Food and Nutrition Sciences
Vol. 2 No. 2 (2011) , Article ID: 4538 , 4 pages DOI:10.4236/fns.2011.22021
Probiotics Bacteria from Egyptian Infants Cause Cholesterol Removal in Media and Survive in Yoghurt
![]()
Genetic Engineering and Biotechnology Research Institute, Menofiya University, Egypt.
E-mail: hmahrous7@yahoo.com
Received January 1st, 2011, revised February 16th, 2011, accepted March 7th, 2011.
Keywords: Probiotics, Lactobacillus, Cholesterol Removal, Yoghurt, Fermentation
ABSTRACT
One of the most significant groups of probiotic organisms are the lactic acid bacteria, commonly used in fermented dairy products. In this study, cultures were isolated from two infants. After screening for the classic properties of probiotic organisms, four promising isolates were identified as two strains of Lactobacillus acidophilus (P106, P110), strain of Lactobacillus plantarum (P164) and Lactobacillus pentosus (P191) which were tested for capability to remove cholesterol and to deconjugate sodium taurocholate from the culture medium. Results showed that a considerable variation existed among cultures in their growth viability in the presence of bile salt, deconjugation of sodium taurocholate and assimilation of cholesterol from the medium. All tested strains removed less cholesterol from the broth (ranged from 4.02% - 24.32%) compared to those grown in broth supplemented with 0.2% bile salts (from 29.02 to 45.3). Lactobacillus acidophilus P106 appeared to be more active in bile salt hydrolase compared to the other strains, and therefore, is regarded as a suitable candidate probiotic and adjunct culture. These strains were employed to make yoghurt and, in order to achieve a short production time; a two-stage fermentation procedure was used with Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus providing the rapid acidification. Storage trials at 4˚C showed that the viability of the probiotic cultures was retained over 15 days.
1. Introduction
Probiotics have a long history of human use, and cultured dairy products, for example, are traditionally consumed in several parts of the world. The FAO/WHO [1] defines probiotics as 'Live microorganisms which when administered in adequate amounts confer a health benefit on the host'. The first recorded probiotic was fermented milk for human consumption. After that, probiotics became popular with animal nutrition. The role of fermented milk in human diet was known even in Vedic times. But, the scientific interest in this area boosted after the publication of the book entitled The Prolongation of Life [2]. After that, probiotics became popular with animal nutrition. These bacteria have been proven to reduce the effects of some gastrointestinal problems including Crohn’s Disease and Irritable Bowel Syndrome by the reduction of the inflammation that is associating with these disorders. Also, through the production of lactose, probiotics can greatly reduce lactose intolerance. Probiotics have also been proven to prevent colon cancers when consumed on a regular basis by decreasing the amount of carcinogens in the intestine.
Probiotic strains, especially lactic acid bacteria have a major role to play in the cholesterol lowering mechanism. As the cholesterol level keeps increasing in the serum, it leads to cardiac diseases. These cholesterol levels could be brought down using probiotics [3]. The mechanisms can be direct or indirect. Direct mechanism is either inhibiting the de novo synthesis or by decreasing the intestinal absorption of dietary cholesterol. Inhibition of de novo synthesis can be attained by hypocholesterolemic factors like lactose, calcium hydroxyl methyl glutarate, uric acid, orotic acid, whey proteins, etc. The dietary cholesterol absorption is reduced by three ways (assimilating, binding or by degradation). Probiotic strains assimilate the cholesterol for their own metabolism. Probiotic strains could be bound to the cholesterol molecule, and they are capable of degrading cholesterol to its catabolic products. The cholesterol level could be reduced indirectly by deconjugating the cholesterol to bile acids, thereby reducing the total body pool. The bile acids commonly occur in the form of bile salts with glycine and taurine. Deconjugation by different lactic acid bacterial cultures was also tried using two forms of bile salts, viz. taurocholates and glycocholates. Most of the strains could deconjugate glycocholates. The plasma cholesterol concentration can be regulated by the biosynthesis of cholesterol from saturated fat, removal of cholesterol from the circulation, absorption of dietary cholesterol, and excretion of cholesterol via bile and feces.Cellular cholesterol homeostasis is very important for the prevention of cardiovascular disease, and numerous studies have been already reported that enzyme inhibitors for 3- hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase and acyl CoA: cholesterol acyltransferase (ACAT) have beneficial effects on hypercholesterolemia and arteriosclerosis [4]. Some natural microor-ganisms in human intestine are beneficial in terms of lowering serum cholesterol [5-7].
Probiotic yogurt is most commonly marketed and consumed as a digestive aid and is perfectly safe for most people to consume as they would regular yogurt and also a natural immune system booster can also be consumed to improve overall health and to prevent illness. This study was conducted to: 1- Study the capability of some probiotic lactic acid bacteria strains to remove cholesterol and to deconjugate sodium taurocholate from the culture medium. 2- Choose the most beneficial strain as adjunct culture in the production of yoghurt that patient with a high cholesterol problem could be fed.
2. Materials and Methods
2.1. Tested Microbial Strains
Probiotic strains candidate: two strains of Lactobacillus acidophilus (P106, P110); strain of Lactobacillus plantarum (P164) and Lactobacillus pentosus (P191) were identify by Mahrous [8], which were isolated from healthy, breastfeeding infants (15-90 days old) and used after the selection had been done according to Bergey’s Manual of Determinative Bacteriology, 9th edition [9] with confirm the identification by SDS-PAGE technique and API System. The strains were tested for their probiotic characteristic i.e. gastric acid resistance, bile salt tolerance, antibacterial activity, adhesion to human mucus. Lactobacillus strains were cultivated in MRS (de Man Rogosa Sharpe) broth (Lab M, IDG, UK) and incubated at 37˚C in BBL anaerobic jar (Becton Dickinson Microbiology Systems, Sparks, MD) provided with disposable BBL gas generating pack (CO2 system envelopes, Oxoid, Ltd., West Heidelberg, Victoria, Canada).
2.2. Cholesterol Removal
Cholesterol solution (10 mg/mL in 96% ethyl alcohol) was prepared and filter sterilized. For each culture to be tested, 70 μL of cholesterol solution was added to 10 mL of MRS broth (final cholesterol concentration 70 μg/mL) with 0.2% (w/v) bile salts (oxgall) or without. To the MRS broth, 1% (v/v) of freshly grown culture was added and incubated anaerobically at 37˚C for 20 h. An uninoculated sample was used as control. After incubation, the cells were removed by centrifugation at 10,000 g for 10 mins at 4˚C and cholesterol was determined in the supernatant using modified Rudel and Morris, 1973 [10] method in which 3 mL of supernatant, 2 mL of 33% (w/v) KOH and 3 ml 96% ethanol were placed in a capped test tube, vortexed for 20 sec and incubated for 15 mins at 60˚C in a water bath. After incubation, the mixture was removed and cooled under tap water, then 5 mL of hexane and 3 mL of water were added and vortexed for one min. One milliliter of the hexane layer was transferred into a dry clean test tube and evaporated under nitrogen gas. One milliliters of cholesterol liquicolor enzymatic kit (Human-Gesellschaft fur Biochemica und Diagnostica mbh-Wiesbaden-Germany) was added. The solution was mixed and left for 5-10 mins at 37˚C and absorbance was measured at 500 nm with a spectrophotometer (LKB Biochrome ultrospec 11, Campridge, England). The ability of bacterial strain to remove cholesterol from media was calculated as percentage from the following equation: A = 100 – (B/C)*100, where A = % of cholesterol removed, B = absorbance of the sample containing the cells and C = absorbance of the sample without cells. It was observed that, the sample without cells has no pellet following centrifugation and cholesterol was therefore determined in the whole sample.
2.3. Bile Salt Hydrolase
Agar plates assay was developed to detect bile salt hydrolase activity in lactobacilli (Bryant and Burkey). Agar plates MRS medium were prepared with 0.5% (w/v) of the taurodeoxycholic acid (TDCA). After boiling and steam sterilization, the plates were poured into sterile plastic Petri dishes (60 by 15 mm). Once solidified, the plates were inverted and placed in the anaerobic condition for at least 72 h before use. All plates were inoculated from an overnight culture by using a 10-µL loop. Plates were incubated in glass screw-cap jars at 37˚C for 72 h. Precipitated bile acid could be seen around colonies representing highly active strains within 48 h., bile salt hydrolysis was manifested at two intensities: (i) the formation of precipitate halos around colonies or (ii) the formation of opaque granular white colonies.
2.4. Acid Producing Ability
The ability of each strain to produce acid when grown in milk was determined. Overnight cultures were previously prepared by inoculation (0.1%, v/v) into MRS broth at 37˚C for 16 h to insure that, the cultures will be in the log phase. The strains were inoculated in reconstituted nonfat dry milk by inoculating each of them separately or in combination with starter yoghurt and the decline in pH were measured after 0, 2, 4, 6 and 24 h.
2.5. Viability of the Probiotic Isolates in Yoghurt
The yoghurt was prepared according to the method of Shah, 2000 [11] in order to avoid the poor texture and acid development sometimes associated with the use of probiotic cultures. Full-cream bovine milk, fortified with skim-milk powder to give a level of 14% solids-non-fat, was pasteurized at 85˚C for 15 mins, cooled to 40˚C - 42˚C and inoculated at a rate of 20 mL/L with a probiotic isolates previously sub-cultured in skim-milk. The milk was then incubated at 37˚C for 2 h and, at this point, a traditional yoghurt starter culture consisting of Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus was added (20 mL/L). The product was then incubated at 42˚C until the desired pH of 4.4 - 4.5 had developed [12]. Samples of yoghurt containing one of the selected isolates and Str. thermophilus and Lb. delbrueckii subsp. bulgaricus were then stored at 4˚C to monitor the effect of storage on the viability of the probiotic species. The counts of the probiotic starter cultures were determined after 0, 1, 2, 4, 6, 8, 10 and 15 days of storage (BA-sorbitol agar for Lactobacillus acidophilus (P106, P110); LPSM for Lactobacillus plantarum selective medium and MRS agar with maltose for Lactobacillus pentosus). The entire procedure was performed twice.
2.6. Statistical Analysis
Data are presented as the mean ± standard deviation, and n represents the number of the probiotics and the control.
3. Results and Discussion
The effects of ingestion of probiotic bacteria such as Lb. acidophilus on serum cholesterol levels have attracted much interest. It has been reported that a culture of Lb. acidophilus actively taking up cholesterol from laboratory media would function in vivo to exert a hypocholesterolemic effect [13]. Our results as well as other published studies reported the beneficial properties of lactobacilli for health. Also through our results the activity of probiotic bacteria did not depend on genera but on strain. The reason for this previous suggestion is that Lb. acidophilus P106 can remove higher level of cholesterol than other tested lactobacilli.
3.1. Cholesterol-Removing Ability
A high level of cholesterol in blood is generally considered to be a risk factor for cardiovascular disease. Therefore, decreasing in serum cholesterol levels is important to prevent the disease. The cholesterol-removing ability of probiotic lactobacilli was assessed (Table 1); all tested strains had the ability to remove cholesterol from laboratory media during growth. Among the Lb. acidophilus P106 removed more cholesterol than other tested strains. All tested strains grown in broth free of bile salts removed less cholesterol (range 4 -24%) than those grown in broth supplemented with 0.2% bile salts (range 29 to 45%). Results revealed that addition of Bile Salts greatly improved the uptake and assimilation of cholesterol from the media. These results agreed with the observations of Pereria and Gibson, 2002 [14] that the uptake of cholesterol by lactic acid bacteria was higher in the medium containing 0.4% oxgall.
The mechanism by which lactic acid bacteria remove cholesterol from laboratory media has been studied. It has been reported that cholesterol removed by some lactobacilli was due to a disruption of the cholesterol micelles caused by the deconjugation and precipitation of cholesterol with the free bile salts as the pH of the media dropped by acid production during growth [15,16]. However, it was also reported that some strains of Lb. acidophilus incorporated some of the cholesterol into the cellular membrane [17].
3.2 Bile Salt Hydrolase
Bile acids are synthesized from cholesterol and conjugated to either glycine or taurine in the liver [18]. They then pass into the intestine, where the amino acid may be hydrolyzed from the conjugated bile acid by bacterial enzymes. These enzymes constitute a class collectively known as conjugated bile salt hydrolases (BSHs). They are expressed by gastrointestinal bacteria of several genera.
When bile salt hydrolase-producing lactobacilli were streaked out on MRS plates containing 0.5% TDCA, the taurine-conjugated bile acid was deconjugated, producing deoxycholic acid. The deconjugation activity of Lactobacilluis colonies was manifested in Figure 1, copious amounts of deoxycholic acid precipitated around active colonies and diffused into the surrounding medium. The greatest precipition showed in the case of Lb. acidophilus P106 caused the strongest precipitation followed by Lb. acidophilus P110; Lb. plantarum P164 and Lb. pentosus P191, respectively. These results are in agreement with other published work that tested the activity of BSH for some probiotic lactobacilli and all tested strains gave BSH-positive [19,20].
3.3. Acid Producing Ability
Acid production by lactobacilli strains was illustrated in Table 2. Generally, the four tested strains were weaker acid producers when inoculated separately than when
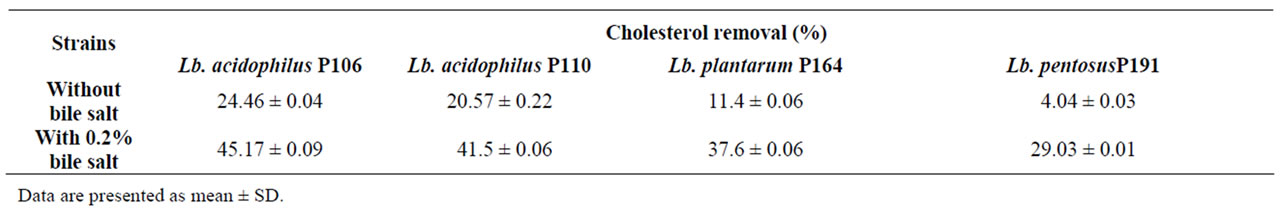
Table 1. (Mean ± SE) of cholesterol removal (%) from media by some probiotic lactic acid bacteria.
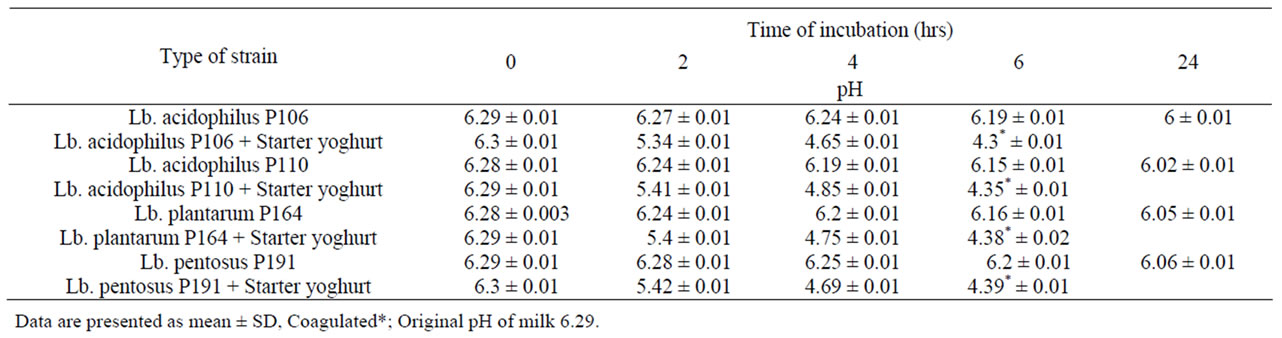
Table 2. (Mean ± SE) of Acid production by Lb. acidophilus P106, Lb. acidophilus P110 Lb. plantarum P164 and Lb. pentosus P191 strains in sterilized skim milk for 6 hrs at 37˚C.
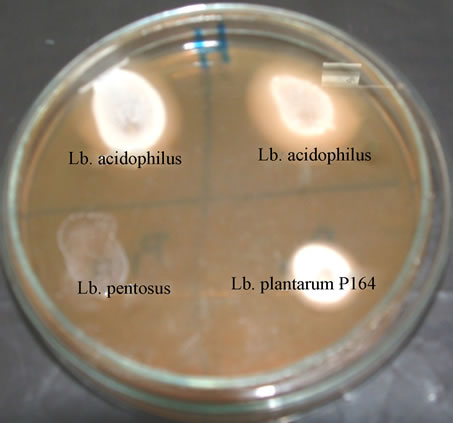
Figure 1. Manifestation of bile salt hydrolase activity by lactobacilli on solid MRS.
inoculated together with starter yoghurt. This is mainly due to the nature ofprobiotic strains which grow in milk slowly. These results are in agreement with Ref. 1 and 21. Which showed that probiotic bacteria grow weakly in milk and the common practice is to add yoghurt bacteria to enhance the fermentation process for making probiotic yoghurt?
3.4. Culture Viability in Yoghurt during Storage
The results of the current trial are shown in Figure 2, and it is clear that the initial counts of all four strains were well above the count of 1.0 × 106 cfu·g–1 which is often quoted as the ‘therapeutic minimum’ [22,23]. Duringstorage, the counts of Lb. acidophilus P110; Lb. plantarum P164 and Lb. pentosus P191 remained broadly stable
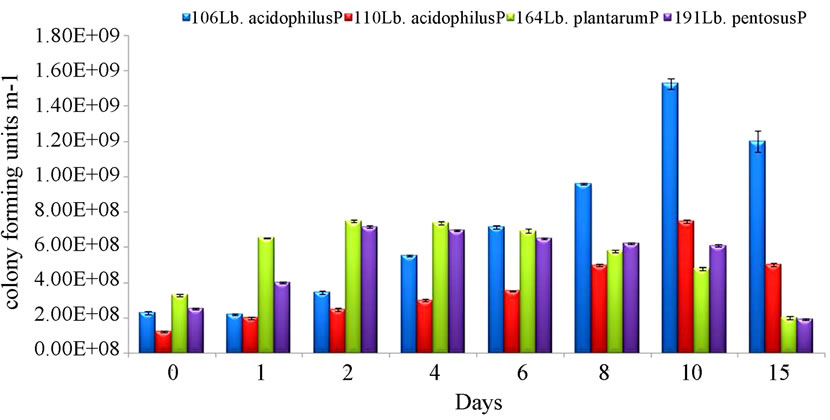
Figure 2. (Mean ± SE) of the viability of Lb. acidophilus P106; Lb. acidophilus P110; Lb. plantarum P164 and Lb. pentosus P191 in the presence of a traditional yoghurt starter culture during the storage of a fermented milk at 4˚C.
over the 15 days but, the count of Lb. acidophilus P106 was increased and reached 1.5 × 109 cfu·g–1 by the tenth day; the highest count achieved by any of the probiotic isolates throughout the whole period of storage. Even at 15 days, the counts for Lb. acidophilus P106 were higher than the initial count, but the reason for this unexpectedly high level of growth and survival needs to be established.
4. Conclusion
Lactobacillus isolates shows ability to uptake cholesterol from media. The degree of cholesterol uptake depends on isolate. Lb. acidophilus P106 could be selected for specific purposes. Indeed the most important factor for removing the cholesterol level is the strain of bacteria. Thus, we postulate that this strain could be used for reducing high cholesterol levels in patient. However, further studies are required to determine the mechanism(s) involved in the removal of cholesterol by these Lactobacillus strains in vitro.
5. Acknowledgments
The author would like to extend sincerest appreciation to Prof. Dr. Morsi Abu El-Seoud El-Soda (Professor of Dairy Microbiology, Faculty of Agriculture, Alexandria University) for his unlimited encouragement.
REFERENCES
- “Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria,” Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria, FAO/WHO, 2001.
- http://www.who.int/foodsafety/publications/fs_management/ probiotics/en/index.html
- E. Metchnikoff, “The Prolongation of Life,” Putmans Sons, New York, 1908, pp. 151-183.
- R. Fuller, “Probiotics in Man and Animals,” Journal of Applied Bacteriolology, Vol. 66, 1989, pp. 365-378.
- J. M. Hay, W. M. Yu and T. Ashraf, “Pharmacoeconomics of Lipidslowering Agents for Primary and Secondary Prevention of Coronary Artery Disease,” Pharmacoeconomics, Vol. 15, No. 1, 1999, pp. 47-74. doi:10.2165/00019053-199915010-00004
- C. F. Fernandes, K. M. Shahani and M. A. Amer, “Therapeutic Role of Dietary Lactobacilli and Lactobacillus Fermented Dairy Products,” FEMS Microbiology Review, Vol. 46, 1987, pp. 343-356.
- M. Fukushima, A. Yamada, T. Endo and M. Nakano, “Effects of a Mixture of Organisms, Lactobacillus Acidophilus or Streptococcus Faecalis on Delta6-desaturase Activity in the Livers of Rats Fed a Fatand Cholesterol-enriched Diet,” Nutrition, Vol. 15, 1999, pp. 373-378. doi:10.1016/S0899-9007(99)00030-1
- A. A. Al-Saleh, A. A. M. Metwalli and H. M. Abu-Tarboush, “Bile Salts and Acid Tolerance and Cholesterol Removal from Media by Some Lactic Acid Bacteria and Bifidobacteria,” Journal of Saudi Society for Food and Nutrition, Vol. 1, No. 1, 2006, pp. 1-17.
- H. Mahrous, “Functionalities of Lactic Acid Bacteria Isolated from Egyptian Environment,” Ph.D. Thesis, Genetic Engineering & Biotechnology Research Institute (GEBRI), Minufiya University, Shibin El Kom, 2006.
- J. G. Holt, N. R. Krieg, P. H. A. Sneath, J. T. Staley and S. T. Williams, “Bergey’s Manual of Determinative Bacteriology,” 9th Edition, Baltimore: Williams and Wilkins, 1994.
- L. L. Rudel and M. D. Morris, “Determination of Cholesterol Using O-phthalaldehyde,” Journal of Lipid Research, Vol. 14, No. 3, 1973, pp. 364-366.
- N. P. Shah, “Probiotic Bacteria: Selective Enumeration and Survival in Dairy Foods,” Journal of Dairy Science, Vol. 83, No. 4, 2000, pp. 894-907. doi:10.3168/jds.S0022-0302(00)74953-8
- R. I. Dave and N. P. Shah, “Viability of Yogurt and Probiotic Bacteria in Yogurts Made from Commercial Starter Cultures,” International Dairy Journal, Vol. 7, No. 1, 1997, pp. 31-41. doi:10.1016/S0958-6946(96)00046-5
- S. E. Gilliland, C. R. Nelson and C. Maxwell, “Assimilation of Cholesterol by Lactobacillus Acidophilus,” Applied of Environment Microbiology, Vol. 49, No. 2, 1985, pp. 377-381.
- D. I. A. Pereira and G. R. Gibson, “Cholesterol Assimilation by Lactic Acid Bacteria and Bifidobacteria Isolated from the Human Gut,” Applied and Environmental Microbiology, Vol. 68, 2002, pp. 46-89.
- F. A. M. Klaver and R. van der Meer, “The Assumed Assimilation of Cholesterol by Lactobacilli a Bifidobacterium Bifidum is Due to Their Bile Salt-deconjugating Activity,” Applied of Environment Microbiology, Vol. 59, No. 4, 1993, pp. 1120-1124.
- D. K. Walker and S. E. Gilliland, “Relationships among Bile Tolerance, Bile Salt Deconjugation and Assimilation of Cholesterol by Lactobacillus Acidophilus,” Journal of Dairy Science, Vol. 76, No. 4, 1993, pp. 956-961. doi:10.3168/jds.S0022-0302(93)77422-6
- D. O. Noh, S. H. Kim and S. E. Gilliland, “Incorporation of Cholesterol into the Cellular Membrane of Lactobacillus Acidophilus ATCC 43121,” Journal of Dairy Science, Vol. 80, No. 12, 1997, pp. 3107-3113. doi:10.3168/jds.S0022-0302(97)76281-7
- D. C. Savage, S. G. Lundeen and L. T. O’Connor, “Mechanisms by which Indigenous Microorganisms Colonise Epithelial Surfaces as a Reservoir of the Lumenal Microflora in the Gastrointestinal Tract,” Microecology Terminology, Vol. 21, 1995, pp. 27-36.
- M. Begley, C. Hill and C. G. M. Gahan, “Bile Salt Hydrolase Activity in Probiotics,” Applied and Environmental Microbiology, Vol. 72, No. 3, 2006, pp. 1729-1738. doi:10.1128/AEM.72.3.1729-1738.2006
- Y. T. Ahn, G. B. Kim, K. S. Lim, Y. J. Baek and H. U. Kim, “Deconjugation of Bile Salts by Lactobacillus Acidophilus Isolates,” International Dairy Journal, Vol. 13, No. 4, 2003, pp. 303-311. doi:10.1016/S0958-6946(02)00174-7
- A. Samona and R. K. Robinson, “Effect of Yogurt Cultures the Survival of Bifidobacteria in Fermented Milks,” Journal of the Society of Dairy Technology, Vol. 47, No. 2, 1994, pp. 58-60.
- “Special Yoghurts - Potential Health Benefits,” In: R. K. Robinson, Ed., Dairy Indian International, John Wiley and Sons, New York, 1989, pp. 23-25.
- G. Gardiner, R. P. Ross, P. M. Kelly, C. Stanton, K. Collins and G. Fitzgerald, “Microbiology of Therapeutic Milks,” In: R. K. Robinson, Ed., Handbook of Dairy Microbiology, John Wiley & Son, 2002. doi:10.1002/0471723959.ch9

