American Journal of Plant Sciences
Vol.3 No.2(2012), Article ID:17595,7 pages DOI:10.4236/ajps.2012.32035
Ethephon-Induced Abscission of “Redhaven” Peach
![]()
1Agriculture and Agri-Food Canada, Saskatoon Research Centre, Saskatoon, Canada; 2Department of Plant Agriculture, Horticultural Experiment Station, University of Guelph, Simcoe, Canada; 3Department of Plant Agriculture, Vineland Research Station, University of Guelph, Vineland, Canada; 4Department of Plant Agriculture, University of Guelph, Guelph, Canada.
Email: Ali.Taheri@agr.gc.ca, JCline@uoguelph.ca
Received October 7th, 2011; revised November 9th, 2011; accepted November 16th, 2011
Keywords: Prunus Persica; Fruit Size; Ethylene; Thinning; Crop Load; AVG
ABSTRACT
Fruit size of peaches is an important quality factor that can be optimized by adjusting the number of fruit on the tree by hand thinning 40 - 60 days after full bloom (dafb). Hand thinning is labor intensive and therefore the development of other strategies to reduce production cost is warranted. Since ethylene plays a key role in peach fruitlet abscission, it is hypothesized that foliar applications of ethephon will induce fruit abscission and increase fruit size. Ethephon (0 to 400 mg·L–1) was applied to “Redhaven” peach trees 45 - 50 days after full bloom in 2005 and 2007 to determine the efficacy and concentration required to induce fruit abscission. Abscission was linearly related to ethephon concentration and as a result reduced fruit set by 70% to 100%. These data indicate that ethephon in the range of 100 - 200 mg·L–1 can be used to induce adequate levels of fruit abscission of “Redhaven” peaches without inducing trunk or limb gummosis.
1. Introduction
Canada’s peach and nectarine production is valued at $37 million and annually 29.5 MT of marketable fruit are produced from 2624 ha grown largely in southern Ontario (85%) and the central interior of British Columbia (15%) [1]. Fruit quality and consumer acceptance of fresh market peaches is largely defined by fruit size, flavour, melting texture, and surface and flesh color [2,3]. For producers, fruit size is the primary factor affecting yield and crop value. While there is a strong genetic (cultivar) influence on fruit size [4], it has long been established that fruit size can be increased by adjusting the crop load early in the growing season [2,3].
Peach trees invariably produce more fruits than are needed for an acceptable commercial crop. Removing fruitlets by hand thinning is typically performed at stage II of fruit development (pit hardening), 40 - 60 days after full bloom (DAFB) [2,3,5]. In Canada, hand thinning can require between 100 - 150 h·ha–1 depending on tree vigour, age, size, and fruit set, resulting in a estimated cost of ~$1235 ha–1 per year [6] based on current wages and benefits. Finding an alternative thinning method to offset this relatively high labor cost is of interest to peach orchardists in Canada and other producing regions.
A practice widely adopted in apple production [7], use of chemicals to induce fruitlet abscission is worthy of consideration for reducing the crop load of peaches. Several studies have been conducted on peaches in the past [8], yet no commercially acceptable approach today exists for peach producers. Three timings, with respect to fruit growth and development, exist when chemical thinning can be accomplished: pre-bloom, bloom, and postbloom.
Pre-bloom chemical “thinning” is accomplished more by regulating flower development through the use of gibberellic acid (GA3), rather than removal of existing flowers or fruitlets. Coneva and Cline [9] and Stern and BenArie [10] found that application of GA3 during flower induction and initiation the year prior to the desired response, reduces the number of flowers, and consequently fruit set and the amount of hand thinning required in “Redhaven” peach. González-Rossia et al. [11] were able to demonstrate a similar response for “Springlady” peach and “Zincal 5” nectarine. Reighard et al. [12,13] found applications of 2-chloroethyl-phosphonic acid (ethephon) plus emulsified soybean oil adjuvant applied to peach trees in the dormant stage (January) reduced flowering at bloom, but the results were cultivar dependent affected by temperature at application, rate of oil, and chill hour accumulation. One of the disadvantages of inhibiting flower bud formation or thinning at bloom is the potential risk of frost damage after thinning to the flowers or young fruits which would alter optimum fruit load.
Chemical blossom or fruitlet thinning has the distinct advantage over hand thinning in that it can be done early during fruit ontogeny, well before the period of natural fruit abscission (“June drop”). Several different “blossom” chemicals including endothal, ammonium thiosulfate (ATS), ammonium nitrate, decyl alcohol, armothin, surfactants and lime sulphur have been reported to be effective as flower thinners during the spring season [9,14,15].
The third and most common approach to adjusting the crop load of peaches is between bloom and natural drop using fruitlet abscission inducing chemicals. Fruitlet thinning has the advantage in that it is conducted 14 - 21 d after bloom, when the risk of spring frost has diminished and also after fruit set is more apparent. Many compounds including 2-chloroethane-phosphonic acid (CEPA) [16,17], ethephon [9], napthaleneacetic acid [18-20], and 3-chlorophenoxy-α-propionamide (3-CPA) [21,22] have been used on peaches in the past. Ethephon is one of the more promising compounds [23-26], however, there are concerns over the formation of gummosis or leaf defoliation when used at higher concentrations. Furthermore, the results can be inconsistent because of cultivar response, environmental conditions, geographical location, tree age and cultivar [8].
Thinning peaches with ethephon in Canada has neither been reported previously nor practiced commercially, in part because it is not labelled for this purpose. Given the previous mentioned concerns about air temperatures during the fruit set period can be extremely variable in southern Ontario, which experiences predominantly a humid continental climate. The Ontario peach and nectarine industry is on the northern frontier for commercial production. The Niagara tender fruit belt climate is characterized by harsh winters (extreme minimum –26˚C), short growing seasons (182 frost free days), and cool summers (30-yr normal average temperature for June, July, and August is 18.1˚C, 21.7˚C, 20.7˚C) [27]. Under these conditions, tree growth and vigour are limited. The purpose of this study was to determine the effectiveness of ethephon in inducing fruitlet abscission of peaches in a northern peach producing region that has growing conditions and cultivars, that are distinctly different from the more arid and warmer regions of the United States and Europe.
2. Materials and Methods
This experiment was conducted in 2005 and repeated in 2007 on fiveand six-year old, respectively “Redhaven” peach trees (Prunus persica L.) on Bailey rootstock. Trees were spaced at 4 × 4 m (625 trees ha–1) and trained using an open centre orchard system.
In 2005, trees were located in a commercial orchard in Beamsville, Ontario (43˚11' 32.92"N latitude, 79˚28' 59.30"W longitude) containing a Toledo series (Aquaic Hapludalf) soil, which consisted of 15 - 40 cm loamy textures over lacustrine silty clay with imperfect drainage (Kingston and Presant, 1989). In 2007, trees were located in a nearby orchard (43˚10' 57.74"N latitude and 79˚24'11.47"W longitude) were used containing a Tavistock series soil which consisted of 40 - 100 cm reddishhued loamy texture over clay loam till [28].
In 2005, six treatments were applied in a randomized complete block design with four replications with two trees per experimental unit. The trees were sprayed with a radial airblast sprayer (GB Irrorazione Diserbo, Model Laser P7, Italy) at a volume of ~2 L·tree–1. Treatments were as follows: 100, 200, 400 mg·L–1 ethephon (2-chloroethyl-phosphonic acid, Bayer CropScience Inc, Calgary, AB) and 500 mg·L–1 aminoethoxyvinyl-glycine (AVG) (Valent BioSciences Corp, Walnut Creek, CA). A hand thinned and water only spray treatments served as controls. In 2007, an additional 50 mg·L–1 ethephon treatment was added to the experiment and treatments were replicated six times. All spray treatments, including the water alone, included 0.10% (v/v) Regulaid surfactant (Kalo Inc, KS, USA). The purpose of the AVG treatment was to study the association between ethylene and fruit abscission, as AVG has been previously demonstrate to be a ethylene biosynthesis inhibitor [29,30].
Trees were sprayed 10 June, 2005 and 13 June, 2007 when fruits were about 15 mm - 20 mm in diameter (30 DAFB). In order to minimize spray drift, experimental units were separated at least with one guard tree. Hand thinning was carried out 45 - 55 DAFB (6 July, 2005 and 27 June, 2007; 30 - 40 mm in diameter) by spacing fruits ~15 cm apart.
Fruits were harvested weekly over a three week period based on fruit background (change from green to yellow) and the appearance of red surface blush color. Fruit set, total yield, mean fruit weight, fruit size, fruit firmness and total soluble solids were measured per experimental unit and crop density per tree was calculated. Fruit set was determined by marking four primary scaffold limbs on each tree and counting the number of fruits before treatment imposition and after fruit abscission (three weeks later). Twenty randomly selected fruits free of blemishes, were selected per tree for fruit quality determination. Fruit flesh firmness was measured twice per fruit from two opposite locations on the equatorial region of the fruit after the skin was removed, using a penetrometer (Fruit Texture Analyser, model GS-14; GUSS, South Africa) equipped with a 7.9 mm probe. Total soluble solids was measured using a digital refractometer (Abbe Model 10450; American Optical Corporation, Buffalo, NY) from the juice extracted after fruit firmness measurements. Fruit size distribution for each treatment was determined using a subsample of ~5 kg of fruit per tree based on the following fruit size categories: <57, 57 - 64, 64 - 70, 70 - 83, and >83 mm in fruit diameter. Trunk circumference was measured 30 cm above the ground in the spring of each year. The incidence of gummosis on tree trunk and branches was rated using a 5 point scale (1 = no gummosis, 3 = moderate, 5 = severe gummosis).
Statistical analyses were carried out using Fisher’s protected analysis of variance (PROC GLM) in SAS software (Ver 9.02, SAS Institute, Cary, NC). The normality of the data was evaluated using PROC UNIVARIATE on data residuals. Percent and rating data were transformed using the square root function. Mean comparisons were performed using Least Significant Difference.
3. Results
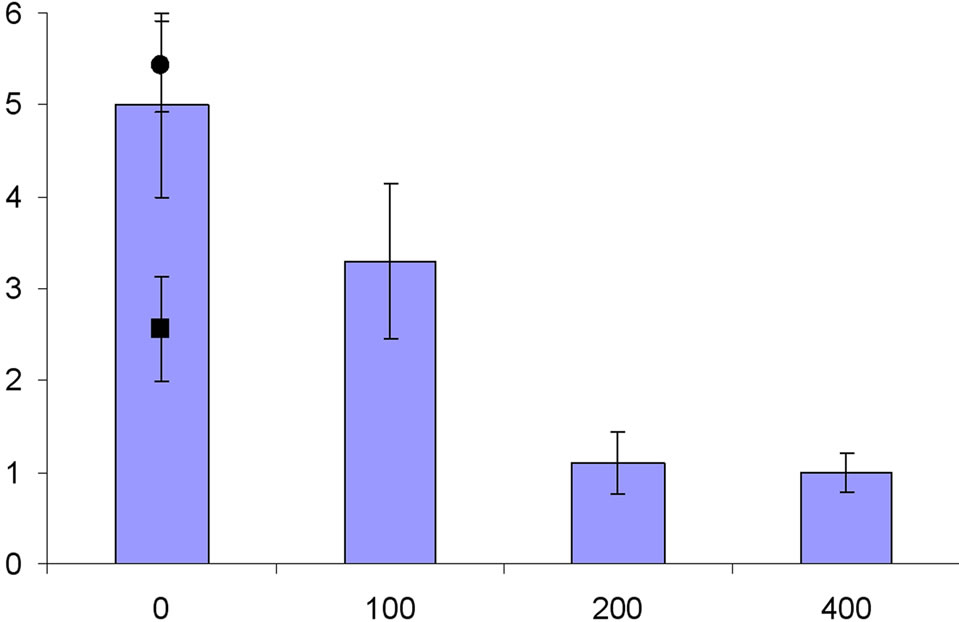
Treatment with ethephon in both 2005 and 2007 significantly reduced fruit set compared to the control and AVG treatments. Increasing concentrations of ethephon was linearly associated with reduced fruit set (Figure 1), with an 18% - 20% reduction in set between 0 and 300 mg/L ethephon. Final fruit set on trees treated with AVG was not statistically different from the water-sprayed control
 (a)
(a) (b)
(b)
Figure 1. Effect of ethephon and AVG on crop density of “Redhaven” peach in 2005 (a) and 2007 (b). Circle and square symbols at 0 mg·L−1 are the crop densities for AVG and hand-thinned treatments, respectively. The error bars are the standard error of the means for each treatment.
treatments. As a result, higher rates of ethephon resulted in significant reductions in yield. Treatment with AVG resulted in yields similar to the water-spray control treatment in both years. Mean fruit weight increased in a linear fashion with higher concentrations of ethephon. Again, there was no significant difference in mean fruit weight between the AVG and water-spray control treatment. Crop density, expressed as the number of fruit per crosssectional area, was significantly reduced with increasing ethephon concentration (Figure 1). In contrast, AVG treated trees numerically had the highest crop density in both years, but this effect was not statistically different from the water control treatment (Table 1).
The effect of ethephon and AVG on fruit size distribution was measured in 2007 (Table 2). Higher rates of ethephon led to an increase in the percentage of larger-sized fruits compared to the AVG and water-sprayed control treatments; where the later had the greatest percent of unmarketable fruits (less than 57 cm diameter). Increasing rates of ethephon, from 100 to 400 mg·L–1 led to an increase in the percentage of fruit in the 57 - 63, 64 - 69, 70 - 83, and >83 mm size categories.
Fruit quality, as determined by firmness and soluble solids, was significantly influenced by the ethephon but not the AVG treatments (Table 3) when measured on the same harvest date. Increasing rates of ethephon resulted in a softer fruit with higher soluble solids. These same treatments resulted in advance ripening 10 to 16 d earlier than the hand-thinned controls (data not shown). Because of the direct effect of ethrel on crop load and subsequently fruit size, determination of firmness and soluble solids from similar sized fruit from the same stage of physiological maturity was not possible in order to adequate measure the direct treatment effect on these quality parameters. Gummosis, a known adverse response to ethephon applications [31,32], was measured on tree branches and tree trunks at harvest time in 2007. The highest concentration of ethephon (400 mg·L–1) resulted in a significant increase (P ≤ 0.0001) in the amount of tree trunk and branch gummosis (Figure 2). Leaf abscission, another potential side effect of foliar ethephon sprays, was also observed and with increasing severity at higher concentrations of ethephon (data not shown). Trees were however able to recover approximately three weeks after ethephon application.
4. Discussion
Fruit abscission was linearly and positively related to ethephon concentrations up to 400 mg·L–1. Concentrations between 100 to 200 mg·L–1 would be considered commercially acceptable without sacrificing excessive marketable yield, as was observed at the higher rates. With each incremental increase of 1 mg/L ethephon (between 0 and 400 mg/L), there was a 18% - 20% reduction
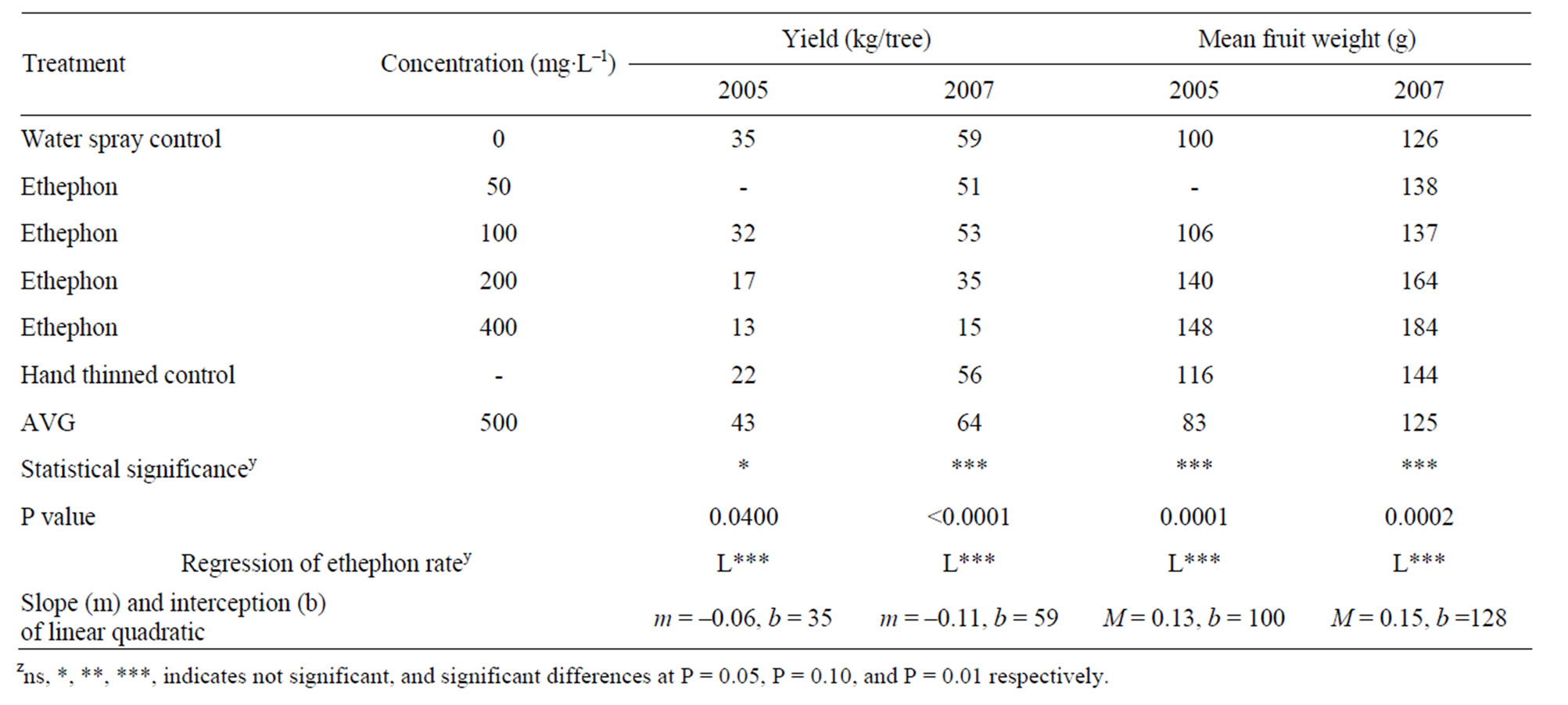
Table 1. Total yield per tree and mean fruit weight of “Redhaven” peaches as affected by different ethephon and AVG treatments in 2005 and 2007z.
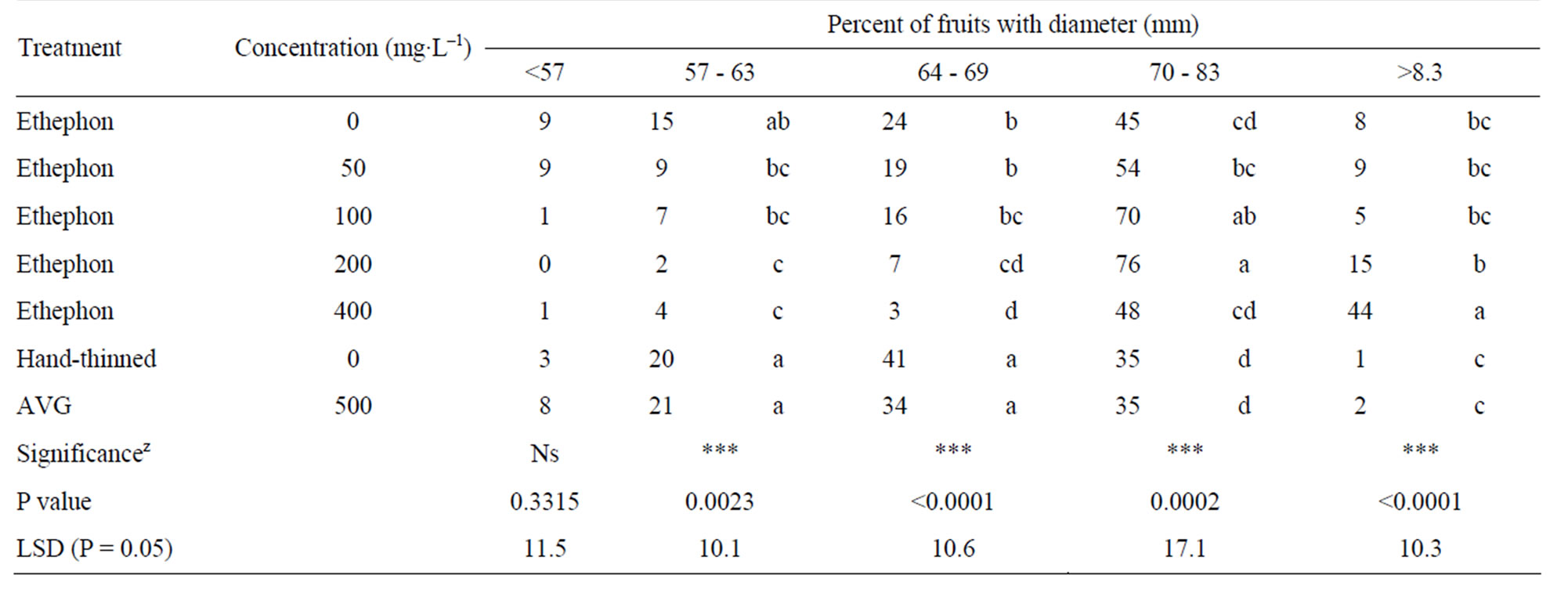
Table 2. Effect of ethephon and AVG on fruit size distribution of “Redhaven” peaches at harvests in 2007.

Table 3. Effect of ethephon and AVG on fruit firmness and soluble solids of “Redhaven” peaches. 16 August, 2005.

Figure 2. Ethephon and AVG effect on trunk and branch gummosis in 2007 (1 = no gummosis, 3 = medium, 5 = high severity). The error bars are the standard error of the means for each treatment.
in fruit set, a 13% - 15% increase in fruit size, and a 6% - 11% reduction in yield over the two years of the study. Clearly, the practicality of selecting ethephon rates would need to be selected based on four factors commercially: cultivar abscission response to ethephon, the target fruit size desired (determined largely by the market), the incremental returns realized for larger sized fruit, and the associated decline in yield with reduced fruit set.
With respect to the effect of ethephon on fruit abscission, in Australia, ethephon applied 39 - 53 DAFB at concentrations of 40 - 100 mg·L–1 resulted in successful thinning of the peach cultivars “Golden Queen”, “Wight” and “Keimos” [33]. In India, applications to “July Elberta” peaches at 250 mg·L–1 resulted in 75% fruit thinning and an increase in fruit weight [25] while applications of 200 - 300 mg·L–1 to “Redhaven” were efficacious for fruit thinning and produced fruit with comparable size of those from trees that were hand-thinned [26].
At higher concentrations of ethephon, fruit maturity was advanced by about 14 d weeks. Similar results were observed on “Redskin” and “Redhaven” peaches that were treated with ethephon 50 d before harvest [8]. Ethephon applications in this study were made about two months before fruit maturity, and considering the half life of ethephon at pH = 7 is 48 hr [34], the observed results on fruit maturity are more likely a reduced crop load effect than a direct effect of ethephon on fruit firmness and soluble solids. In order to separate out the crop load effect from ethephon effects on fruit maturity, additional studies should be conducted to compare ethephon treatments to hand-thinned controls that have a similar crop load. Measurements of ethephon evolution from treated fruits and from hand thinned control fruits, as well as the investigation of genes involved in the ethylene biosynthesis pathway could also reveal if the ethephon-induced advancement in maturity was a direct effect of ethephon or a result of lower yields in trees treated with 200 - 400 mg·L–1 ethephon.
There are concerns over the use of ethephon in a number of tree fruit crop species including peaches. One of the primary concerns is ethephon’s inconsistency when used as a thinning agent [2,3]. Ethephon degradation and subsequent release of ethylene gas is highly temperature dependent [35,36], at least outside the window of 21˚C - 32˚C [37]. Another concern, a direct consequence of thinning either by hand or with ethephon, is the decrease in total yield per tree. This however can be partially offset with concomitant increases in fruit size, fruit weight, and a higher percentage of fruits in larger size categories—all contributing to potentially higher marketable and economic yields (unless the trees are “over-thinned”).
Gummosis is another concern when using ethephon on Prunus species, particularly at higher concentrations [31, 32]. In the present study, ethephon application at 400 mg·L–1 resulted in significant gummosis of the trunk and primary scaffold limbs. Since 400 mg·L–1 most likely exceeds the optimum concentration for thinning, because its marked negative effect on yield, ethephon at commercial rates of 100 - 150 mg·L–1 should be inconsequential when used on “Redhaven”. However, other peach cultivars may respond differently, and therefore caution should be exercised for ethephon use on new cultivars. Pre-mature leaf yellowing and abscission as a result of ethephon treatment in peach has been reported previously [3] and was also observed in this experiment. However, trees developed a full canopy of new leaves approximately three weeks after application with no apparent long-term effects on the health of the trees, but long-term effects should be considered in future studies.
In both years of this experiment, AVG had no effect on the fruit abscission in comparison with the water only control treatment. It was hypothesized, based on the mechanism that AVG inhibits ethylene production at the biosynthesis level [29,30,38], that trees treated with AVG would have increased fruit set (less natural fruit abscission) in comparison with those left untreated. However, the data indicate that foliar sprays of 500 mg·L–1 AVG had no effect on fruit set when applied approximately 30 DAFB (~15 mm fruit size). At this phenological stage, perhaps elevated concentrations of ethylene-mediated synthesis of polygalacturonase enzymes had already been initiated in the fruit abscission zone [39]. Further research to block ethylene perception at the receptor level using 1-methylcyclopropene would be of interest to further test the association of ethylene with fruit abscission.
In conclusion, these results indicate that ethephon can be used at 100 - 200 mg·L–1 to induce fruit abscission with no negative effect on tree gummosis. Using ethephon as a thinning agent can reduce hand-thinning labor cost. Collectively, the literature suggests that the thinning response of peaches with ethephon may vary by both cultivar and environmental conditions during and following application. Further studies are necessary to evaluate the cultivar response to ethephon in various growing regions. It also should be mentioned that these results are only for “Redhaven” peaches and the optimum range should be evaluated for other cultivars.
5. Acknowledgements
We gratefully acknowledge the technical assistance of Ms. Debbie Norton. Financial and in-kind support has been provided through the University of Guelph and the Ontario Ministry of Agriculture, Food and Rural Affairs, and Mr. Jamie Warner, Warner Orchards for providing the commercial orchards in which to conduct this research.
REFERENCES
- Anonymus, “OMAFRA, Horticultural Statistics in: Ontario Ministry of Agriculture,” Food and Rural Affairs, Toronto, 2007.
- R. E. Byers, “Flower and Fruit Thinning and Vegetative: Fruiting Balance,” In: D. C. Ferree, Ed., Apples: Botany, Production and Uses, CAB International, Oxon, 2003, pp. 409-438. doi:10.1079/9780851995922.0409
- R. E. Byers, G. Costa and G. Vizzotto, “Flower and Fruit Thinning of Peach and Other Prunus,” Horticultural Reviews, Vol. 28, 2003, pp. 351-392.
- R. Scorza and W. Sherman, “Peaches,” In: J. Janick and M. Jn, Eds., Fruit Breeding: Tree and Tropical Fruits, John Wiley, New York, 1996, pp. 325-440.
- A. Zanchin, C. Bonghi, G. Casadoro, A. Ramina and N. Rascio, “Cell Enlargement and Cell Separation during Peach Fruit Development,” International Journal of Plant Sciences, Vol. 155, 1994, pp. 49-56. doi:10.1086/297146
- K. Slingerland, “Establishment and Production Costs for Tender Fruit in Ontario,” In: M. Jn, Ed., 2006 Economic Report, Ontario Ministry of Agriculture, Food and Rural Affairs, K. Slingerland and Queen’s Printer for Ontario, Toronto, 2006.
- M. N. Westwood, “Temperate-Zone Pomology: Physiology and Culture,” Timber Press, Portland, 1993.
- G. E. Stembridge and C. E. Gambrell, “Thinning Peaches with Bloom and Postbloom Applications of 2-Chloroethylphosphonic Acid,” Journal of American Society for Horticultural Sciences, Vol. 96, 1971, pp. 279-283.
- E. Coneva and J. A. Cline, “Gibberellic Acid Inhibits Flowering and Reduces Hand Thinning of ‘Redhaven’ Peach,” HortScience, Vol. 41, No. 7, 2006, pp. 1596- 1601.
- R. A. Stern and R. Ben-Arie, “GA3 Inhibits Flowering, Reduces Hand-Thinning, and Increases Fruit Size in Peach and Nectarine,” Journal of Horticultural Science and Biotechnology, Vol. 84, No. 2, 2009, pp. 119-124.
- D. Gonzalez-Rossia, M. Juan, C. Reig and M. Agusti, “The Inhibition of Flowering by Means of Gibberellic Acid Application Reduces the Cost of Hand Thinning in Japanese Plums (Prunus Salicina Lindl.),” Scientia Horticulturae, Vol. 110, No. 4, 2006, pp. 319-323. doi:10.1016/j.scienta.2006.07.022
- G. L. Reighard, D. R. Ouellette and K. H. Brock, “PreBloom Thinning of Peach Flower Buds with Soybean Oil in South Carolina,” Acta Horticulturae, Vol. 727, 2006, pp. 345-351.
- G. L. Reighard, D. R. Ouellette and K. H. Brock, “Peach Flower Bud Thinning by Dormant Season Applications of Ethephon Plus Vegetoil,” In: Anonymous, Ed., Plant Growth Regulator Society of America, Research Triangle Park, 2006, pp. 220-224.
- C. Tsipouridis and T. Thomidis, “Evaluation of the Effectiveness of Armothin for Chemical Peach Thinning,” Australian Journal of Experimental Agriculture, Vol. 45, No. 1, 2005, pp. 103-105. doi:10.1071/EA03078
- R. E. Byers, “Effects of Bloom-Thinning Chemicals on Peach Fruit Set,” Journal of Tree Fruit Production, Vol. 2, No. 2, 1999, pp. 59-78. doi:10.1300/J072v02n02_06
- D. W. Buchanan and R. H. Biggs, “Peach Fruit Abscission and Pollen Germination as Influenced by Ethylene and 2-Chlorothan Phosphonic Acid,” Journal of the American Society for Horticultural Science, Vol. 94, 1969, p. 3.
- L. J. Edgerton and W. J. Greenhalgh, “Regulation of Growth, Flowering, and Fruit Abscission with 2-Chloroethanephosphonic Acid,” Journal of the American Society for Horticultural Science, Vol. 4, 1969, pp. 327-329.
- O. A. Bradt, “Chemical Thinning of Peaches,” Biennial Report, Vineland Horticultural Experimental Station and Horticultral Products Laboratory, 1952, pp. 19-25.
- S. J. Leuty and M. J. Bokovac, “The Effect of Naphthalene Acetic Acid on Abscission of Peach Fruits in Relation to Endosperm Development,” Journal of the American Society for Horticultural Science, Vol. 92, 1968, pp. 124-133.
- P. B. Lombard and A. E. Mitchell, “Anatomical and Hormonal Development in Redhaven Peach Seeds as Related to the Timing of Naphthaleneacetic Acid for Fruit Thinning,” Proceedings of the American Society for Horticultural Science, Vol. 80, 1962, pp. 163-172.
- G. C. Martin and M. Nelson, “The Thinning Effect of 3-Chlorophenoxy-α-propionamide (3-CPA) in Paloro Peach,” HortScience, Vol. 4, No. 3, 1969, pp. 206-208.
- G. E. Stembridge and C. E. Gambrell, “Thinning Peaches with 3-Chlorophenoxy-a-Propionamide,” Journal of the American Society for Horticultural Science, Vol. 94, No. 3, 1969, pp. 570-573.
- N. Veinbrants and J. F. Hutchinson, “Studies on the Use of 2-Chloroethylphosphonic Acid (Ethephon) as a Thinning Agent for Jonathan Apples,” Australian Journal of Experimental Agriculture and Animal Husbandry, Vol. 16, No. 83, 1976, pp. 937-942. doi:10.1071/EA9760937
- G. E. Stembridge and C. D. Gambrell Jr., “Thinning Peaches with Ethylene-Releasing Chemicals,” Acta Horticulturae, Vol. 80, 1978, pp. 257-263.
- M. R. Sharma and D. R. Gautam, “Note on Ethephon for Thinning Peach Fruit,” Indian Journal of Agricultural Sciences, Vol. 51, 1981, pp. 362-364.
- N. Sharma, R. P. Singh and B. Singh, “Effects of Chemical and Manual Thinning on the Productivity and Fruit Size of Redhaven Peach,” Indian Journal of Horticulture, Vol. 60, 2003, pp. 239-243.
- Environment-Canada, “Canadian Climate Normals 1971- 2000—Vineland Station,” 2010. http://www.climate.weatheroffice.gc.ca/climate_normals/index_e.html
- M. S. Kingston and E. W. Presant, “Soil of the Regional Municipality of Niagara, Report No. 60,” Ontario Ministry of Agriculture, Food and Rural Affairs, Guelph, 1989.
- R. D. Belding and G. R. W. Lokaj, “Aminoethoxyvinylglycine Treatment of Peach Fruit Reduces Ethylene Production and Softening,” HortScience, Vol. 37, No. 7, 2002, pp. 1065-1068.
- A. M. Bregoli, S. Scaramagli, G. Costa, E. Sabatini, V. Ziosi, S. Biondi and P. Torrigiani, “Peach (Prunus persica) Fruit Ripening: Aminoethoxyvinylglycine (AVG) and Exogenous Polyamines Affect Ethylene Emission and Flesh Firmness,” Physiologia Plantarum, Vol. 114, No. 3, 2002, pp. 472-481. doi:10.1034/j.1399-3054.2002.1140317.x
- W. C. Olien and M. J. Bukovac, “Interaction between Temperature and Ethylene in Sour Cherry Fruit Abscission,” HortScience, Vol. 17, 1982, pp. 795-796.
- W. C. Olien and M. J. Bukovac, “Ethylene Generation, Temperature Responses, and Relative Biological Activities of Several Compounds with Potential for Promoting Abscission of Sour Cherry Fruit,” Journal of the American Society for Horticultural Science, Vol. 107, 1982, pp. 1085-1089.
- F. J. Gathercole, “Thinning of Cling Peaches with Ethephon in the Riverland Area of South Australia,” Australian Journal of Experimental Agriculture and Animal Husbandry, Vol. 21, 1981, pp. 354-356. doi:10.1071/EA9810354
- J. Efer, S. Müller, W. Engewald, T. Knobloch and K. Levsen, “Indirect GC Determination of Ethephon in Drinking Water by a Combination of Reactive Headspace Sampling with Adsorptive Enrichment/Thermal Desorption,” Chromatographia, Vol. 37, No. 7-8, 1993, pp. 361-364. doi:10.1007/BF02272249
- W. C. Olien and M. J. Bukovac, “Ethephon-Induced Gummosis in Sour Cherry (Prunus cerasus L.). II. Flow Characteristics of Gum Solutions,” Plant Physiology, Vol. 70, No. 2, 1982, pp. 556-559. doi:10.1104/pp.70.2.556
- W. C. Olien and M. J. Bukovac, “Ethephon-Induced Gummosis in Sour Cherry (Prunus cerasus L.). I. Effect on Xylem Function and Shoot Water Status,” Plant Physiology, Vol. 70, No. 2, 1982, pp. 547-555. doi:10.1104/pp.70.2.547
- R. C. Yuan and D. H. Carbaugh, “Effects of NAA, AVG, and 1-MCP on Ethylene Biosynthesis, Preharvest Fruit Drop, Fruit Maturity, and Quality of ‘Golden Supreme’ and ‘Golden Delicious’ Apples,” HortScience, Vol. 42, No. 1, 2007, pp. 101-105.
- L. Alexander and D. Grierson, “Ethylene Biosynthesis and Action in Tomato: A Model for Climacteric Fruit Ripening,” Journal of Experimental Botany, Vol. 53, No. 337, 2002, pp. 2039-2055. doi:10.1093/jxb/erf072
- C. Bonghi, N. Rascio, A. Ramina and G. Casadoro, “Cellulase and Polygalacturonase Involvement in the Abscission of Leaf and Fruit Explants of Peach,” Plant Molecular Biology, Vol. 20, 1992, pp. 839-848. doi:10.1007/BF00027155

