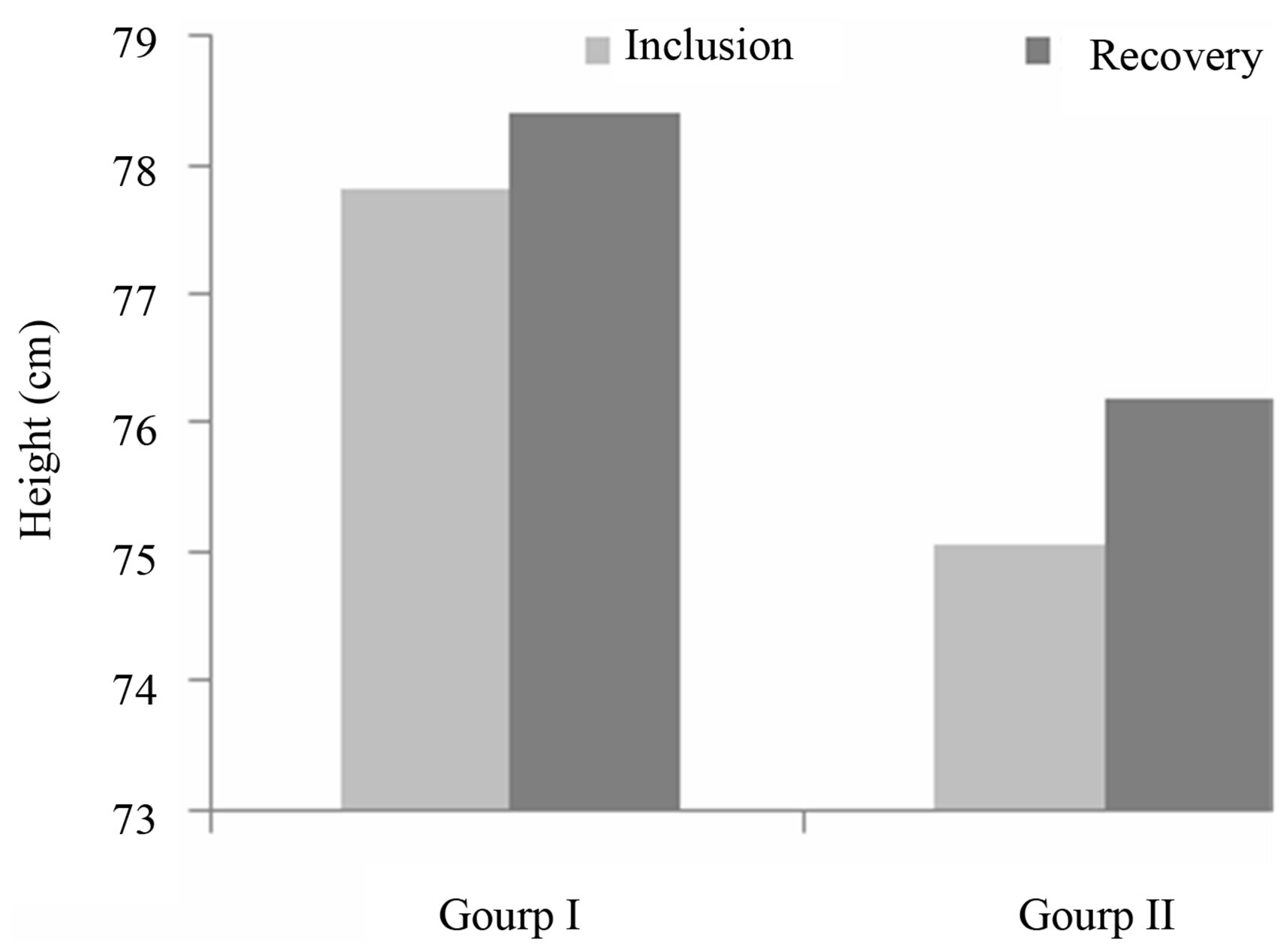
Figure 3. Variation in height of children during the recovery study.
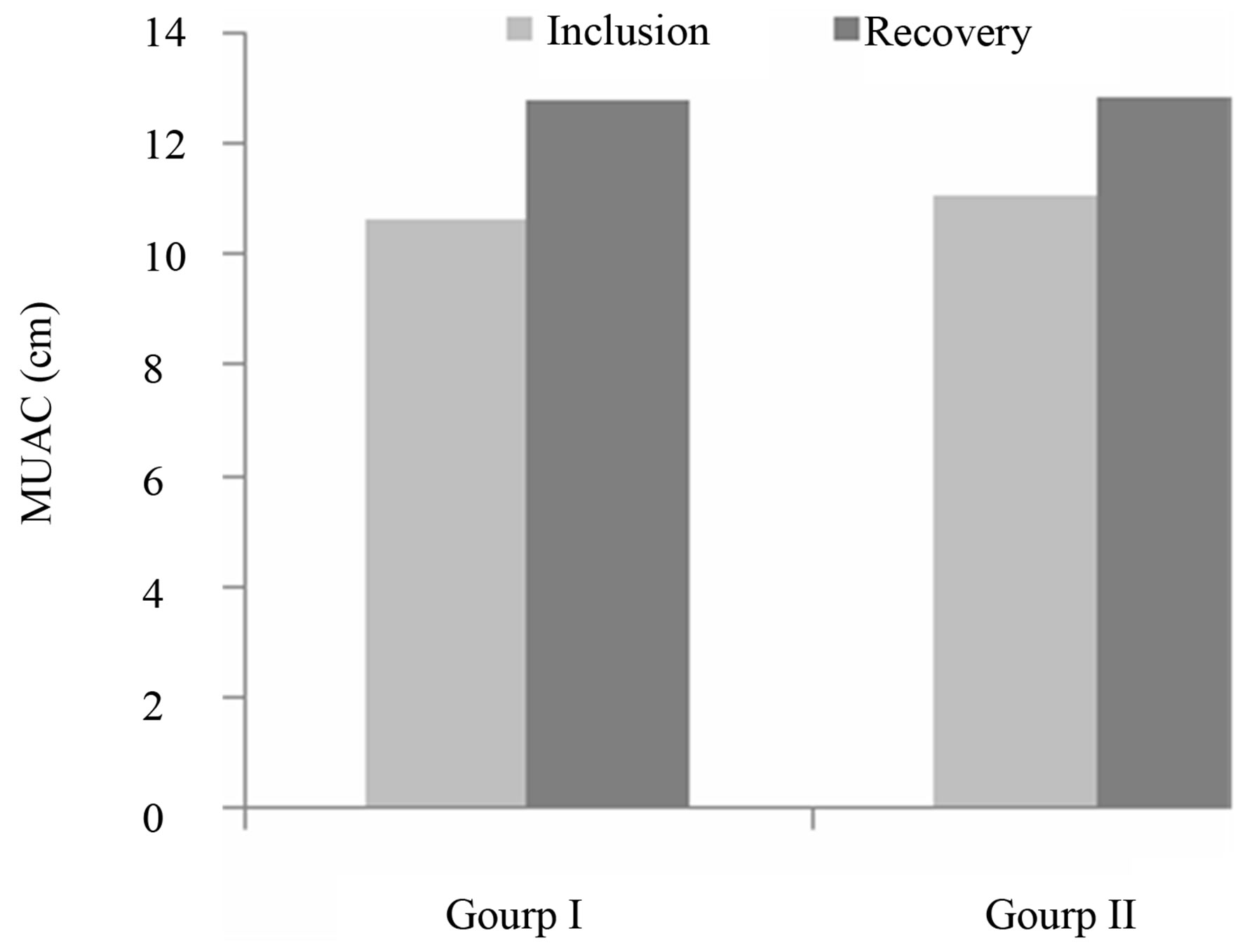
Figure 4. Variation of MUAC.
sumed, especially when mixed with Moringa leaf powder. This situation can be explained by the physico-chemical composition and energetic content of Moringa leaves, which has more Proteins, carbohydrates and micronutrients [6,24].
3.2.4. Variation in Hemoglobin Rate during the Recovery Study
Anemia, which is often, associated with malnutrition, also usually results from a state of nutritional deficiency. Iron deficiency anemia, the most prevalent nutritional problem in the world, adversely affects physical growth, cognitive and behavioral performance, the immune status, and morbidity from infections in infants. Therefore, early identification of the risk factors for iron deficiency anemia is essential for the prevention not only of anemia but also the numerous and long-term consequences caused by iron deficiency [25]. To detect anemia, age-appropriate cut-offs of hemoglobin (Hb) values are necessary and, if present, any physiological changes should be defined [26].
The hemoglobin rate results are shown in Table 2.
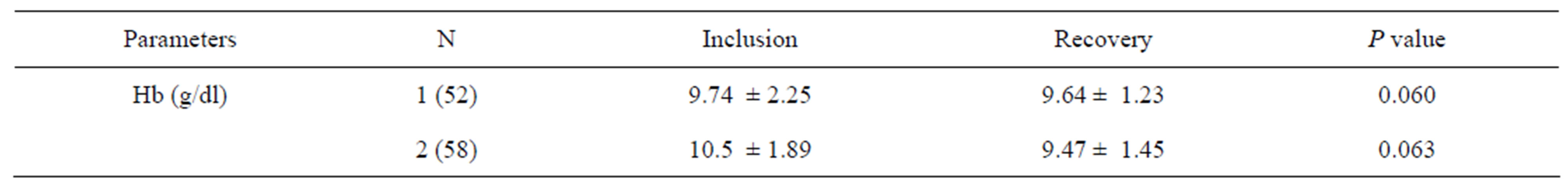
Table 2. Results of hemoglobin rate during recovery.
Given the normal hemoglobin levels in children of between 11.5 and 14.5 g/dl, the average rate of hemoglobin shown in the table above probably reflects anemia among those children studied, both as inpatients and outpatients. Diagnostic criteria for anemia in young infants are poorly defined in infants aged 6 months, and the high proportion of anemic children indicates the need to reevaluate the definition of anemia. In clinical practice, commonly used cut-off values for identifying iron deficiency and iron deficiency anemia at 6 - 12 months of age are Hb < 11 g/dl, but these values are extrapolated from older age groups and may not be appropriate for infants [27].
There was no significant improvement in hemoglobin levels in either group (p = 0.060 Group I, p = 0.063 Group II) in this study, but the interpretation of these results should be complemented by an analysis of other iron deficiency parameters such as ferritin serum, transferrin and protein in the inflammatory phase. Improvement in the overall nutritional status does not necessarily signify changes in biological parameters such as hemoglobin. Iron supplementation during nutritional rehabilitation normally improves Hb levels significantly, but this variation is in turn affected by weight gain, as high weight gain is not conducive to significant changes in Hb levels [28,29]. In fact, a negative correlation was found, and this corroborates with the results of Compaoré et al. [30].
This can be firsly explained by the low bioavailability of iron, due to synthesis of cells and red blood cells [21] and secondly by the dose of Moringa used or when the duration of the study. Indeed, N’dong et al., [6] has demonstrated a low bioavailability of iron in the Moringa leaf powder, around 2.24% ± 0.65%. The addition of large quantities of Moringa could increase this value of iron present, but could also change the organoleptic characteristics of food such as porridge (color, taste), a factor which would affect its acceptability.
Beta-carotene rich Moringa leaves can thus be an important source of vitamin A, can be used for releasing the bound iron status and thus, help in reducing anaemia as well as prevalence of vitamin A deficiency. The mineral composition of the leaves (Table 1) unravels a high concentration of iron and calcium. Children, women of reproductive age and pregnant women are most vulnerable to micronutrient deficiency and anemia.
3.2.5. Variation of Clinical Parameters
The evolution of the clinical forms of malnutrition was more rapid and very pronounced in Group I. This can be explained by the nutritional properties [4,24,31] and pharmacological of Moringa oleifera leaves and can be used either for prevention and correction of malnutrition, or to reduce oxidative stress and inflammatory reactions [32].
In addition to monitoring the nutritional quality of the porridge, the acceptability to patients of the Moringa leaf powder-enriched porridge was evaluated, as well as any possible side effects in the children. Indeed, a large amount of powdered Moringa leaf would have greatly improved the nutrient-content of the porridge, but may have influenced its organoleptic quality. The following table (Table 3) presents the results of the acceptability and side effects quality. Moringa was mixed into porridge. With virtually no taste difference, the kids easily accepted it into their diet. Tolerability was considered to be good, as there were no recorded cases of medical admittance, nor any occurrence of digestive disorders, skin allergies or respiratory disorders. These results support the conclusion of Tete-Bénissan et al., [32] who noticed that the powder did not result in any of the children a digestive problem or allergic reactions. Despite the profound metabolic disturbances caused by malnutrition and infections, Moringa oleifera provides good nutritional recovery in malnourished patients infected with HIV or not. No infection occurred in children receiving Moringa. The level of Moringa crude protein content is of particular nutritional significance as it may meet animal’s protein and energy requirements and boost the immune system against diseases [20,33].
Whilst a small number of children (3.84%) showed resistance to their first bowls of porridge mixed with Moringa powder, most children consumed it without an issue. However this was only observed during the first week of the study. This can be attributed firstly to the color of the slurry mixed with the Moringa leaf powder. The use of moringa however, imparted green coloration to the product, making them appear greenish. Moringa leaf powder is dark green in appearance and therefore, its addition to the products resulted in their appearing greenish.
The comparative frequency of diarrhea was 7.8% among children in Group I against about 80.3% of those in Group II. Exception has diarrhea and vomiting which accountability to Moringa has been demonstrated, no
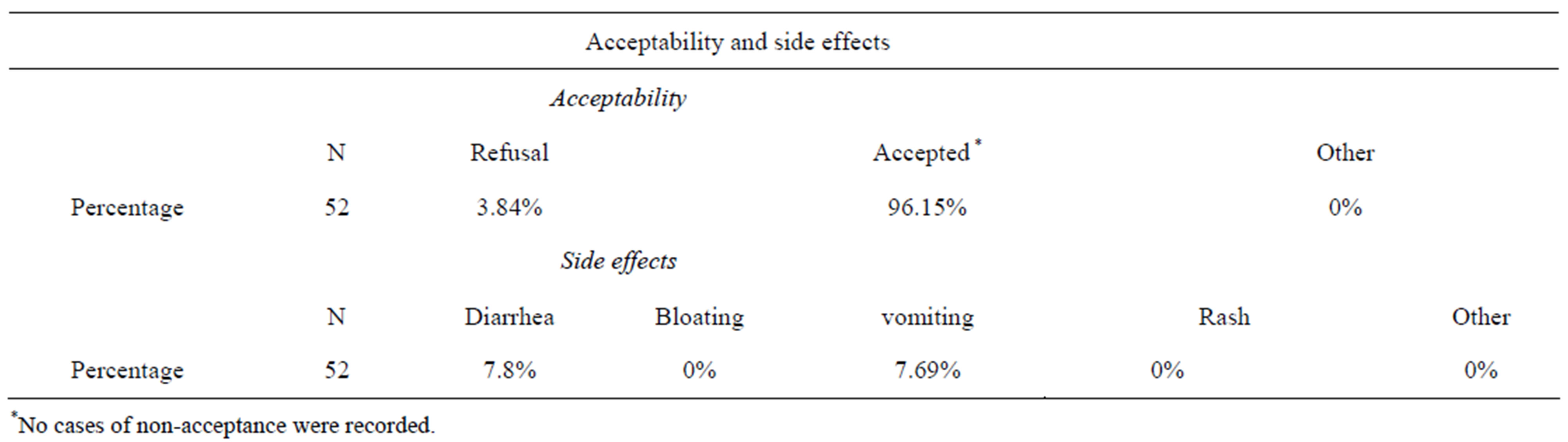
Table 3. Evaluation of acceptability and side effects associated with the consumption of porridge mixed with Moringa leaf powder.
adverse effects associated with the consumption of porridge mixed with Moringa leaf powder were observed. This frequency of diarrhea among children in Group I may be attributed to Moringa especially as 80% of children in group II made diarrhea. Vitamin A, a potent antiinfective and E are some of the specific nutrients that assist animals and human body to develop disease resistance. Moringa powder is, however, rich in vitamin such that it is one of the richest plant sources of vitamin [9].
With the exception of the above, no intolerance to Moringa was recorded. Indeed, Yang [4] highlighted the lack of flatulent sugars such as raffinose and stachyose in mature Moringa leaves, a fact which could explain the absence of bloating in children in group I.
4. Conclusions
This study was designed to evaluate the impact of Moringa oleifera in improving the nutritional status of vulnerable groups, including children. The monitoring of severely malnourished children allowed us to objectively assess the impact of Moringa leaf powder consumption on these patients.
This study allowed us to appreciate the benefits to nutritional recovery of the Moringa leaf powder, by assessing parameters such as daily weight gain, recovery speed, hemoglobin rate, and side effects. The acceptability of Moringa amongst patients was good.
This study shows that Moringa, packed with nutriational potential, can be used as a dietary supplement, and may even contribute to the fight against malnutrition in Burkina Faso.
5. Acknowledgements
The author acknowledges the CMA Paul VI of Ouagadougou (Burkina Faso), and all workers of CREN for providing facilities for this work. Thanks to the Ethical Committee for Health Research of Burkina Faso (ECHRBF). Particular thanks to SEKONE L. Placide from Espace Moringa and Philippe Arnold phD from Foundation DREYER for the financial support. Thanks to KABORE P. Antoine, phD student in law (University of Geneve), and KY Lawakilea for his technical support.
REFERENCES
- ECOWAS, “La Planification et le Financement des Programmes de Nutrition dans la CEDEAO,” Douzième Forum, Septembre 2010.
- DN, “National Nutritional Investigation,” Final Report, Health Ministry of Burkina Faso, 2010, p. 45.
- C. P. Nana, I. Brouwer, N. M. Zagre, F. J. Kok and A. S. Traore, “Impact of Promotion of Mango and Liver as Sources of Vitamin A for Young Children: A Pilot Study in Burkina Faso,” Public Health Nutrition, Vol. 9, No. 6, 2006, pp. 808-813. doi:10.1079/PHN2005911
- R. Y. Yang, C. Lien-Chung, H. Jenn-Chung, B. C. Brian Weng, C. Manuel Palada, M. L. Chadha and Virginie Levasseur, “Propriétés Nutritionnelles et Fonctionnelles des Feuilles de Moringa; Du Germoplasme, à la Plante, à l’aliment et à la santé,” The World Vegetable Center, 2006.
- F. Ezzati, A. D. Lopez and A. Rodgers, “Selected Major Risk Factors and Global and Regional Burden of Disease,” Lancet, Vol. 360, No. 9343, 2002, pp. 1347-1360.
- M. N’dong, S. Wade, N. Dossou, A. T. Guiro and R. G. Diagne, “Valeur Nutritionnelle du Moringa Oleifera, Etude de La Biodisponibilité, Effet de L’enrichissement de divers plats Traditionnels Sénégalais avec la Poudre de Feuilles,” African Journal of Food Agriculture Nutrition and Development, Vol. 7, No. 3, 2007, pp. 1684-5374.
- J. M. Sawadogo and J. Kinda, “Role of the Misola Flour in the Prevention of Malnutrition and the Nutritional Recovery of the Children,” 2nd International Workshop: Food-Based Approaches for a Healthy Nutrition in West Africa: The Role of Food Technologists and Nutritionists, 2004, p. 13.
- O. Bruyeron, M. Denizeau, J. Berger and S. Trêche, “Marketing Complementary Foods and Supplements in Burkina Faso, Madagascar, and Vietnam: Lessons Learned from the Nutridev Program,” Food Nutrition Bulletin, Vol. 31, No. 2, 2010, pp. S154-S167.
- F. Anwar, L. Sajid, A. Muhammad and H. G. Anwarul, “Moringa Oleifera: A Food Plant with Multiple Medicinal Uses,” Phytotherapy Research, Vol. 21, No. 1, 2007, pp. 17-25. doi:10.1002/ptr.2023
- L. R. Ott and M. Longnecker, “An Introduction to Statistical Methods and Data Analysis,” 5th Edition, Thomson Learning, Duxbury, 1999.
- L. J. Fuglie, “The Natural Nutrition for the Tropics,” In: L. J. Fuglie, Ed., The Miracle Tree, the Multiple Attributes of Moringa, CTA, CWS, Dakar, 2001, pp. 103-115.
- A. Shakir and D. Morley, “Measuring Malnutrition,” Lancet, Vol. 1, No. 7860, 1974, pp. 758-759. doi:10.1016/S0140-6736(74)92987-0
- World Health Organization, “Management of Severe Malnutrition. A Manual for Physicians and Other Senior Health Workers,” WHO, Geneva, 2006, p. 72.
- United Nations Children’s Funds, “La Situation des Enfants dans le Monde,” UNICEF, Geneva, 1998, pp. 70-90.
- N. Foidl, P. S. Harinder and K. B. Markar, “The Potential of Moringa Oleifera of Agricultural and Industrial Uses,” In: J. Fuglie, Ed., The Miracle Tree, CTA, Dakar, 2001, pp. 45-76.
- S. Gervais,“Perception des Paysans et des Paysannes de leur Insécurité Alimentaire dans le département de Boulsa au Burkina Faso,” MSI, Université de Laval, 1993.
- A. Ashworth, and D. J. Millward, “Catch-up growth in children,” Nutition Reviews, Vol. 44, 1986, pp. 157-163. doi:10.1111/j.1753-4887.1986.tb07613.x
- C. T. Lockett, C. C. Calvert and L. E. Grivetti, “Energy and Micronutrient Composition of Dietary and Medicinal Wild Plants Consumed during Drought. Study of Rural Fulani, Northeastern Nigeria,” International Journal of Food Sciences Nutrition, Vol. 51, No. 3, 2000, pp. 195- 208. doi:10.1080/09637480050029700
- OMS/PAM/UNSCN/UNICEF, “Prise en Charge Communautaire de la Malnutrition Aiguë Sévère. Une Déclaration Commune de l’Organisation Mondiale de la santé, du Programme Alimentaire Mondial, Comité Permanent de la Nutrition du Système des Nations Unies et du Fonds des Nations Unies pour l’enfance,” 2007. http://www.who.int/nutrition/topics/Statement_community_based_man_sev_acute_mal_fre.pdf
- I. Kyriazakis and J. G. Houdijk, “Nutritional Control of Parasites,” Small Ruminant Research, Vol. 62, No. 1, 2006, pp. 79-82. doi:10.1016/j.smallrumres.2005.07.036
- E. H. I. Diop, N. I. Dossou, M. M. Ndour, A. Briend and S. Wade, “Comparison of the Efficacy of a Solid Readyto-Use Food and a Liquid, Milk-Based Diet for the Rehabilitation of Severely Malnourished Children: A Randomized Trial,” American Journal of Clinical Nutrition, Vol. 78, 2003, pp. 302-307.
- C. G. Neumann, S. Whaley and M. D. Sigman, “Animal source Foods Improve Cognitive Performance, Activity and Nutrition Status in Kenyan School Children,” Pediatric Research, Vol. 51, 2002, p. 192.
- M. G. Sall, Kuakuvi, B. Diaham, M. Fall, “Evolution Anthropométrique d’enfants Sénégalais Hospitalises pour Malnutrition Protéino-Energétique (MPE) Grave au cours de la Récupération Nutritionnelle,” Médécine Afrique Noire, Vol. 38, No. 8-9, 1991, pp. 596-601.
- W. C. Yaméogo, D. M. Bengaly, A. Savadogo, P. A. Nikièma and S. A. Traoré, “Determination of Chemical Composition and Nutritional Values of Moringa oleifera Leaves,” Pakistan Journal of Nutrition, Vol. 10, No. 3, 2011, pp. 264-268. doi:10.3923/pjn.2011.264.268
- S. Grantham-McGregor and C. Ani, “A Review of Studies on the Effect of Iron Deficiency on Cognitive Development in Children,” Journal of Nutrition, Vol. 131, 2001, 649S-666S.
- O. Hernell and B. Lonnerdal, “Is Iron Deficiency in Infants and Young Children Common in Scandinavia and Is There a Need for Enforced Primary Prevention,” Acta Paediatrica, 2004, Vol. 93, 2004, pp. 1024-1026.
- A. M. Emond, N. Hawkins, C. Pennock and J. Golding “Haemoglobin and Ferritin Concentrations in Infants at 8 Months of Age,” Archive of Diseases Childhood, Vol. 74, No. 1, 1996, pp. 36-39. doi:10.1136/adc.74.1.36
- M. K. Georgieff, S. W. Wewerka, C. A. Nelson and R.-A. de Regnier, “Iron Status at 9 Months of Infants with Low Iron Stores at Birth,” Journal of Pediatrics, Vol. 141, No. 3, 2002, pp. 405-409. doi:10.1067/mpd.2002.127090
- I. Thorsdottir, B. S. Gunnarsson, H. Atladottir, K. F. Michaelsen and G. Palsson, “Iron Status at 12 Months of Age-Effects of Body Size, Growth and Diet in a Population with High Birth Weight,” European Journal of Clinical Nutrition, Vol. 57, No. 4, 2003, pp. 505-513. doi:10.1038/sj.ejcn.1601594
- W. R. Compaoré, P. A. Nikièma, A. Savadogo, H. I. N. Bassolé, J. Mouecoucou, S. Pignatelli, J. Simporé and S. A. Traoré, “Nutritional and Hematological Recovery of Severely Malnourished Children with New Flour Formulas Developed at Ouagadougou (Burkina Faso),” British Journal of Dairy Sciences, Vol. 2, No. 1, 2011, pp. 11-17.
- U. Zongo, “Détermination de la Qualité Nutritionnelle des Feuilles de Moringa et étude de L’évolution des Paramètres Cliniques et Biologiques des Enfants Malnutris dont le Régime Alimentaire Inclut le Moringa,” M.S.II Thesis, University of Ouagadougou, Burkina Faso, 2012, p. 59.
- A. Tété-Bénissan, M. L. A. Quashie, K. Lawson-evi, K. Kokou and M. Gbeassor, “Récupération Nutritionnelle chez les Sujets Malnutris VIH Positifs et VIH Négatifs Après Utilisation de Feuilles de Moringa Oleifera Lam,” Journal of Animal &Plant Sciences, Vol. 15, No. 2, 2012, pp. 2184-2199.
- E. A. Brisibe, U. E. Umoren, F. Brisibe, P. M. Magalhaes, J. F. S. Ferreira, D. Luthria, X. Wu and R. L. Prior, “Nutritional Characterization and Antioxidant Capacity of Different Tissues of Artemisia annua L.,” Food Chemistry, Vol. 115, No. 4, 2009, pp. 1240-1246. doi:10.1016/j.foodchem.2009.01.033
NOTES
*Corresponding author.

