American Journal of Plant Sciences
Vol.5 No.13(2014), Article
ID:46995,11
pages
DOI:10.4236/ajps.2014.513208
Identification of Potential F1 Hybrids in Maize Responsive to Water Deficient Condition
Fahad Masoud Wattoo1, Muhammad Saleem1, Muhammad Sajjad2*
1Department of Plant Breeding and Genetics, University of Agriculture Faisalabad, Faisalabad, Pakistan
2Department of Plant Breeding and Genetics, University of Arid Agriculture, Rawalpindi, Pakistan
Email: *msajjadpbg@gmail.com, msajjad@uaar.edu.pk
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 21 April 2014; revised 20 May 2014; accepted 13 June 2014
ABSTRACT
The assessment of heterotic F1 combinations is a basic requisite for hybrid seed development. A set of 30 F1 hybrids along with their parental inbred lines were evaluated under both normal and water deficit conditions for various physiological and agronomic traits. Highly significant mean squares due to general combining ability, specific combining ability and reciprocal effect were observed for all traits under both water regimes. Components of variation exhibited greater estimates for GCA variance (б2g) than SCA variance (б2s) for majority of the traits under both normal and stress conditions depicting the predominant role of additive genetic component. Inbred lines NCIL-20-20, D-157 and OH-8 were found to be the best general combiner on the basis of performance regarding grain yield per plant under water deficit condition. The F1 combinations namely, NCIL-20-20 × D-109, NCIL-20-20 × OH-8 and D-157 × NCIL-20-20 were out-performers based on yield and yield attributes under water deficit conditions. On the basis of our results, we recommend these hybrids for further exploitation to assess their potential for commercial cultivation under water deficit condition.
Keywords:Maize, General Combining Ability (GCA), Specific Combining Ability (SCA), Water Stress, Inbred Lines

1. Introduction
Maize (Zea mays L.), is a versatile plant amongst cereal crops with a wide range of agro-climatic adaptability. It is a multipurpose crop consumed as food for human, feed for poultry, fodder for animals and fuel for industry. In Pakistan, it is mostly cultivated in Punjab and KPK province in spring and autumn seasons [1] . A number of biotic and abiotic stresses affect standing crop in the field. Overall one major factor that constrains crop growth in the world is water availability [2] and it is anticipated that by 2025 about one third of human population will be affected by water deficit [3] . Due to water deficit conditions in maize about 24 million tons is being lost annually and high yield potential genotypes under potential regions cannot compensate the projected increase in demand for the next decade [4] . Breeding cultivars tolerant to water deficit condition appear to be the only option for stress prone areas. Hybrids were found to be more tolerant to water deficit condition as compared to inbred lines [5] . Interpretation of various agro-physiological that provide tolerance against water deficit is essential for fruitful selection of genotypes under stress environments [6] . Existence of variability for drought tolerance in crop plant has already been reported [7] . Combining ability analysis based on progeny test is useful and reliable approach for screening inbred lines and F1 hybrids [8] . Among the available conventional techniques, diallel cross analysis as developed by Hayman [9] [10] and Jinks [11] is an efficient tool furnishing information on genetic mechanism conditioning various plant traits in one generation. Therefore, combining ability estimates provide information on mechanism controlling quantitative characters and further help in selecting suitable parents for developing superior hybrids or varieties [12] . General and specific combining ability provide estimates for additive and non-additive components, respectively [13] [14] . In literature, additive [15] -[18] and non-additive [12] [19] genetic effects have been reported for grain yield and yield related traits under various environmental conditions. Therefore, information regarding genetic mechanism for water deficit tolerance is pre-requisite for development of maize hybrids and synthetics for sustainable agriculture. The present research 6 × 6 diallel analysis was pursued to evaluate maize inbred lines and F1 hybrid for water deficit regimes using some agro-physiological traits.
2. Materials and Methods@NolistTemp# The research work was conducted in the Department of Plant Breeding and Genetics, University of Agriculture Faisalabad during the years 2010-2011. Six inbred lines including M-14, OH-8, D-157, D-114, D-109 and NCIL-20-20 were selected out of fifty collected inbred lines at seedling stage using physio-agronomic parameters under water deficit conditions. Selected inbred lines were crossed in all possible combinations in the field during spring 2011 in a complete diallel mating design. Both male and female inflorescences of maize plants were covered with kraft paper bag and butter paper bags at the time of inflorescence, respectively, to make controlled crosses. Pollens collected in petri dish were applied on silks with the help of camel hair brush. Silks were pollinated twice on consecutive days to ensure required seed setting. After pollination, the inflorescences were again covered with their respective bags. The instruments were sterilized after each pollination. The F1 and their reciprocal crosses along with the parents were planted in the research field in autumn 2011 under normal and water deficit stress conditions using Randomized Complete Block Design (RCBD) with three replications. Each experimental unit comprised of two rows of 5.3 m each keeping row to row distances of 75 cm while plant to plant spacing was 23 cm. Two seeds were dibbled per hill to ensure good plant population. Thinning was done to keep one healthy plant per hill after 15 days of sowing. Six rows of non-experimental lines were planted on each side of experimental area to minimize border effect. Recommended insecticide was applied to counter for shoot fly and stem borer. Except for irrigation schedule, all recommended agronomic, cultural practices and plant protection measures were kept uniform. Normal experimental set received standard irrigation whereas 50% of normal irrigation was supplied to the water deficit set [20] . Ten equally competitive plants were ear-marked from each entry from both sets and data for pertaining to various physio-agronomic traits like cell membrane thermostability [21] , Stomatal conductance (Steady state porometer, Model L-1 1600 SSP1674 Li cor. Ink, USA), plant height, number of days to 50% tasseling, number of days to 50% silking, anthesis-silking interval and grain yield per plant. Data relating to various agro-physiological traits were enumerated and compared using statistical analysis according to Steel et al. [22] . Combining ability studies were performed by using Method I Model I following Griffing’s approach [23] . Genetic variability in the material was divided into components of general combining ability (GCA), specific combining ability (SCA), reciprocal effects and error mean squares for the traits. Sum of squares for these components were calculated as under:SS due to GCA 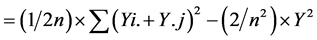
SS due to SCA = 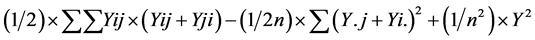
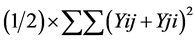 where, Y i. and Y. j = total of the ith and jth arrays in the mean table Y. = Grand total of the mean table Yij = mean value of the cross of ith parent with jth parent Yji = mean value of the cross of jth parent with ith parent (reciprocal cross) n = number of parents Sum of Square due to error Mean sum of squares due to error obtained in the Analysis of variance (ANOVA) were used after dividing by number of replications because mean values are used here. Thus, SS due to error = SS (error) in ANOVA/r where, r = number of replications. Keeping in view the probability of the mean squares for fixed model I, estimates of genetic components due to SCA, GCA and reciprocals are obtained as under:
where, Y i. and Y. j = total of the ith and jth arrays in the mean table Y. = Grand total of the mean table Yij = mean value of the cross of ith parent with jth parent Yji = mean value of the cross of jth parent with ith parent (reciprocal cross) n = number of parents Sum of Square due to error Mean sum of squares due to error obtained in the Analysis of variance (ANOVA) were used after dividing by number of replications because mean values are used here. Thus, SS due to error = SS (error) in ANOVA/r where, r = number of replications. Keeping in view the probability of the mean squares for fixed model I, estimates of genetic components due to SCA, GCA and reciprocals are obtained as under: ANOVA for combining ability (Griffing method I model I)
| SOV | df | SS | MS | F-value | Exp (MS) |
| GCA SCA Reciprocal Error | (p-1) p(p-1)/2 p(p-1)/2 (r-1)(p2-1) | Sg Ss Sr Se | Mg Ms Mr Me′ | Mg/Ms Ms-M′e (Mr-M′e)/2 | 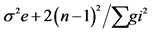 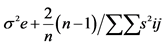 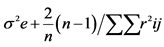 |
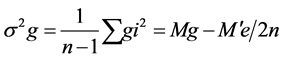
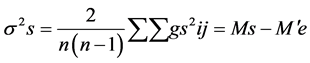
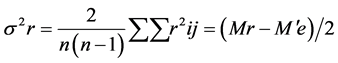
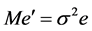 where,
where, 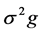 ,
, 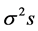 ,
, 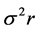 and
and 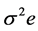 are the estimates of variance due to general combining ability (GCA), specific combining ability (SCA), reciprocal effects and environment, respectively. General combining ability (GCA) effects were calculated using the expression:
are the estimates of variance due to general combining ability (GCA), specific combining ability (SCA), reciprocal effects and environment, respectively. General combining ability (GCA) effects were calculated using the expression: 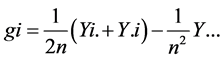 Specific combining ability effects (SCA) were calculated using the expression:
Specific combining ability effects (SCA) were calculated using the expression: 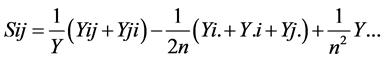 Reciprocal effects were calculated following:
Reciprocal effects were calculated following: 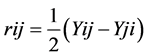 Variances were calculated as under:
Variances were calculated as under: 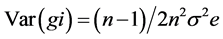
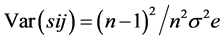
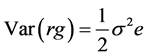 Standard errors were calculated by taking the square root of the respective variance as under:
Standard errors were calculated by taking the square root of the respective variance as under: 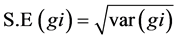
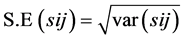
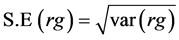 Critical difference between two parents = S.E × T tab at 0.05 probability. OR = (σ2) 0.5 × T tab at 0.05 probability
Critical difference between two parents = S.E × T tab at 0.05 probability. OR = (σ2) 0.5 × T tab at 0.05 probability Morpho-physiological characteristics of selected maize inbred lines
| SR. NO. | Inbred lines | Plant height | Maturity | Leaf | Pith | Tasselling | CL | CMT | RLWC | Grain yield |
| 1 | NCIL-20-20 | Tall | Early | Erect broad and large size leaves | White | Scattered tassel | 6.20 | % cell injury (68.5) | RLWC (0.82) | High yielder |
| 2 | D-157 | Tall | Early | Semi droopy medium size leaves | White | Tassel long and compact | 9.20 | % cell injury (70.0) | RLWC (0.83) | High yielder |
| 3 | OH-8 | Tall | Early | Erect, broad and vigorous leaves | White | Tassel compact | 7.70 | % cell injury (71.5), | RLWC (0.79) | High yielder |
| 4 | D-114 | Tall | Medium | Droopy leaves | White | Thin scattered tassel | 8.10 | % cell injury (70.65) | RLWC (0.85) | High yielder |
| 5 | M-14 | Tall | Medium | Semi droopy medium size leaves | White | Tassel compact | 8.10 | % cell injury (72.5) | RLWC (0.86) | High yielder |
| 6 | D-109 | Tall | Medium | Erect broad and medium size leaves | White | Thin and compact tassel | 7.05 | % cell injury (69.5) | RLWC (0.82) | High yielder |
CL = Coleoptiles length. CMT= Cell membrane thermostability. RLWC = Relative leaf weight content.
3. Results and Discussion
3.1. Cell Membrane Thermostability
Estimation of combining ability revealed highly significant estimates for general and specific combining ability revealing the significance of both additive and non-additive gene action for the expression of trait under normal and water deficit conditions ( Table 1). General combining ability (GCA) variance (б2g) was found higher than Table 1. Mean squares due to general combining ability (GCA), specific combining ability (SCA) and reciprocal effects under normal (N) & moisture stress (S) condition. ** = Highly Significant. specific combining ability (SCA) variance (б2s) indicated additive gene action under both normal and water deficit conditions (Table 2). Higher additive genetic variance for a trait suggested early selection with significant genetic gain. Half of the parents showed positive GCA estimates while half parents displayed negative GCA estimates. Inbred line NCIL-20-20 showed maximum general combining ability effect (5.438) whereas inbred D-109 (-6.589) exhibited lowest GCA effects under normal condition (Table 3(a)). NCIL-20-20 proved to be the best general combiner on the basis of GCA effects. Among the crosses, most useful combination was OH-8 × D-114 (2.270) with maximum specific combining ability (SCA) effects whereas combination M-14 × D-114 showed maximum negative effects (−2.12). Regarding reciprocal crosses, 6 were observed with positive while 9 crosses displayed negative effects. Maximum positive effects (0.673) were produced by cross D-114 × D-157 while maximum negative value (−1.11) recorded for cross NCIL-20-20 × D-157 under normal condition for cell membrane thermo-stability. Inbred NCIL-20-20 proved to be the best general combiner under both conditions while inbred D-109 reflected poor combiner among the parents. Cross D-109 × NCIL-20-20 exhibited maximum SCA (2.04) effects under stress condition. Among the reciprocal crosses, cross D-157 × OH-8 displayed maximum positive (0.15) effects while combination D-109 × OH-8 showed maximum negative effects (−0.39) under water deficit condition. Chohan et al. [17]
3.2. Stomatal Conductance
General combining ability (GCA) effects were observed more under normal condition than specific combining ability (SCA) effects revealing the role of predominant additive genetic effect (Table 1). Variance due to general combining ability (GCA) (б2g) was more than specific combining ability (SCA) variance (б2s) indicated additive genetic effects (Table 2). Under normal conditions, half parental lines showed positive and rest showed negative effects (Table 3(B)). Parent NCIL-20-20 exhibited maximum GCA effect (0.011) while parent D-109 displayed minimum (−0.011) GCA effects under both regimes. Cross D-157 × NCIL-20-20 produced maximum SCA value (0.003) while cross M-14 × NCIL-20-20 gave minimum SCA effects (−0.005). Eleven reciprocal crosses exhibited positive effect while other six produced negative effects. Under water stress condition, 4 inbred lines showed positive while two displayed negative GCA effect (Table 3(B)). Cross combination M-14 × OH-8 and OH-8 × NCIL-20-20 exhibited maximum SCA effects (0.007) while cross OH-8 × D-157 showed minimum SCA effect under stress condition. The results are comparable with Rebetzke [25] and Rahman [26] who reported both additive and non-additive gene action for the control of the trait while Akbar [24] reported additive type of gene action.
Table 2. General combining ability (GCA) variance (б2g), Specific combining ability (SCA) variance (б2s) and reciprocal effects (б2r) in 6 × 6 diallel crosses in maize under normal (N) and water stress condition (S).
Table 3. Estimates of GCA (diagonal), SCA (above diagonal) and their reciprocal effects (below diagonal) under normal and stress (S) conditions. (a) Cell membrane thermo-stability; (b) Stomatal conductance; (c) Plant height; (d) Plant height; (e) Days to silking; (f) Anthesis-silking interval; (g) Grain yield per plant.
(a)
(b)
(c)
| Inbred lines | M-14 | OH-8 | D-157 | D-114 | D-109 | NCIL-20-20 | ||||||||||||
| N | S | N | S | N | S | N | S | N | S | N | S | |||||||
| M-14 | −5.696 | −5.544 | 0.782 | 2.772 | 0.496 | 4.200 | −0.226 | −2.311 | −0.393 | −1.903 | 0.116 | 1.747 | ||||||
| OH−8 | −0.217 | −0.050 | −1.571 | −1.572 | −0.729 | 0.761 | 0.449 | 0.200 | 0.249 | −2.408 | −0.859 | −1.075 | ||||||
| D-157 | 1.467 | −0.150 | 0.033 | 0.083 | 6.531 | 7.767 | 0.230 | −0.389 | 0.113 | −0.747 | 0.088 | −1.831 | ||||||
| D-114 | 0.333 | −0.017 | 0.333 | 0.167 | 0.150 | 0.017 | 1.020 | −0.756 | −1.009 | 1.758 | 0.299 | −0.075 | ||||||
| D-109 | −0.033 | 0.217 | 0.167 | −0.183 | 0.033 | −0.083 | −1.567 | −0.400 | −13.980 | −14.664 | 0.282 | 0.933 | ||||||
| NCIL-20-20 | 0.383 | 0.000 | −0.267 | 0.083 | −0.383 | 0.000 | 0.817 | 0.033 | −0.100 | −0.333 | 13.695 | 14.769 | ||||||
(d)
| Inbred lines | M-14 | OH-8 | D-157 | D-114 | D-109 | NCIL-20-20 | ||||||||||||
| N | S | N | S | N | S | N | S | N | S | N | S |
| ||||||
| M-14 | −0.843 | 2.361 | 0.120 | −0.472 | 0.065 | 0.472 | −0.630 | −1.417 | 0.065 | 0.417 | −0.241 | 0.861 |
| |||||
| OH-8 | 0.000 | 0.000 | −0.676 | 0.306 | −0.102 | −0.472 | −0.130 | 0.306 | 0.065 | 0.306 | 0.093 | −0.250 |
| |||||
| D-157 | 0.167 | 0.000 | −0.167 | 0.000 | −1.120 | −1.972 | 0.315 | 0.417 | −0.491 | −0.417 | 0.037 | −0.472 |
| |||||
| D-114 | −1.167 | 0.000 | −0.167 | 0.000 | 0.167 | 0.167 | 0.907 | −1.750 | 0.315 | −0.639 | 0.009 | 0.972 |
| |||||
| D-109 | 0.000 | 0.000 | −0.167 | 0.167 | 0.167 | −0.167 | 0.000 | −0.167 | 1.046 | −0.250 | −0.130 | −0.694 |
| |||||
| NCIL-20-20 | −0.333 | 0.000 | −0.167 | −0.167 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.167 | 0.685 | 1.306 |
| |||||
(e)
| N | S | N | S | N | S | N | S | N | S | N | S | ||||||
| M-14 | −1.019 | 1.546 | −0.009 | −1.04 | −0.231 | −0.02 | −0.454 | −0.49 | 0.269 | −0.296 | 0.046 | 0.26 | |||||
| OH-8 | −0.167 | 1.833 | −0.463 | −0.98 | 0.213 | 0.34 | −0.009 | 0.53 | −0.454 | −0.10 | −0.009 | 0.29 | |||||
| D-157 | 0.000 | −0.83 | −0.333 | 0.33 | −1.407 | −2.01 | 0.102 | −0.60 | −0.176 | 0.92 | −0.065 | −1.68 | |||||
| D-114 | 0.167 | −0.50 | −0.167 | 0.33 | 0.000 | −0.50 | 0.648 | 0.13 | 0.102 | 0.28 | −0.120 | −0.15 | |||||
| D-109 | 0.000 | −0.16 | −0.167 | 0.16 | −0.167 | 0.16 | 0.167 | 0.33 | 1.426 | 0.27 | 0.102 | 0.03 | |||||
| NCIL-20-20 | −0.167 | 0.50 | 0.000 | 0.33 | 0.333 | 0.00 | 0.000 | 0.66 | 0.000 | 0.000 | 0.815 | 1.04 | |||||
(f)
| Inbred lines | M-14 | OH-8 | D-157 | D-114 | D-109 | NCIL-20-20 | |||||||||||||
| N | S | N | S | N | S | N | S | N | S | N | S |
| |||||||
| M-14 | −0.333 | −0.167 | −0.00 | −0.667 | −0.194 | 0.028 | 0.14 | −0.083 | 0.167 | 0.250 | 0.11 | 0.028 |
| ||||||
| OH-8 | −0.167 | 0.333 | 0.278 | −1.389 | 0.194 | −0.750 | 0.03 | 0.806 | −0.11 | 0.139 | −0.00 | 0.583 |
| ||||||
| D-157 | −0.167 | 0.333 | −0.167 | 0.000 | −0.194 | 0.250 | −0.00 | −0.000 | 0.194 | 0.333 | −0.03 | 0.111 |
| ||||||
| D-114 | 1.167 | 0.167 | 0.00 | −0.167 | −0.167 | 0.000 | −0.194 | 0.528 | −0.306 | −0.278 | −0.03 | −0.167 |
| ||||||
| D-109 | 0.000 | 0.000 | 0.167 | 1.333 | −0.333 | 0.500 | 0.167 | 0.500 | 0.444 | 0.028 | 0.167 | −0.500 |
| ||||||
| NCIL-20-20 | 0.167 | −0.167 | 0.167 | 0.167 | 0.333 | 0.000 | 0.000 | 0.000 | 0.167 | 0.167 | 0.000 | 0.750 |
| ||||||
(g)
| Inbred lines | M-14 | OH-8 | D-157 | D-114 | D-109 | NCIL-20-20 | |||||||||||
| N | S | N | S | N | S | N | S | N | S | N | S | ||||||
| M-14 | −7.75 | −18.52 | 4.338 | 2.494 | −1.843 | −2.211 | 1.374 | 2.906 | −0.248 | 0.436 | 1.191 | −2.328 | |||||
| OH-8 | 0.183 | 3.567 | −0.62 | −0.347 | −2.476 | −2.553 | −2.476 | −1.519 | −2.748 | 3.628 | 3.474 | −0.703 | |||||
| D-157 | −0.367 | −0.150 | 0.100 | 0.517 | 10.31 | 14.342 | 5.010 | −2.108 | 7.688 | 1.572 | −2.473 | 1.958 | |||||
| D-114 | 0.133 | −0.150 | 0.083 | −0.167 | 0.133 | 0.267 | 0.390 | 6.092 | −0.662 | −4.494 | −7.106 | 5.975 | |||||
| D-109 | −0.067 | −0.150 | 0.100 | −2.783 | 0.233 | −0.017 | −0.033 | −0.033 | −20.155 | −27.54 | 9.188 | 0.189 | |||||
| NCIL-20-20 | 0.000 | 0.167 | 0.350 | −0.067 | −0.367 | 0.050 | 0.150 | −0.117 | −3.500 | 0.000 | 17.84 | 25.97 | |||||
3.3. Plant Height
Highly significant mean squares for general combining ability (GCA) and specific combining ability (SCA) under both regimes indicated both additive and non-additive type of gene action (Table 1). Higher GCA (б2g) than SCA (б2s) variance (Table 2) shows additive gene action for the control of the trait inheritance under both environmental conditions. The result are in agreement with the findings of Saeed et al. [27] , Saleem et al. [28] ,Yuan et al. [29] , Prakash and Ganguli [30] , Rezaei et al. [31] , Muraya et al. [32] , Hussain [33] , Chohan et al. [17] and Iqbal et al., [18] . Inbred line NCIL-20 showed high estimates (13.7) for general combining ability and proved to be the good combiner while inbred line D-109 showed lowest value for general combing ability and thus a poor general combiner for plant height. Cross M-14 × OH-8 exhibited maximum value (0.78) followed by M-14 × D-157 (0.49) (Table 3(C)). The maximum value regarding reciprocal effects was given by D-157 × M-14 (1.46) followed by NCIL-20-20 × D-114 (0.817) while highest negative effects (−1.56) were recorded for cross D-109 × D-114. Under water stress condition, (Table 3(C)) highest GCA effects (7.76) were observed for D-157. Lowest GCA effects for the trait were observed for inbred D-109 (−14.66). Six single crosses displayed positive SCA with maximum effects by M-14 × D-157 (4.2) followed by M-14 × OH-8 (2.77) and OH-8 × D-114 (0.2). Crosses OH-8 × D-109 (−2.41) showed maximum negative specific combining ability effects followed by M-14 × D-114 (−2.31) and M-14 × D-109 (−1.9). Highest positive reciprocal effects were observed for D-109 × M-14 (0.217) followed by D-114 × OH-8 (0.167) and D-157 × OH-8 (0.083) indicating the effect of cytoplasmic genetic constituents. The lowest reciprocal effects (−0.05) were recorded for OH-8 × M-14.
3.4. Days to Tasseling
Mean squares for general combining ability (GCA) and specific combining ability (SCA) indicated the presence of both additive and non-additive gene action for the trait (Table 1). High estimates of GCA than SCA under both conditions indicated the role of additive gene action for inheritance of trait. Greater estimates of GCA variance (б2g) (Table 2) than SCA variance (б2s) indicated additive genetic effects. Prakash and Ganguli [30] , Bello and Olaoye [15] , Chohan et al. [17] and Iqbal et al., [18] reported additive gene action while Akbar et al. [24] reported non-additive gene action for this trait. Half of the parents displayed positive value while the rest showed negative GCA effects (Table 3(D)). Parent D-109 (1.04) showed maximum GCA effects and was the best combiner followed by parent D-114 (0.91) and NCIL-20-20 (0.685). Regarding single crosses cross D-157 × D-114 and D-114 × D-109 showed maximum positive SCA effects (0.315) while minimum was displayed by cross D-114 × NCIL-20-20 (0.01). In case of reciprocal crosses, cross D-157 × M-14, D-114 × D-157 and D-109 × D-157 showed maximum effects (0.167). Parental line M-14 (2.36) showed the maximum GCA effects followed by NCIL-20-20 (1.31) and OH-8 (0.306), indicating good general combiner, respectively under water stress condition. In case of specific combining ability effects, seven crosses showed positive SCA effects while other eight displayed negative SCA effects. Cross D-114 × NCIL-20-20 showed the maximum SCA (0.97) effects while negative SCA effects were shown by OH-8 × NCIL-20-20 (−0.25). Cross D-109 × OH-8, D-114 × D-157 and NCIL-20-20 × D-109 displayed the maximum reciprocal effects whereas high negative effects were shown by NCIL-20-20 × OH-8, D-109 × D-157 and D-109 × D-114.
3.5. Days to Silking
Variance for general combining ability (б2g) was observed greater than specific combining ability effects (б2s) (Table 2) indicated the presence of additive genetic effects. The results are compatible with those of Reddy et al. [34] , Olaoye [35] , Gichuru et al. [16] , Chohan et al. [17] , and Iqbal et al. [18] who reported additive gene effects for days to silking under both conditions. Half inbred lines depicted positive while the rest showed negative GCA effects under normal water condition (Table 3(E)). The best general combiner was D-109 (1.43) and lowest general combiner was OH-8 (−0.463). Cross M-14 × D-109 (0.269) showed the maximum positive value while M-14 × OH-8 (−0.009) displayed the maximum negative value for specific combining ability (SCA). Nine of the reciprocal crosses displayed positive value while the six showed negative reciprocal effects. Cross combination D-114 × M-14 and D-109 × D-114 showed maximum positive (0.167) value whereas D-114 × OH-8 (−0.333) displayed maximum negative reciprocal effects. Four genotypes showed positive and two showed negative general combining effects (Table 3(E)). Genotypes M-14 (1.54) showed the maximum GCA positive value followed by NCIL-20-20 (1.04) and D-109 (0.27). Combination D-157 × D-109 showed the maximum positive value (0.92) whereas cross D-157 × NCIL-20-20 showed the minimum negative value (−1.68) under water deficit condition. In case of reciprocal crosses, ten of the crosses showed positive value while rest of the five exhibited negative value.
3.6. Anthesis-Silking Interval
Analysis of variance for anthesis-silking interval (ASI) revealed significant mean squares due to general combining ability (GCA) and specific combining ability (SCA) effects (Table 1). GCA variance (б2g) was observed more than SCA variance (б2s) under both conditions which indicated the significance of additive genetic effects for the inheritance of the trait (Table 2). Similar findings have been reported by Ahmad [36] , Bello and Olaoye [16] , Chohan et al. [17] and Iqbal et al., [18] who reported additive gene action for this trait. Half inbred lines showed positive GCA value while other three parents displayed negative GCA value under normal water condition (Table 3(F)). Inbred line D-109 (0.44) showed maximum value and proved to be the best general combiner while inbred line M-14 (−0.33) indicated minimum value depicting poor combiner for the trait. Cross OH-8 × D-157 showed maximum SCA value (0.19) while M-14 × D-157 showed minimum (−0.19) SCA effects. Maximum reciprocal effect was observed for D-114 × M-14 (1.16) whereas minimum value was shown by cross D-109 × D-157 (−0.33). General combining ability estimates showed four positive and two negative GCA estimates for parental inbred lines under water deficit condition (Table 3(F)). Inbred line NCIL-20-20 (0.75) exhibited maximum GCA effects proving to be the best general combiner followed by D-114 (0.53) and D-157 (0.25) whereas inbred line M-14 and OH-8 showed negative GCA estimates of −0.167 and −1.39, respectively. Eight of the single crosses showed positive SCA effects while seven displayed negative estimates regarding anthesis-silking interval (ASI) under water deficit condition. The best combination was (Table 3(F)) OH-8 × D-114 (0.81) while poorest results were obtained for cross OH-8 × D-157 (−0.75). In case of reciprocal crosses, the maximum reciprocal effects was observed for D-109 × OH-8 (1.33) whereas minimum reciprocal effects was noted for cross D-114 × OH-8 and NCIL-20-20 × M-14 which was −0.167.
3.7. Grain Yield per Plant
Under both conditions, analysis of variance gave highly significant estimates for general (GCA) and specific combining ability (SCA) revealing the significance of both additive and non-additive genetic effects (Table 1). GCA variance (б2g) was higher than SCA variance (б2s) indicating the predominant role of additive gene action for the expression of trait (Table 2). Betran et al. [19] , Makumbi et al. [37] , Rezaei et al. [31] , Santos et al. [38] , Derera et al. [39] , Bello and Olaoye, [15] , Chohan et al. [17] and Iqbal et al. [18] concluded that additive gene action was more important than non-additive gene action for grain yield. On the other hand, non-additive effects for grain yield was reported by Akbar et al. [24] , Shiri et al. [40] and Gichuru et al. [16] reported grain yield under the control of both additive and non-additive gene action. Estimates of general combining ability (Table 3(J)) showed that half of the parents possessed positive value while remaining half displayed negative GCA effects. Parent NCIL-20-20 showed maximum general combining ability effects (17.84) proving to be best combiner under normal condition, whereas parent D-109 displayed maximum negative GCA effects (−20.15) which indicated as poor combiner for grain yield per plant. Specific combining ability effects (SCA) indicated that seven crosses displayed positive SCA effects whereas eight crosses showed negative SCA effects. D-109 × NCIL-20-20 exhibited maximum SCA effects (9.18) followed by D-157 × D-109 (7.68) and D-157 × D-114 (5.01) whereas cross D-114 ×NCIL-20-20 displayed maximum negative estimate (−7.11). Highest positive value (0.35) for reciprocal effects was displayed by cross NCIL-20-20 × OH-8 while maximum negative value (−3.500) was exhibited by cross NCIL-20-20 × D-109 under normal water application condition. While under water stress conditions, Parent NCIL-20-20 (25.975) showed maximum GCA effects followed by D-157 (14.342) and D-114 (6.092). Inbred line NCIL-20-20 was best combiner on the basis of GCA effects while inbred line D-109 displayed poor performance under water deficit condition. Eight of the crosses showed positive specific combining ability effects (SCA) whereas seven displayed negative SCA effects (Table 3(J)). Combination D-114 × NCIL-20-20 showed maximum estimates (5.97) while cross OH-8 × D-157 exhibited maximum negative SCA effect. Cross D-157 × OH-8 showed maximum reciprocal effect (0.517) while cross D-109 × OH-8 displayed maximum negative (−2.78) effect under water deficit condition.
4. Conclusion
Highly significant mean square estimates due to specific combining ability (SCA), general combining ability (GCA) and reciprocal effects for the traits under both conditions suggested significant contribution of genetic components of variation attributable to general combining ability, specific combining ability and reciprocal effects. Components of variation exhibited greater estimates for GCA variance (б2g) than SCA variance (б2s) for majority of the traits under both conditions depicting the predominant role of additive genetic component except for days to silking under water deficit condition which displayed more SCA variance (б2s) than GCA variance (б2g). Inbred lines NCIL-20-20, D-157 and OH-8 were recorded as the best general combiner on the basis of performance regarding grain yield per plant under both conditions i.e. normal and water deficit condition. These inbred lines can be exploited and utilized in future breeding program. On the basis of mean grain yield per plant the best combination was NCIL-20-20 × D-109 followed by NCIL-20-20 × OH-8 and D-157 × NCIL-20-20, respectively under normal and water stress condition. These well performing combinations can be utilized for developing new hybrids for drought affected areas. Prediction of additive gene action would be expected to be more reliable as compared to the traits which were controlled by non-additive type of gene action.
References
- Anonymous (2011-2012) Economic Survey of Pakistan. Finance Division, Government of Pakistan, Islamabad.
- Araus, J.L., Slafer, G.I., Reynolds, M.P. and Royo, C. (2002) Plant Breeding and Drought in C3 Cereals: What Should We Breed for? Annals of Botany, 89, 925-940. http://dx.doi.org/10.1093/aob/mcf049
- FAO (2007) Coping with Water Scarcity. Challenge of the Twenty-First Century, UN-Water.
- Heisey, P.W. and Edmeades, G.O. (1999) Maize Production in Drought-Stressed Environments: Technical Options and Research Resource Allocation. World Maize Facts and Trends 1997/1998.
- Dass, S., Arora, P., Kumari, M. and Dharma, P. (2001) Morphological Traits Determining Drought Tolerance in Maize. Indian Journal of Agricultural Research, 35, 190-193.
- Araus, J.L., Salfer, G.A., Royo, C. and Serret, M.D. (2008) Breeding for Yield Potential and Stress Adaptation in Cereals. Critical Reviews in Plant Science, 27, 377-412. http://dx.doi.org/10.1080/07352680802467736
- Frova, C., Krajewski, P., di Fonzo, N., Villa, M. and Sari-Gorla, M. (1999). Genetic Analysis of Drought Tolerance in Maize by Molecular Markers. I. Yield Components. Theoretical and Applied Genetics, 99, 280-288.http://dx.doi.org/10.1007/s001220051233
- Ortegon-Morales, A.F., Escobedo-Mendoza, A. and Villarreal, L.Q. (1992) Combining Ability of Sunflower (H. annuus L.) Lines and Comparison among Parent Lines and Hybrids. In: Proceedings of the 13th International Sunflower Conference, Pisa, 7-11 September 1992, 1178-1193.
- Hayman, B.I. (1954) The Theory and Analysis of Diallel Crosses. Genetics, 39, 789-809.
- Hayman, B.I. (1954) The Analysis of Varience of Diallel Tables. Biometrics, 10, 235-244.http://dx.doi.org/10.2307/3001877
- Jinks, J.L. (1954) Analysis of Continous Variation in a Diallel cross of Nicotiana Rustica Varieties. Genetics, 39, 767-788
- Singh, S.B. and Gupta, B.B. (2008) Combining Ability Analysis for Some Morpho-Physiological and Yield Components Related to Drought Tolerance in Maize (Zea mays L.). Progressive Research, 3, 181-186.
- Sprague, G.F. and Tatum, L.A. (1942) General vs Combining Ability in Single Crosses of Corn. Agronomy, 34, 923-932. http://dx.doi.org/10.2134/agronj1942.00021962003400100008x
- Rajos, B.A. and Sprague, G.F. (1952) A Comparison of Variance of Components in Corn Yield Traits: III. General and Specific Combining Ability and Their Interaction with Locations and Years. Agronomy, 44, 462-466.http://dx.doi.org/10.2134/agronj1952.00021962004400090002x
- Bello, O.B. and Olaoye, G. (2009) Combining Ability for Maize Grain Yield and Other Agronomic Characters in Typical Southern Guinea Savanna Ecology of Nigeria. African Journal of Biotechnology, 8, 2518-2522.
- Gichuru, L., Njoroge, K., Ininda, J. and Peter, L. (2011) Combining Ability of Grain Yield and Agronomic Traits in Diverse Maize Lines with Maize Streak Virus Resistance for Eastern Africa Region. Agriculture and Biology Journal of North America, 2, 432-439.
- Chohan, M.S.M., Saleem, M., Ahsan, M. and Asghar, M. (2012) Genetic Analysis of Water Stress Tolerance and Various Morpho-Physiological Traits in Zea mays L. Using Graphical Approach. Pakistan Journal of Nutrition, 11, 489-500. http://dx.doi.org/10.3923/pjn.2012.489.500
- Iqbal, J., Saleem, M., Ahsan, M. and Ali, A. (2012) General and Specific Combining Ability Analysis in Maize under Normal and Moisture Stress Conditions. Journal of Animal and Plant Sciences, 22, 1048-1054.
- Betran, F.J., Beck, D., Banziger, M. and Edmeades, G.O. (2003) Genetic Analysis of Inbred and Hybrid Grain Yield under Stress and Non Stress Environments in Tropical Maize. Crop Science, 43, 807-817.http://dx.doi.org/10.2135/cropsci2003.8070
- Khan, I.A., Habib, S., Sadaqat, H.A. and Tahir, M.H.N. (2004) Selection Criteria Based on Seedling Growth Parameter in Maize Varies under Normal and Water Stress Conditions. International Journal of Agriculture and Biology, 6, 252-256.
- Ibrahim, A.M. and Quick, J.S. (2001) Heritability of Heat Tolerance in Winter and Spring Wheat. Crop Science, 41, 1401-1405. http://dx.doi.org/10.2135/cropsci2001.4151401x
- Steel, R.G.D., Torrie, J.H. and Discky, D.A. (1997) Principles and Procedures of Statistics: A Biometrical Approach. 3rd Edition, McGraw Hill Book Co., New York.
- Griffing, B. (1956) Concept of General and Specific Combining Ability in Relation to Diallel/Crossing Systems. Australian Journal of Biological Sciences, 9, 463-493.
- Akbar, M. (2008) Genetic Control of High Temperature Tolerance in Zea mays L. Ph.D. Thesis, Department of Plant Breeding and Genetics, University of Agriculture, Faisalabad.
- Rebetzke, G.J., Condom, A.G., Richards, R.A. and Furquhar, G.D. (2003) Gene Action for Leaf Conductance in Three Wheat Crosses. Australian Journal of Agricultural Research, 54, 381-387. http://dx.doi.org/10.1071/AR02151
- Rahman, H.U. (2005) Genetic Analysis of Stomatal Conductance in Upland Cotton (Gossypium hirsutum L.) under Contrasting Temperature Regimes. Journal of Agricultural Science, 143, 161-168.
- Saeed, M.T., Saleem, M. and Afzal, M. (2000) Genetic Analysis of Yield and Its Components in Maize Diallel Crosses (Zea mays L.). International Journal of Agriculture and Biology, 2, 376-378.
- Saleem, M., Shahzad, K., Javid, M. and Ahmed, A. (2002) Genetic Analysis for Various Quantitative Traits in Maize (Zea mays L.) Inbred Lines. International Journal of Agriculture and Biology, 4, 379-382.
- Yuan, D.H.W., Wei, G., DeXiang, L., JunJie, L., Qiang, L., DY, H., Wu, G.W., Long, D.X., Lu, J. and Liu, Q. (2003) Analysis of Combining Ability and Hereditary Parameters of Main Quantitative Characters of 10 Maize Inbred Lines. Journal of Maize Science, 11, 26-29.
- Prakash, S. and Ganguli, D.K. (2004) Combining Ability for Various Yield Component Characters in Maize (Zea mays L.). Journal of Research, Birsa Agricultural University, 16, 55-60.
- Rezaei, A.H., Yazdisamadi, B., Zali, A., Rezaei, A.M., Tallei, A. and Zeinali, H. (2005) An Estimate of Heterosis and Combining Ability in Corn Using Diallel Crosses of Inbredlines. Iranian Journal of Applied Animal Science, 36, 385-397.
- Muraya, M.M., Ndirangu, C.M. and Omolo, E.O. (2006) Heterosis and Combining Ability in Diallel Crosses Involving Maize (Zea mays L.) S1 Lines. Australian Journal of Experimental Agriculture, 46, 387-394. http://dx.doi.org/10.1071/EA03278
- Hussain, I. (2009) Genetics of Drought Tolerance in Maize (Zea Mays L.). Ph.D. Thesis, Department of Planting Breeding and Genetics, University of Agriculture, Faisalabad.
- Reddy, A.R., Chaitanya, K.V. and Vivekanandan, M. (2004) Drought-Induced Responses of Photosynthesis and Antioxidant Metabolism in Higher Plants. Journal of Plant Physiology, 161, 1189-1202.http://dx.doi.org/10.1016/j.jplph.2004.01.013
- Olaoye, G., Bello, O.B., Abubaker, A.Y., Olayiwola, L.S. and Adesina, O.A. (2009) Analysis of Moisture Deficit Grain Yield Loss in Drought Tolerant Maize (Zea mays L.) Germplasm Accessions and Its Relationship with Field Performance. African Journal of Biotechnology, 8, 3229-3238.
- Ahmad, A. (2002) Genetics of Growing Degree Days, Yield and Its Components in Maize. Ph.D. Thesis, Department of Plant Breeding and Genetics, University of Agriculture, Faisalabad.
- Makumbi, D., Banziger, M., Ribaut, J.M. and Betran, F.J. (2004) Diallel Analysis of Tropical Maize Inbreds under Stress and Optimal Conditions. In: Polland, M., Sawkins, J., Ribaut, J.M. and Hoisington, D., Eds., Resilent Crops for Water Limited Ennvirments: Proceedings of a Workshop Held at Cuernavaca, Mexico City, 24-28 May 2004, 112-113.
- Santos, M.F., Camara, T.M.M., Moro, G.V., Costa, E.F.N. and De Souza, C.L. (2007) Responses to Selection and Changes in Combining Ability after Three Cycles of a Modified Reciprocal Recurrent Selection in Maize. Euphytica, 157, 185-194. http://dx.doi.org/10.1007/s10681-007-9410-x
- Derera, J., Tongoona, P., Vivek, B.S. and Liang, M.D. (2008) Gene Action Controlling Grain Yield and Secondary Traits in Southern African Maize Hybrids under Drought and Non-Drought Environments. Euphytica, 162, 411-422.http://dx.doi.org/10.1007/s10681-007-9582-4
- Shiri, M., Aliyev, R.T. and Choukan, R. (2010) Water Stress Effects on Combining Ability and Gene Action of Yield and Genetic Properties of Drought Tolerance Indices in Maize. Research Journal of Environmental Sciences, 4, 75-84.http://dx.doi.org/10.3923/rjes.2010.75.84
NOTES

*Corresponding author.


