Food and Nutrition Sciences
Vol. 3 No. 2 (2012) , Article ID: 17526 , 9 pages DOI:10.4236/fns.2012.32035
Olive and Corn Oil Enriched Diets Changed the Phospholipid Fatty Acid Composition in Mice Liver after One-Third Hepatectomy
![]()
1Department of Biotechnology, University of Rijeka, Rijeka, Croatia; 2Department of Biology and Medical Genetics, Medical Faculty, University of Rijeka, Rijeka, Croatia; 3Department of Chemistry and Biochemistry, Medical Faculty, University of Rijeka, Rijeka, Croatia; 4Department of Physiology, Immunology and Pathophysiology, Medical Faculty, University of Rijeka, Rijeka, Croatia.
Email: jgiacometti@biotech.uniri.hr
Received October 29th, 2011; revised December 9th, 2011, accepted December 16th, 2011
Keywords: Fatty Acids; Phospholipids; One-Third Partial Hepatectomy; Olive Oil; Corn Oil; Diet
ABSTRACT
We examined the effect of dietary fats on the kinetics of liver regeneration after one-third partial hepatectomy (PH) in male Balb/c mice. Corn and olive supplemented diets were chosen as a model of n-6 and n-9 diets. Phospholipid fatty acids were determined by gas chromatography (GC) after previous fractionation by solid-phase extraction using aminopropyl (NH2) column, and data were analysed by nonparametric Kruskal-Wallis test. Diet enriched with corn and olive oil did not affect the balance of lipid disorders during liver regeneration after 1/3 PH. Desaturases activity direction has changed depending on the used diet. The key role in the alteration of the polar fatty acids profile at all stages of liver regeneration and examined dietary fats is played by activity of stearoyl-CoA desaturase (SCD).
1. Introduction
Phospholipids (PL) are a class of lipids that are important in the structure and function of all biological membranes. Changes in the phospholipid fatty acids (PL-FA) profiles have a wide range of effects related to the integrity of cellular membranes, such as the membrane structure and fluidity, the activity of membrane enzymes and the affinity of growth factor receptors. Fatty acids (FA) act as signalling molecules involved in cell proliferation and/or apoptosis [1,2]. Polyunsaturated fatty acids (PUFA) coordinate hepatic lipid synthesis and oxidation by suppressing the transcription of hepatic genes [3,4].
Dietary fats play a dual role in the human and animal physiology; as a source of energy and structural components of cells. As a regulator of gene expression that impacts lipid, carbohydrate and protein metabolisms, as well as cell growth and differentiation [5].
It was observed that the liver is highly responsive to changes in dietary lipid composition [6]. The quantity of fat intake or its composition impact several physiological systems, many of which are cell and tissue specific. Unbalanced dietary fat intake has been implicated in the induction and progression of several chronic diseases both in liver and most other intact tissues.
Liver regeneration is a complex physiological response to hepatic injury during which the remnant organ initiates a series of reactions in order to re-establish the hepatic-dependent homeostasis and promote cell growth. Partial hepatectomy (PH) is the most frequently used model to study liver regeneration and the molecular signalling and factors involved in the proliferation of all existing mature cell populations resident in the remaining organ. Cell proliferation begins very early during rat liver regeneration, peaking for hepatocytes at 24 h and the remnant liver undergoes almost complete restoration of the lost mass and function by about one week. The degree of hepatocyte proliferation is directly proportional to the degree of injury. Membrane proliferation is a common feature of programmed cell death and implies major changes in the metabolism, traffic and re-modelling of intracellular membranes and their lipid constituents [7-9].
Our in vivo study was primarily directed to understanding possible alterations of the disorders in the lipid homeostasis in the liver caused by 1/3 PH. Further analysis was included into examination of the differences in the ongoing regeneration of the liver under the influence of n-6 and n-9 diets reflected to the polar fatty acid profiles.
2. Materials and Methods
2.1. Chemicals and Reagents
All the used chemicals and reference compounds for gas chromatography (GC) were purchased from Sigma (St. Louis, MO, USA) and were of the highest reagent grade available. A compound used for the formulation of diets, the extra virgin olive oil sample was obtained from Agroprodukt d.o.o. (Pula, Croatia) and the refined corn oil sample was obtained from Oleifico Zucchi S.p.A (Cremona, Italy). Extra virgin olive oil had 279 mg/kg of total polyphenols.
The solid-phase extraction cartridges, NH2 (Bond Elut, 3 ml volume, 500 mg sorbents) used for the lipid classes separation were purchased from Varian (Harbor City, CA, USA).
2.2. Diets and Animals
Stock standard diet (pellet, type 4RF21 GLP, Mucedola, Settimo Milanese, Italy) was used as the standard diet in all experiments. Olive oil (FOO) and corn oil (FCO) were added to the stock standard diet to a 5% w/w.
Diets were freshly prepared once a week by the addition of the appropriate amounts of oils, gasses with N2 and stored at 0˚C - 4˚C to minimize fatty acid degradation. FA composition in the used dietary oils (as FA percent) is shown in Table 1.

Table 1. Fatty acid composition of the used dietary oils.
Male Balb/c mice (Medical Faculty, Rijeka, Croatia), at the age of 2 - 3 months and weight of at least 25 to 30 g, were acclimated for 1 week at the temperature (21˚C - 23˚C) and in a humidity controlled facility on a 12 h light/dark cycle. After the acclimatisation period, animals were divided in three dietary groups: group 1 (Control) standard diet fed mice; group 2 (FOO) olive oil fed mice; group 3 (FCO) corn oil fed mice. Groups 2 and 3 were fed FOO and FCO diet for 3 weeks. Dietary groups had their own separate control group that was killed before implemented hepatectomy. After they were hepatictomized, each dietary group animals were further divided into three groups having 6 animals each and killed 1-d, 2-d and 7-d post PH. 1/3 PH (or 30% PH) consisted of the removal of the median liver lobe. To avoid possible diurnal variability, all operations were performed between 8:00 - 9:00 a.m. The non-regenerated regions of the liver was removed by plastic instruments for analysis from the same region in all cases, washed several times with a saline solution (0.9% w/w, NaCl) to remove blood, immediately weighed and stored at –80˚C until the analysis. Sham operated animals (SH, n = 6) received the same treatment as partial hepatectomized mice, but without hepatectomy (laparotomy only). Body weight and food intake were monitored during the study.
All procedures with animals were designed and performed in consideration of its relevance to the improvement of human or animal health and advances of knowledge for the good of society. The acquisition, care and use of animals were in accordance with current federal laws and regulations. The use of animals in research conformed to the highest ethical, humane and scientific standards. Experimental procedures followed the guidelines for Animal Experiments of the Medical Faculty University of Rijeka, as well as the relevant Laws (NN 135/2006) and Notifications (paragraph 25) of the Croatian Government, and were approved by the Medical Faculty Ethics Committee.
2.3. Lipid Analyses
The FA composition of the dietary oil was determined according to the modified EC Regulation 2568/91 [10] by GC analysis within 5% coefficient of variance. Oils, which were subjected to analysis, were heated under reflux with 2 M methanol-hydrochloric acid at 100˚C during 4 h. The obtained FAME were extracted with petroleum ether, passed through anhydrous sodium sulphate and evaporated in a rotating evaporator to dryness. Test portions, in the form of the FAME were performed in duplicate and 1 ml of each sample solute in hexane was injected.
Total lipids were extracted from tissues with chloroform/methanol (2:1, by vol) according to Folch et al. [11] containing 0.01% butylated hydroxytoluene (BHT) as antioxidant. The PL FA composition in the examined tissues was determined according to Giacometti et al. [12]. The lipid extracts were fractionated and purified by solid-phase extraction (SPE) and polar lipids separated on the NH2 column. FA of the polar lipids were transmethylated with methanol/n-hexane/sulphuric acid (75:25:1, by vol) at 90˚C for 90 min, extracted in petrol-ether and analyzed by gas chromatography (GC). GC analyses of FAME were carried out using an Autosystem XL from Perkin-Elmer with flame-ionization detection (FID). Chromatography software from Perkin-Elmer Nelson (Turbochrom 4, rev. 4.1.) was used for data acquisition from the FID. An SP-2330 capillary column (Supelco, Bellefonte, PA, USA), 30 m × 0.32 mm I.D., 0.2 mm film thickness, was used. Helium was used as the carrier gas with split injection (100:1). The analyses were carried out in programmed temperature mode from 140˚C to 220˚C, at 5˚C·min–1 and then isothermal for 25 min. The detector temperature was 350˚C and injector temperature was 300˚C. The results were expressed as a percentage of individual fatty acids in polar lipid fractions.
2.4. Calculations and Statistics
SFA were calculated as SFA = S% (14:0 + 16:0 + 18:0 + 20:0 + 22:0 + 24:0), MUFA as MUFA = S% (14:1 + 16:1 + 18:1 + 20:1) and PUFA as PUFA = S% (PUFAn-3 + PUFAn-6).
PUFAn-3 were calculated as PUFAn-3 = S% (20:5n-3 + 22:5n-3 + 22:6n-3) and PUFAn–6 as PUFAn–6 = S% (18:2n-6 + 18:3n-6 + 20:2n-6 + 20:3n-6 + 20:4n-6 + 22:4n-6). Delta-9-desaturation index of the C16 (PCD) was calculated as 16:1n-7/16:0 ratio and delta-9-desaturation index of the C18 (SCD) as 18:1n-9/18:0 ratio. The rate-limiting step in 20:4n-6 and 22:6n-3 synthesis is the desaturation of 18:2n-6 and 18:3n-3 by delta-6 desaturase index (D6D). D6D was calculated as D6D = [(18:3n-6 + 20:3n-6)/18:2n-6] and delta-5 desaturation index (D5D) was calculated as the 20:4n-6/20:3n-6 ratio.
GC data were evaluated with the StatSoft, Inc. (2008) STATISTICA (data analysis software system), version 8.0. Numerical data were expressed as mean ± S.D. Differences between the origin and time-points, and between themselves during PH depending diets in the examined tissues were analyzed by the nonparametric Kruskal-Wallis test for multiple comparisons of mean ranks for all groups. Statistical significance was assumed with a P < 0.05.
3. Results
FA analyses of dietary oils showed (Table 1) that corn oil mainly contained 18:2n-6 (57.06%) with an n-6/n-3 FA ratio approximately 190:1, consisting of total n-6 and n-3 levels of 57.06% and 0.3%, respectively. Analysis of the dietary olive oil showed that it mainly contained 18:1n-9 (74.48%), and the n-6/n-3 ratio was 13.43:1. These differences, as well as the presence of olive oil minor substances were the basis for the research impact of diet during liver regeneration after one-thirds heaptectomy.
Figure 1 shows the dynamics of liver regeneration in relation to lipids, gravimetrically determined TLL and PLL in partially hepatectomized mice fed different diets. Statistically significant changes during PH were found in the TLL in the FCO diet (P = 0.011), while PLL were observed in the standard diet (P = 0.0007). Compared with the control, PLL levels increased significantly on the 2-d and 7-d post PH in the group fed standard diet. Significant differences were also found in the level of PLL on the 7-d in the FCO and 1-d and 7-d in the FOO compared to the same point in time in all used diets. We did not find significant differences either in the level of TLL or PLL in SH animals and groups control. Similarly there were no significant changes in liver weight in any of the groups.
We assessed the extent of liver injury 1-d, 2-d and 7-d post PH by monitoring changes in the PL-FA composition in the liver, as well as the n-6 and n-9 diets impact on the regenerating liver.
3.1. PL-FA during Liver Regeneration
As presented in Tables 2 and 3, the n-6 FAs were major FAs in the PUFA fraction in all used diets, where they found the largest proportion of 18:2n-6 and 20:4n-6. Compared to the control in the group that was fed standard diet, the level of 18:2n-6 elevated during PH on the 2-d, and thereafter was reduced on the 7-d. However, a significant enhance in 18:2n-6 was found in the liver on the 2-d and 7-d. No significant differences were found in the total liver MUFA during liver regeneration, although 16:1n-7 was significantly increased on the 7-d, and 20:1n-9 on the 2-d post PH. Since the lipid homeostasis is disrupted by surgical liver injury, in the early stage of liver regeneration, the PUFA/SFA ratio was significantly reduced after the 1-d, when PUFA significantly decreased and SFA increased. However, the n-3/n-6 ratio was markedly reduced on the 7-d. The observation that 1/3 PH promoted the growth of initiated hepatocytes is in itself interesting in view of the fact that only a half of the remaining 2/3 of hepatocytes need to respond to proliferation following 1/3 PH. We found a minor change in desaturase activities during liver regeneration in the mice fed with standard diet.
3.2. n-6 and n-9 Diet Effect on PL-FA during Liver Regenerations
The dynamics of the PH in mice were significantly dif-
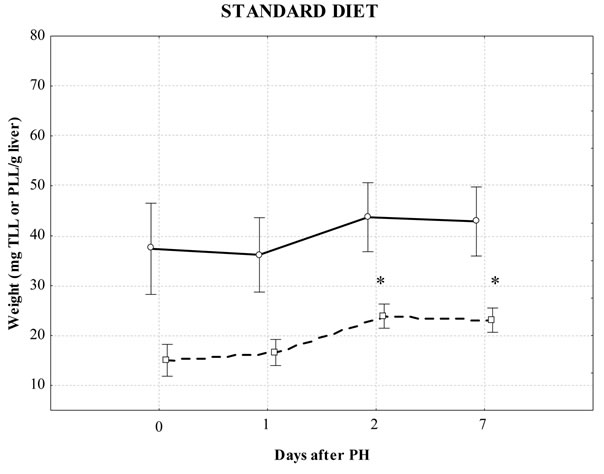

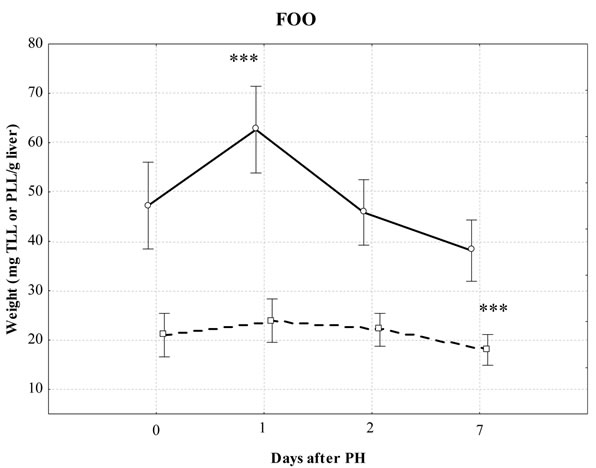
Figure 1. Effect of different diets on total liver lipids (TLL) and total polar liver lipids (PLL) after partial hepatectomy (PH) gravimetrically determined. Values are mean ± S.D. of 6 - 8 mice/group; *significantly different from the control during PH using Kruskal-Wallis test: Multiple comparisons of mean ranks for all groups (P < 0.05); ***significant differences between same time-point PH in the standard diet, FCO and FOO-diet groups using Kruskal-Wallis test: Multiple comparisons of mean ranks for all groups (P < 0.05). solid line: TLL; dotted line: PLL.
ferent depending on studied diets, as shown in Table 3. Compared to the standard diet control liver samples, corn oil significantly elevated n-6 FA (as 18:2n-6, 20:2n-6, 20:4n-6) and reduced n-3 FA (as 20:5n-3, 22:5n-3, 22:6n-3) in the liver control-FCO samples. Also, 16:1n-7 was decreased, while 18:0 and 20:0 were increased in the liver control-FCO samples. Olive oil supplemented diet significantly decreased n-3 FA (as 22:5n-3 and 22:6n-3) in the liver control-FOO samples compared to the control fed standard diet.
As Table 3 shows, fatty acid profile of liver regeneration differs depending on dietary fats in the liver. Since the FCO diet is a model for n-6 diet, we looked for significantly higher amounts of 20:4n-6. Contrary to expectations, a significantly reduced amount of the 20:4n-6 was found only on the 1-d in the FCO diet. Later stage of liver regeneration significantly increased MUFA and decreased SFA (on the 7-d) in the groups supplemented with corn oil in the liver. Dietary oleate, as a major component of olive oil, had beneficial effects on significantly lowering 18:1n-9 in the early regeneration period (on the 1-d). The level of MUFA in the groups with supplemented diets had a consequence in significant PUFA/ MUFA ratio changes, such as significantly elevated MUFA and reduced PUFA/MUFA ratio in the later stage (7-d) in the FCO diet, and significantly reduced MUFA and elevated PUFA/MUFA ratio in the early stage (1-d) in the FOO diet group.
Hepatocyte levels of oleic acid are controlled by both elongation and desaturation pathways, where the elongation pathway is not a constitutive. Depending on the used diets, important differences in hepatic activity were found neither in delta-5 desaturase (D5D) nor delta-6 desaturase (D6D) in the control-FCO and control-FOO samples. However, significant changes were found depending used diet. In the standard diet group, palmitoyl-CoA desaturase (PCD) was significantly increased on the 7-d. FCO diet affect on the D6D lowering on the 1-d and 2-d, and PCD enhancement on the 7-d. FOO diet significantly reduced stearoyl-CoA desaturase (SCD) and D6D activity on the 1-d and PCD elevated on the 7-d as reported in Table 3 and Figure 2.
4. Discussion
After liver damage, the subsequent restoration of liver function depends on the balance between replication and metabolism, while synthesis ensures the survival of the affected organism. The initiation and synchronization of replication in different types of hepatic cells depend on the extent of the resection, tissue damage, or both [13,14]. Many studies are based on a 2/3 hepatectomy, where a greater damage excites a stronger stimulus. The present smaller stimulus and the absence of liver function of
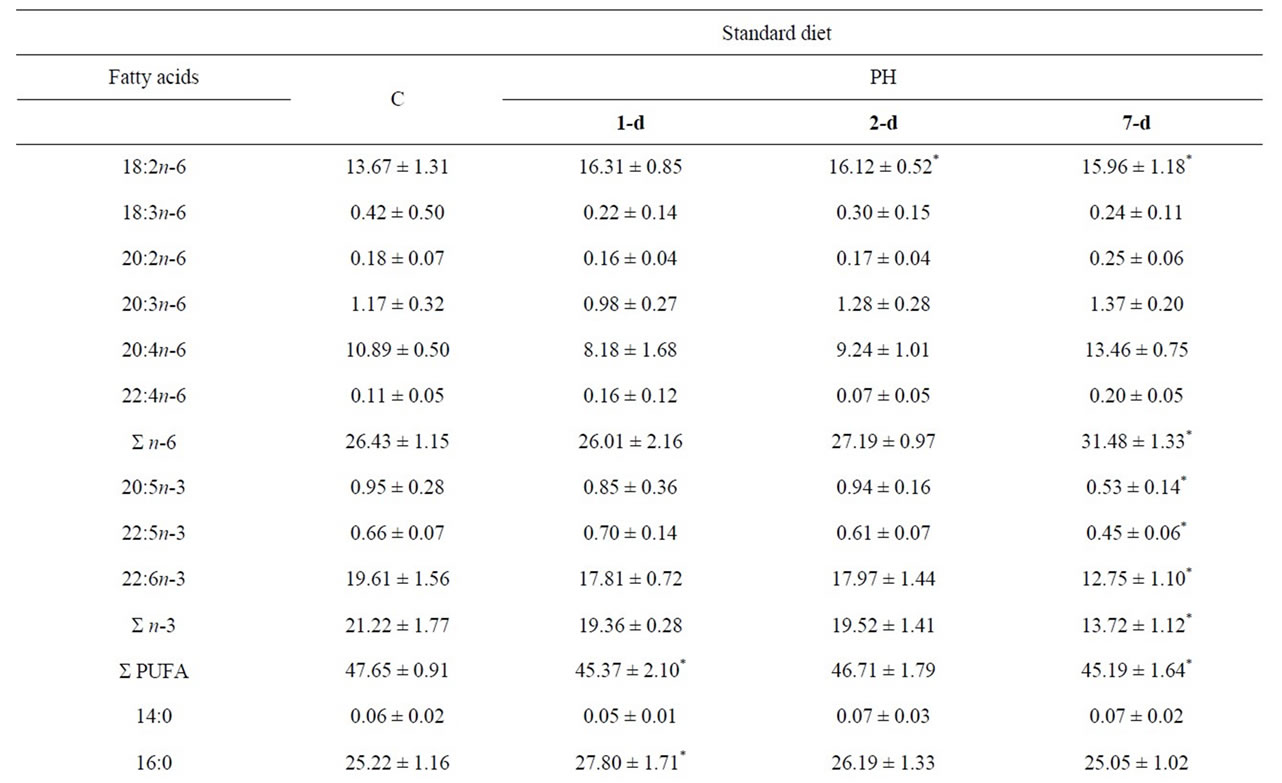
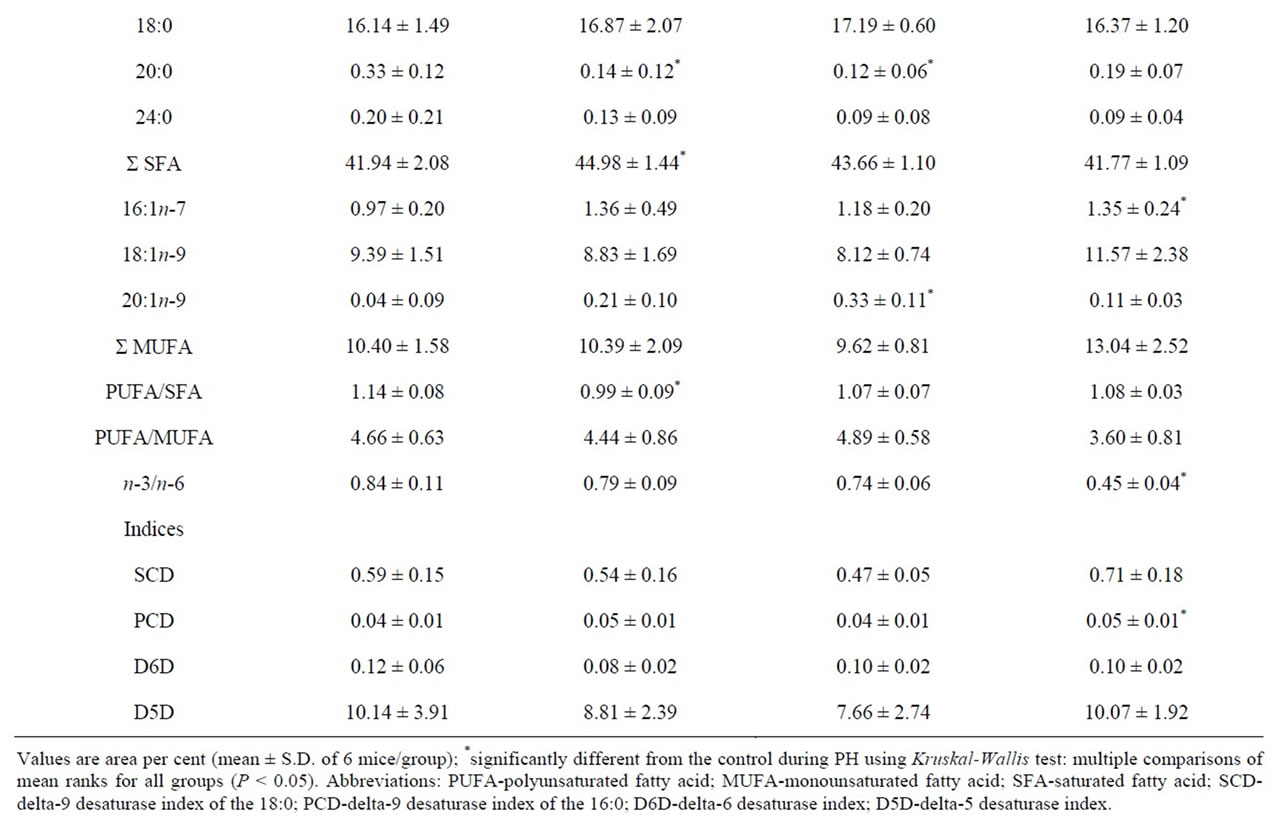
Table 2. Fatty acid composition (%) of the total PL and general indices related to the PL FA composition in the mice liver tissue samples during partial hepatectomy fed standard diet.
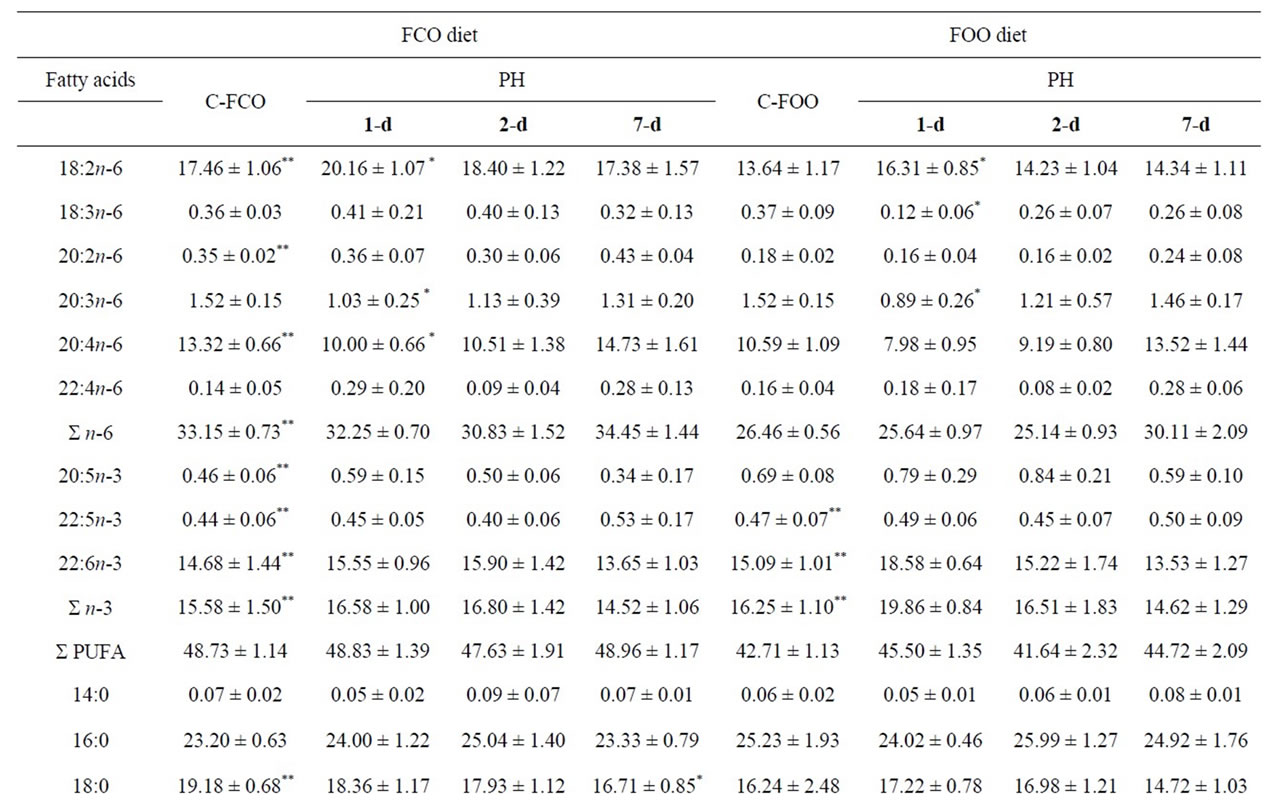
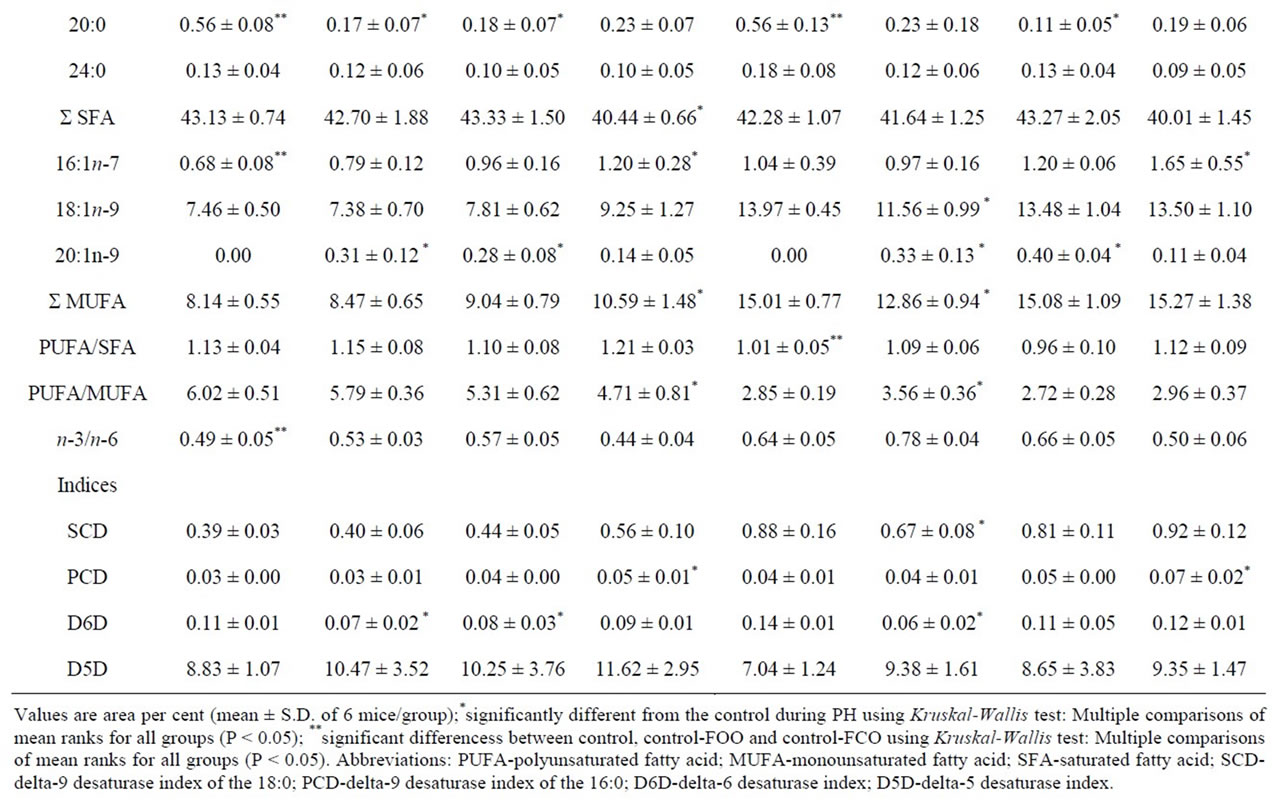
Table 3. Fatty acid composition (%) of the total PL and general indices related to the PL FA composition in the mice liver tissue samples during partial hepatectomy fed FCO and FOO diets.


Figure 2. Desaturases activity direction of in the FCO and FOO diet compared to the standard diet during PH. *Significantly different from the control during PH using Kruskal-Wallis test: Multiple comparisons of mean ranks for all groups (P < 0.05); **Significant differencess between control, control-FOO and control-FCO using Kruskal-Wallis test: Multiple comparisons of mean ranks for all groups (P < 0.05); ***Significant differences between same time-point PH in the standard diet, FCO and FOO-diet groups using Kruskal-Wallis test: Multiple comparisons of mean ranks for all groups (P < 0.05).
small-scale at 1/3 hepatectomy lead to poor possibility of liver regeneration, and long-term may cause liver damage. This phenomenon is related to the occurrence of fatty liver and its reduced ability to regenerate. The low intensity of growth stimuli, such as 1/3 PH, can be very effective in promoting the liver mass growth, undetectable at 24 h, still low at 48 h, peaked at day 3 and remained well above baseline for at least 5 days. However, the peak labeling index in response to 1/3 PH was 50% of that seen with 2/3 PH [15].
The present experimental study was carried out to determine the alterations in the liver polar fatty acids profile after surgical 1/3 partial hepatectomy and possible changes that were under the influence of dietary fats during liver regeneration in mice. We hypothesized that during liver regeneration lipid disorders occur which affects the desaturase activity, and that would be balanced by the n-6 and n-9 diets. A profile of the timesequential changes in the lipid metabolism as phospholipid fatty acid changes during liver regeneration in the residual liver were investigated and compared between animal groups that underwent hepatectomy. Compared with the control group of each diet, sham-operated mice showed no significant differences.
FA delivered to the regenerating liver act as a substrate for the synthesis of phospholipids and cholesterol. The saturated FA in the residual liver are intensively converted to unsaturated acids, which are essential for the synthesis of phospholipids and cholesterol [16]. Since the largest proportion of phospholipids is present in cell membranes, phospholipid fatty acid profiles provided knowledge about the activities of membrane enzymes and membrane fluidity. We found that the conversion of saturated to unsaturated fatty acids was more intense in all phases of liver regeneration in the groups fed with corn and olive oil.
It has long been recognized that the regenerating liver generates signals that couple FA release from peripheral adipose stores to augmented hepatic FA uptake, which in turn promotes hepatic lipogenesis [17] and leads to a rapid accumulation of intracellular triglyceride (TG) within the regenerating liver [18]. The accumulation of fats in the liver depends on the type and grade of tissue damage. Liver steatosis is a major factor determining the outcome after surgery, a consequence of altered lipid metabolism. Diet and nutrition, in particular the amount and type of fat intake, are linked to the risk of impaired lipid metabolism and the possibility of regeneration. The major part of the beneficial effect of Mediterranean diet is a high supply of energy from MUFA, mainly from live oil (n-9 diet). Linoleic acid as the major n-6 PUFA in the diet is metabolised in many tissues and tumour cells to arachidonic acid, as a precursor for eicosanoids, which are directly responsible for growth stimulation and cytokines promotion by n-6 PUFA. Corn oil, rich in linoleic acid, increases cell proliferation ~3.5-fold in hepatocytes, so that olive oil and saturated fats have no significant effect on hepatocyte proliferation [18]. Moreover, corn oil activates NFkB in Kupffer cells via oxidant-dependent mechanisms and in this way probably result early in subsequent increase in TNFa leading to increased cell proliferation in the liver [18].
Our study showed that desaturases, SCD, PCD and D6D have a key role in the lipid metabolism during liver regeneration. Desaturases activities were dependent on the used diet (n-9 or n-6) and in particular on SCD. During the early stages of liver regeneration (1st day), when the greatest amount of accumulated fats in the liver was found, the significant decrease of 18:1n-9 in the FOOgroup compared to the control of the same group was found. Diet enriched with olive oil significantly reduces activity SCD and thus lowers the triglyceride levels. Hepatocyte levels of 18:1n-9 is controlled by both the elongation and desaturation pathways, but the strain path is not a constitutive pathway and cellular levels 18:1n-9 is not determined solely by changes in the delta-9 desaturase expression, but also in the change involving D6D. Regulation of SCD is of physiological importance because the ratio of saturated fatty acids to unsaturated fatty acids is thought to modulate the membrane fluidity by changing the composition of the phospholipids of the cell membranes. Significant decrease of 18:1n-9 on the 1st day after PH in the group fed olive oil and an increase of 22:6n-3 (and n-3) leads to increased membrane fluidity. Similarly, in the later phase of PH, these fatty acids affect the reduction of membrane fluidity. These results connect the input of n-9 diet and the level of n-3 fatty acids in the membranes as results of intracellular metabolism of fatty acids and membrane fluidity in the liver during regeneration. Changes in membrane fluidity can lead to alteration in the cell differentiation.
We found a diversity of the SCD activity comparing the n-6 and n-9 diets during all stages of liver regeneration. The SCD activity was inhibited by the n-9 diet and activated by the n-6 diet during all examined stages of liver regeneration. The SCD activity was inhibited by the n-9 diet and activated by the n-6 diet during all examined stages of liver regeneration. The n-6 diet activated PCD more and D6D less in the early stage of liver regeneration while the n-9 diet contributed to the PCD and D6D inhibition in the later stage.
D6D catalyses the first and rate-limiting reaction of high unsaturated fatty acid (HUFA) synthesis and thus is subject to regulation by dietary fatty acids. Dietary PUFA suppress the induction of lipogenic genes in the liver, whereas essential fat-deficient diets such as fat-free diet and triolein diet (FOO diet) induce these genes [4,19]. When the diet is supplemented with polyenoic FA of the n-6, n-3 and n-9 families, enzymatic activities of hepatic D5D and D6D desaturase are reduced in the liver. Thus, increased degradation of HUFA and demand for membrane phospholipids may at least in part account for the marked induction of D6D by peroxisomal proliferators. Consistent with this hypothesis, the induction of desaturases by peroxisomal proliferators was slower than the induction of fatty acid oxidation enzymes [20,21]. Two transcription factors, sterol regulatory element-binding protein-1c (SREBP-1c) and PPARa are involved in the regulation of the D6D gene. SREBP-1c induces a set of genes for fatty acid and glycerolipid synthesis [22], including all three mammalian desaturases: SCD for MUFA synthesis [23,24] and D6D and D5D for HUFA synthesis [25,26].
Our initial hypothesis is partially proved to be true. Corn and olive oil supplemented diets affect the desaturases activities and can be lead to different course of liver regeneration. The key role in the liver regeneration and examined dietary fats was SCD activity.
5. Acknowledgements
This study was supported by the Croatian Ministry of Science, Projects No. 0062002, 335-0000000-0221 and 062-0621341-0061. None of the authors has conflicts of interest with respect to this work.
Contributions to this work were as follows: J.G. designed the study, conducted the experiment contributed to the chemical analysis of the FAMEs used in the study and wrote the manuscript. A.B.T. helped in sampling and discussion writing. C.M. and M.C. contributed to the successful execution of the experimental work. B.R.S. helped to design the experiment.
We thank N. Peraic, K. Georgiú, D. Kovacic, J. Eskinja, Lj. Crnac for their technical assistance and M. Mrkovic, D. Lisko, I. Dobrila, M. Sulic for their help to the successful execution of the experimental work.
REFERENCES
- S. M. Mahler, P. A. Wilce and B. C. Shanley, “Studies of Regenerating Liver and Hepatoma Plasma Membranes-I. Lipid and Protein Composition,” International Journal of Biochemistry, Vol. 20, No. 6, 1988, pp. 605-611. doi:10.1016/0020-711X(88)90100-0
- S. M. Mahler, P. A. Wilce and B. C. Shanley, “Studies of Regenerating Liver and Hepatoma Plasma Membranes-II. Membrane Fluidity and Enzyme Activity,” International Journal of Biochemistry, Vol. 20, No. 6, 1982, pp. 613- 619. doi:10.1016/0020-711X(88)90101-2
- S. D. Clarke, M. K. Armstrong and D. B. Jump, “Dietary Polyunsaturated Fats Uniquely Suppress Rat Liver Fatty Acid Synthase and S14 mRNA Content,” Journal of Nutrition, Vol. 120, No. 2, 1990, pp. 225-231.
- S. D. Clarke and D. B. Jump, “Dietary Polyunsaturated Fatty Acid Regulation of Gene Transcription,” The Annual Review of Nutrition, Vol. 14, No. 1, 1994, pp. 83-98. doi:10.1146/annurev.nu.14.070194.000503
- D. B. Jump, “Fatty Acid Regulation of Gene Transcription,” Critical Reviews in Clinical Laboratory Sciences, Vol. 41, No. 1, 2004, pp. 41-78. doi:10.1080/10408360490278341
- J. Giacometti, C. Milin, M. Tota, M. Cuk and B. Radosevic-Stasic, “Incorporation of Fatty Acids into Tissue Phospholipids in Mice Fed with Diets Rich in n-9 and n-6 Fatty Acids,” Croatica Chemica Acta, Vol. 78, No. 3, 2005, pp. 397-404.
- N. Fausto, “Liver Regeneration,” Journal of Hepatology, Vol. 32, Sup. 1, 2000, pp. 19-31. doi:10.1016/S0168-8278(00)80412-2
- P. S. Satdarshan Monga, P. Pediaditakis, K. Mule, D. Beer Stolz and G. K. Michalopoulos, “Changes in WNT/- Catenin Pathway during Regulated Growth in Rat Liver Regeneration,” Hepatology, Vol. 33, No. 5, 2001, pp. 1098-1109. doi:10.1053/jhep.2001.23786
- J. Kountouras, P. Boura and N. Lygidakis, “Liver Regeneration after Partial Hepatectomy,” Hepatogastroenterology, Vol. 48, 2001, pp. 556-562.
- EEC Directive 2568, No. L248, 1991. http://eur-lex.europa.eu/LexUriServ/site/en/consleg/1991/R/01991R2568-20031101-en.pdf
- J. Folch, M. Lees and G. H. S. Stanley, “A Simple Method for the Isolation and Purification of Total Lipids from Animal Tissues,” Journal of Biological Chemistry, Vol. 226, 1957, pp. 497-509.
- J. Giacometti, A. Milosevic and C. Milin, “Gas Chromatographic Determination of Fatty Acids Contained in Different Lipid Classes after Their Separation by SolidPhase Extraction,” Journal of Chromatography A, Vol. 976, No. 1-2, 2002, pp. 47-54. doi:10.1016/S0021-9673(02)01159-7
- M. M. Markiewski, R. A. DeAngelis and J. D. Lambris, “Liver Inflammation and Regeneration: Two Distinct Biological Phenomena or Parallel Pathophysiologic Processes?” Molecular Immunology, Vol. 43, No. 1-2, 2006, pp. 45-56. doi:10.1016/j.molimm.2005.06.019
- R. Taub, “Liver Regeneration: From Myth to Mechanism,” Nature Reviews Molecular Cell Biology, Vol. 5, No. 10, 2004, pp. 836-847. doi:10.1038/nrm1489
- A. Yusuf, E. Laconi, P. M. Rao, S. Rajalakshmi and D. S. R. Sarma, “The Effect of 1/3 Partial Hepatectomy on the Growth of Glutathione S-Transferase Positive Foci,” Carcinogenesis, Vol. 20, No. 6, 1999, pp. 1143-1145. doi:10.1093/carcin/20.6.1143
- J. P. Carreau, P. Mazliak and D. Frommel, “Stearoyl-CoA Desaturation Capacity of the Rat Liver during Regenaration after Partial Hepatectomy,” International Journal of Biochemistry, Vol. 13, No. 6, 1981, pp. 765-767. doi:10.1016/0020-711X(81)90050-1
- I. M. Cristea and M. D. Esposti, “Membrane Lipids and Cell Death: On Overview,” Chemistry and Physics of Lipids, Vol. 129, No. 2, 2004, pp. 133-160. doi:10.1016/j.chemphyslip.2004.02.002
- I. Rusyn, C. A. Bradham, L. Cohn, R. Schoonhoven, J. A. Swenberg, D. A. Brenner, et al., “Corn Oil Rapidly Activates Nuclear Factor-kB in Hepatic Kupffer Cells by Oxidant-Dependent Mechanisms,” Carcinogenesis, Vol. 20, No. 11, 1999, pp. 2095-2100. doi:10.1093/carcin/20.11.2095
- D. B. Jump and S. D. Clarke, “Regulation of Gene Expression by Dietary Fat,” The Annual Review of Nutrition, Vol. 19, 1999, pp. 63-90. doi:10.1146/annurev.nutr.19.1.63
- C. W. Miller and J. M. Ntambi, “Peroxisome Proliferators Induce Mouse Liver Stearoyl-CoA Desaturase 1 Gene Expression,” Proceedings of the National Academy of Sciences of the USA, Vol. 93, No. 18, 1996, pp. 9443- 9448. doi:10.1073/pnas.93.18.9443
- W. S. He, T. Y. Nara and M. T. Nakamura, “Delayed Induction of Delta-6 and Delta-5 Desaturases by a Peroxisome Proliferator,” Biochemical and Biophysical Research Communications, Vol. 299, No. 5, 2002, pp. 832- 838. doi:10.1016/S0006-291X(02)02743-2
- J. D. Horton, J. L. Goldstein and M. S. Brown, “SREBPs: Activators of the Complete Program of Cholesterol and Fatty Acid Synthesis in the Liver,” The Journal of Clinical Investigation, Vol. 109, No. 9, 2002, pp. 1125-1131. doi:10.1172/JCI200215593
- D. E. Tabor, J. B. Kim, B. M. Spiegelman and P. A. Edwards, “Identification of Conserved Cis-Elements and Transcription Factors Required for Sterol-Regulated Transcription of Stearoyl-CoA Desaturase 1 and 2,” Journal of Biological Chemistry, Vol. 274, 1999, pp. 20603-20610. doi:10.1074/jbc.274.29.20603
- G. Liang, J. Yang, J. D. Horton, R. E. Hammer, J. L. Goldstein and M. S. Brown, “Diminished Hepatic Response to Fasting/Refeeding and Liver X Receptor Agonists in Mice with Selective Deficiency of Sterol Regulatory Element-Binding Protein-1c,” Journal of Biological Chemistry, Vol. 277, 2002, pp. 9520-9528. doi:10.1074/jbc.M111421200
- T. Matsuzaka, H. Shimano, N. Yahagi and M. AmemiyaKudo, T. Yoshikawa, A. H. Hasty, et al., “Dual Regulation of Mouse D5- and D6-Desaturase Gene Expression by SREBP-1 and PPARa,” Journal of Lipid Research, Vol. 43, No. 1, 2002, pp. 107-114.
- T. Y. Nara, W. S. He, C. Tang, S. D. Clarke and M. T. Nakamura, “The E-Box Like Sterol Regulatory Element Mediates the Suppression of Human Delta-6 Desaturase Gene by Highly Unsaturated Fatty Acids,” Biochemical and Biophysical Research Communications, Vol. 296, No. 1, 2002, pp. 111-117. doi:10.1016/S0006-291X(02)00851-3

