American Journal of Plant Sciences
Vol.3 No.8(2012), Article ID:22194,4 pages DOI:10.4236/ajps.2012.38138
Conocephalum conicum (L.) Dumort: A Case of Unique Reproductive Biology
![]()
Department of Botany, University of Jammu, Jammu, India.
Email: *asanilsharma3@gmail.com
Received May 25th, 2012; revised June 22nd, 2012; accepted June 30th, 2012
Keywords: Reproductive Biology; Liverworts; Dioecious; Sporophyte; Spore-Elater Ratio
ABSTRACT
This paper reports spore-elater ratio per capsule in three populations of Conocephalum conicum collected from different regions of Jammu and Kashmir (Doda, Ladakh and Bhaderwah). Spore-elater ratio came out to be 0.40-0.43:1, far less than expected for Marchantialian taxa. The ratios thus obtained were compared with that present in herbarium specimen collected in 1958 from Kyushu. The ratios have remained constant since many decades, thereby indicating that the sexual reproduction has lesser role to play in the propagation of this species.
1. Introduction
Bryophytes are believed to have originated from algal ancestors 476 - 432 million years ago [1] and acquired a number of characters for successful land habit (jacketed sex organs being one of these). Because the sperm and egg cells of bryophytes are produced in two distinct organs (antheridia and archegonia, respectively) and even on two different plants in the case of dioecious species, the sperm must reach the egg for fertilization to occur. Owing to their algal ancestry, sperms are flagellated and bryophytes therefore, have absolute dependence on water for fertilization. But it has been observed that dioecious species often fail to reproduce sexually. In a study on the reproductive biology of British mosses, Longton [2] found that 87% of the species, wherein sporophytes are unknown are dioecious, whereas sporophytes are regarded as occasional to common in 83% of the monoecious species. Study on reproductive biology of dioecious bryophytes, therefore, calls for special attention. Conocephalum conicum is one of the threatened dioecious hepatic taxa in which preliminary studies have revealed extremely low production of sporophytes and spores.
2. Material and Methods
Sporophytes bearing female thalli of Conocephalum conicum were collected from Gandoh (Doda district of J & K). A capsule was crushed on a clean glass slide with the help of a needle and mounted in glycerine and a rectangular cover slip compartmentalized into quadrats placed over it. Total number of spores and elaters were counted in individual quadrats. Total spore elater output per capsule was determined by adding the number of spores and elaters in all the quadrats. Spore elater ratio/ capsule was determined by applying the formula:
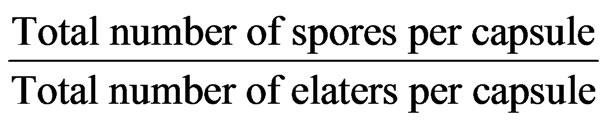
Repeated the same procedure for five capsules and took out the mean value. Similarly, the ratios were determined from undehisced capsules taken from herbarium specimens collected in1958 and deposited in the herbarium of Panjab University, Chandigarh. The figures thus obtained were compared with those earlier determined by Iqbal et al. [3] and Dolma (unpublished data).
3. Results and Discussion
Conocepahalum conicum is commonly called snakewort due to the resemblance of its dorsal epidermis with the snake skin (Figure 1). It is worldwide in distribution [4]. In India it is reported from Eastern [5-7] and Western Himalaya [8-13]. Kashyap [9] reported very common occurrence of Conocephalum conicum in the Kumaon and the outer Himalaya up to Kashmir in the West, lying between 5000 to 8000 feet and in middle Himalaya and Pangi.
J & K state which forms a part of North West Himalaya is divided into three regions, i.e. Jammu, Kashmir and Ladakh; Conocephalum conicum has been reported from

Figure 1. Vegetative thalli of Conocephalum conicum showing hexagonal areolae on its dorsal surface.
all of these. Tanwir and Langer [14] recorded 5 accessions of the species from different areas of district Kargil (Ladakh) located over an altitude of 2600 - 4300 while Koul and Dhar [15] reported it from Kashmir (Aharbal, Nilnag and Pahalgam). From Jammu region, as many as 15 populations have been collected from various localities in district Poonch, situated over 1000 - 2300 m [16] and five from district Udhampur [1530 - 2360] [17]. Maximum numbers of populations [46] were, however, collected from Bhaderwah (district Doda of Jammu) from an altitudinal range of 1650 - 1720 m by Iqbal et al. [3]. From Jammu district, this taxon has not been collected so far, because it grows at least 1000 m above sea level and the altitude of Jammu is far less than that (350 m).
C. conicum is an economically important liverwort. It is used as food for slugs [18], for curing eczema, cuts, burns, boils, fractures, swellings, poisonous snake bites and gall stones [19,20] etc. Pant [13] included the species in the list of hepatics which she observed to have disappeared from Kumaon Himalaya over the years, and become threatened. She, therefore, raised a voice to conserve it. Prior to chalking out strategies for conservation of any taxon, it is prerequisite to assess its population status. Work in this direction was, therefore, initiated in our laboratory to assess the status of this species in Bhaderwah; a small hilly town in Jammu (1650 - 1720 m) by Iqbal who collected 46 populations from diverse habitats such as epilithic (rock crevices, caves, under chilled snow cover, stream banks, submerged under water), non-epilithic (cool and moist shady soil, loamy soil) and corticolous (on the decaying log of Cedrus deodara). Out of 46 populations recorded, 15 were fertile. Of these, 12 showed both male and female thalli while remaining 3 had only female plants. Although, female thalli were observed in 15 populations, mature receptacles were borne by only 3. In the remaining 12 populations receptacles persisted for a few days and then degenerated. Surprisingly, healthy sporophytes with viable spores and elaters were observed only in a single population. Furthermore, spore elater ratio came out to be far lesser than expected i.e. approximately 0.4:1 (Table 1) meaning thereby, that the plant produces far more elaters than the spores. Apparently, such a unique sexual behaviour appeared to be an outcome of breakdown of some pre/postzygotic mechanism of sexual cycle in this particular population.
It would be pertinent to mention here that archesporium in hepatics gets differentiated into two types of cells-sporocytes and elaterocytes. Each sporocyte undergoes meiosis and gives rise to four spores while the elaterocytes simply elongate, acquire spiral bands and get transformed into elaters. The basic ratio between spores and elaters in the group is therefore, 4:1. Ratios approaching 4:1 (3-3.7:1) were obtained by Schuster [21] who investigated various members of Marchantiales including Reboulia, Mannia, Targionia, Sauteria and Conocephalum. Highest ratios among Marchantiales and Jungermanniales are reported for Marchantia polymorpha (128:1) and in Schistochila (200:1) respectively. In Lophocolea cuspidata, Marchesinia, Frullania, Monoclea, Fossombronia the calculated ratios are 12:1, 18:1, 20:1, 46-58:1, 32-36:1 respectively. In such taxa which possess spore elater ratios higher than 4:1, the sporocytes undergo one or more mitotic divisions before undergoing meiosis. Increase in spore output is also accompanied by reduction in spore size. Production of small, abundant spores is an adaptation for wind dispersal of spores and, therefore, more successful life on land.
Failure of sporophyte production in dioecious hepatics is not uncommon. In such taxa which represent about 70% of liverworts, the antheridial and archegonial gametophytes are often spatially separated and male gametes have a limited dispersal range. Rydgren et al., [22] found that 85% of the female plants with sporophytes were situated within a distance of 5 cm from nearest male and the maximum distance travelled by antherozoids was 11.6 cm. In case of moss Dawsonia superba, the greatest distance between fruiting plants and the nearest male plants has been recorded at a maximum of 3.8 m. However, fertilization ranges reported for majority of taxa are generally much shorter [23]. Conocephalum conicum seems to have evolved a unique mechanism to overcome this problem. In this species, the mature antherozoids may be projected 15 cm upward and become airborne, greatly increasing the chances of reaching a female plant [24]. Furthermore, the problems of spatial distribution and limited dispersal range are not encountered in C. conicum, as both male and female plants are found growing in close vicinity (Figures 2 and 3). In order to ascertain the reasons behind such an extreme drop in spore output, studies were extended to two more populations collected from other places (Gandoh and Ladakh) in J & K state. While Gandoh is approximately
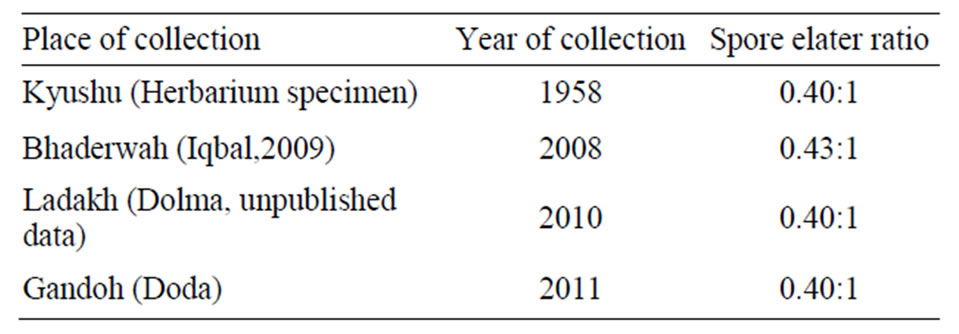
Table 1. Spore elater ratios in populations of Conocephalum conicum collected from different areas.

Figure 2. Male thalli of C. conicum showing dehisced antheridia.

Figure 3. Female thalli showing conical archegoniophores.
21 km away from Bhaderwah, distance between Bhaderwah and Ladakh is about 217 km. To our surprise, spore elater ratio in these three populations was also observed to be 0.40:1 (Table 1, Figures 4 and 5). This clearly indicated that all these populations represent the same genotype. In order to understand whether the ratios have declined over the years in response to climate change, the female receptacles of the species collected from herbarium of Panjab University, Chandigarh were investigated. This population was collected from Kyushu (Eastern Himalaya) in 1958 and even in this population the spore elater ratio was 0.4:1 (Table 1, Figure 6). This clearly indicated that a single genotype has spread over the country at least for the last five decades. This inference also gets support from the observations of Bischler and Dubayle [25] and Iqbal et al. [3] on isozyme analysis of various populations of C. conicum growing under diverse

Figure 4. Whole mount of spores and elaters from mature capsules of populations from Doda.
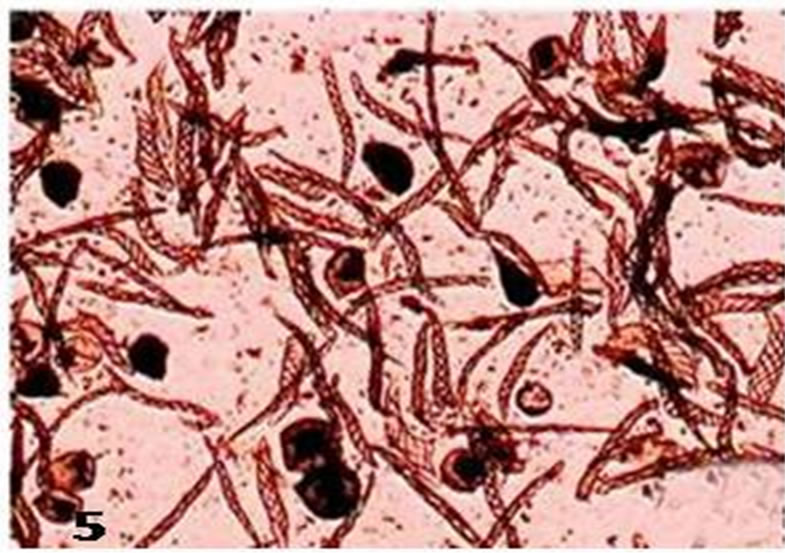
Figure 5. Whole mount of spores and elaters from mature capsules of populations from Bhaderwah.
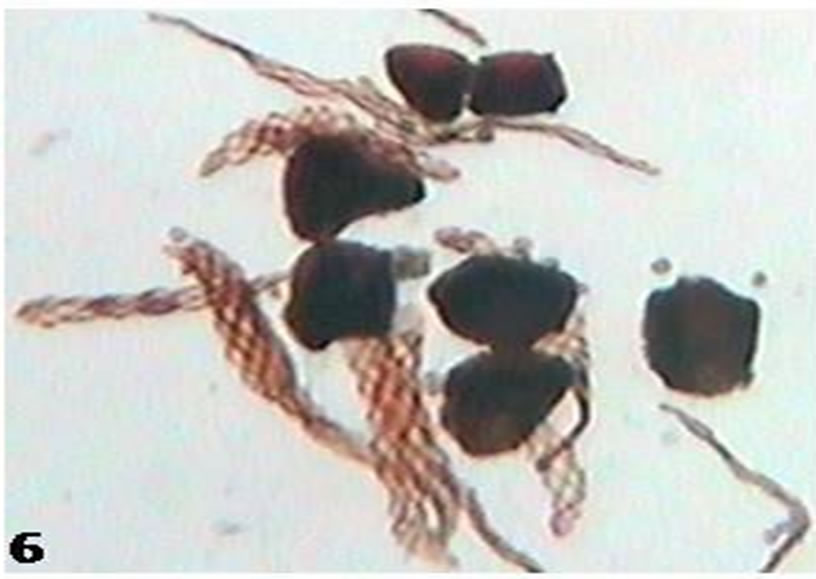
Figure 6. Whole mount of spores and elaters from mature capsules of populations from Herbarium specimen (Kyushu).
habitats. These authors concluded that all the populations were genetically homogenous and variation exhibited by them was environment induced. On the other hand, among populations of C. conicum from Poland, two forms were found that differed in their isoenzyme patterns, phenolic compounds as well as in morphological and physiological characters (ventral scale structure and growth rate in culture). Both forms grew sympatrically but no recombinants were found, in spite of a regular and abundant spore production [26-28]. Monoecious taxa have been found to have a higher genetic variability then dioecious taxa e.g. Plagiochasma, Mannia [29], Marchantia [30]. Species therefore, calls for studies on more Indian populations before arriving at any final conclusion on its genetic structure.
4. Acknowledgements
We wish to thank Head, Dept. of Botany, University of Jammu for providing necessary laboratory facilities. The senior author is also thankful to the Head, Dept. of Botany, Panjab University, Chandigarh for granting permission to study the herbarium bryoflora of the Department and Dr. Sunita Kapila, for her valuable suggestions.
REFERENCES
- M. Groth-Malonek, D. Pruchner, F. Grewe and V. Knoop, “Ancestors of Trans-Splicing Mitochondrial Introns Support Serial Sister Group Relationships of Hornworts and Mosses with Vascular Plants,” Molecular Biology and Evolution, Vol. 22, No. 1, 2005, pp. 117-125. doi:10.1093/molbev/msh259
- R. E. Longton, “Reproductive Biology and Life History Strategies,” Advances in Bryology, Vol. 6, 1997, pp. 65- 101.
- M. Iqbal, A. Langer and A. Alam, “Conocephalum conicum (L.) Dumort. (Snake Liverwort) Threatened in Bhaderwah (J & K) Due to Environmental Shock,” American Journal of Plant Sciences, Vol. 2, No. 4, 2011, pp. 554- 560. doi:10.4236/ajps.2011.24066
- S. Sato and N. Yamada, “Scanning Electron Microscopy on the Antheridium of Conocephalum conicum,” Journal of the Hattori Botanical Laboratory, Vol. 47, 1980, pp. 333-344.
- W. Mitten, “Hepaticae Indiae Orientalie, an Enumeration of the Hepaticae of the East Indies,” Journal of the Proceedings of the Linnean Society, Vol. 5, 1861, pp. 109- 128.
- R. S. Chopra, “Notes on Indian Hepatics,” Proceedings of the Indian Academy of Sciences—Section B, Vol. 7, No. 5, 1938, pp. 239-251.
- S. K. Pandé and D. C. Bharadwaj, “Studies in Indian Hepaticae VI. On Some Liverworts New to Indian Flora,” Journal of Indian Botanical Society, Vol. 28, 1949, pp. 1-13.
- F. Stephani, “Enumeration des Hepatique Connues Dons les Iles de la Societe (Principalement a Tahiti) et Dans les Iles Marquises,” Journal de Botany (Morot), Vol. 12, 1898, pp. 136-150.
- S. R. Kashyap, “Liverworts of Western Himalayas and Panjab Plains. Part I,” The Chronica Botanica, Delhi, 1929.
- N. S. Parihar, “An Annotated Revised Census of Indian Hepatics,” University of Allahabad Studies (India), Botany Section, 1961-1962, pp. 1-56.
- N. S. Parihar, B. Lal and N. Katiyar, “Hepatics and Anthocerotes of India—A New Annotated Checklist,” Central Book Depot, Allahabad, 1994.
- S. K. Pande, “Some Aspects of Indian Hepaticology,” Journal of the Indian Botanical Society, Vol. 37, 1958, pp. 1-26.
- G. B. Pant, “Threatened Bryophytes of Nainital,” In: S. K. Jain and R. R. Rao, Eds., Assessment of Threatened Plants of India, Botanical Survey of India, Department of Environment, Botanical Garden, Howrah, 1983, pp. 313- 317.
- M. Tanwir and A. Langer, “Liverworts of Ladakh, J & K State (North West Himalaya), India,” Journal of Indian Botanical Society, Vol. 85, 2006, pp. 71-73.
- R. K. Koul and G. L. Dhar, “Some Bryophytes of Kashmir Valley,” Kashmir Science, Vol. 5, 1968, pp. 233-237.
- M. Tanwir, “Studies on the Diversity of Hepatic Flora of District Poonch (North West Himalaya),” Ph.D. Thesis, University of Jammu, Jammu, 2005.
- M. Tanwir, A. Langer and M. Bhandari, “Liverwort and Hornwort of Patnitop and Its Adjoining Areas (J & K), Western Himalaya, India,” Geophytology, Vol. 37, No. 1-2, 2008, pp. 35-41.
- H. Ochi, “Conocephalum conicum, as a Food for Slug,” Hikobia, Vol. 2, No. 2, 1960, pp. 154-155.
- Y. Asakawa, “Biologically Active Substances from Bryophytes,” In: R. N. Chopra and S. C. Bhatla, Eds., Bryophytes Development: Physiology and Biochemistry, CRC Press, Florida, 1990, pp. 259-287.
- J. M. Glime and D. Saxena, “Uses of Bryophytes,” Today and Tomorrow’s Printers & Publishers, New Delhi, 1991.
- R. M. Schuster, “The Hepaticae and Anthocerotae of North America,” Columbia University Press, New York, Vol. 1, 1966, pp.1-802.
- K. Rydgren, N. Cronberg and R. H. Okland, “Factors Influencing Reproductive Success in the Clonal Moss, Hylocomium splendens,” Oecologia, Vol. 147, No. 3, 2006, pp. 445-454. doi:10.1007/s00442-005-0290-2
- A. Vanderpoorten and B. Goffinet, “Introduction to Bryophytes,” Cambridge University Press, New York, 2009. doi:10.1017/CBO9780511626838
- M. Shimamura, T. Yamaguchi and H. Deguchi, “Airborne Sperm of Conocephalum conicum (Conocephalaceae),” Journal of Plant Research, Vol. 121, No. 1, 2008, pp. 69- 71. doi:10.1007/s10265-007-0128-6
- H. Bischler and M. C. Boisselier-Dubayle, “Population genetics and Variation in Liverworts,” Advances in Bryology, Vol. 6, 1997, pp. 1-34.
- I. Odrzykoski, M. A. Bobowicz and M. Krzakowa, “Variation in Conocephalum conicum—The Existence of Two Genetically Different Forms in Europe,” In: J. Szweykowski, Ed., New Perspectives in Bryotaxonomy and Bryogeography, Poznan Adam Mickiewicz University, Poznan, 1981, pp. 29-32.
- J. Szweykowski, “Genetic Differentiation of Liverwort Populations and Its Significance for Bryotaxonomy and Bryogeography,” Journal of the Hattori Botanical Laboratory, Vol. 53, 1982, pp. 21-28.
- I. J. Odrzykoski, “Genetic Evidence for Reproductive Isolation between Two European ‘Forms’ of Conocephalum conicum,” Symposia Biologica Hungarica, Vol. 35, 1987, pp. 577-587.
- M. C. Boisselier-Dubayle, “Etude du Polymorphisme Enzymatique Chez Plagiochasma rupestre (Forst.) Steph. et Mannia androgyna (L.) Evans,” Cryptogamie, Bryologie-Lichénologie, Vol. 9, No. 4, 1988, pp. 283-296.
- M. C. Boisselier-Dubayle and H. Bischler, “Electrophoretic Studies in Marchantia polymorpha L,” Journal of the Hattori Botanical Laboratory, Vol. 67, 1989, pp. 297- 311.
NOTES
*Corresponding author.

