Pharmacology & Pharmacy
Vol.3 No.2(2012), Article ID:18735,8 pages DOI:10.4236/pp.2012.32032
Investigations on the Significance of Inductable Cyclooxygenase in Atherogenesis in Cholesterol-Fed Rabbits
![]()
1Clinic for Small Animal Surgery, Foundation of Veterinary University, Hannover, Germany; 2Institute of Clinical Pharmacology, Medical University in Hannover, Hannover, Germany.
Email: lando13@hotmail.de
Received January 15th, 2012; revised February 19th, 2012; accepted March 6th, 2012
Keywords: Celecoxib; DHEA; Plaques; Prostanoide
ABSTRACT
Atherosclerosis is the most prevalent disease in middle and later years of human life. Heart attacks and strokes are among the most frequent causes of death. New approaches in therapy are the use of NSAID’s (nonsteroidal antiphlogistical drugs) of the new generation (selective inhibitors of COX-2) and DHEA (Dehydroepiandrostenon). The key enzyme of prostaglandin synthesis, the COX-2 isoenzyme, is predominantly found in inflammatory tissue. Out of this results a possible importance of COX-2-inhibitors in prophylaxis of atherosclerosis. Our study intended to examine the significance of COX-2 and the COX-2-formed prostanoids, as well as the significance of COX-2-independent isoprostanes on atherosclerosis. For that purpose we tested the effect of the selective COX-2-inhibitor Celecoxib on rabbits fed with cholesterol and compared this with the effect of the steroid hormone DHEA in 4 groups: healthy control, cholesterol-fed control, DHEA-group and Celecoxib group. In the test prostanoids, nitrate and nitrite were measured by gas chromatograpy/tandem-mass-spectrometry (GC-MS/MS) in 24-hours-collected urine. Additionally we measured cholesterol and triglycerides in plasma. The aortas of the examinated animals were measured optically using a planimetrical method. The measurement of prostanoids, isoprostanes, nitrate and nitrite showed considerable variations and particulary significant differences (p < 0.01) even in the initial values. By the treatment with Celecoxib the rate of atherosclerosis was reduced in a highly significant way in comparison to the cholesterol group and the DHEA-group. Consequently the test demonstrated a significant role of COX-2 in the development of atherogenesis.
1. Introduction
Atherosclerosis is one of the most frequent diseases in middle aged and elder humans. In consequence heart and circulation failure are the most frequent causes of death. According to current research the risk factors for atherosclerosis are preventively affected by treatment of hypertension and by lowering cholesterol levels [1]. Existing atherosclerosis is treated with thrombocyte-aggregationinhibitors like acetylsalicylic acid. New options for treatment are being searched for continuously. Two of the new approaches are the treatment with NSAID’s of the new generation and the treatment with the steroid hormone DHEA [2-5]. Under medication with the steroid hormone DHEA a significant reduction (up to 50% compared to the control group) of plaque size was proven in an animal model [6-9]. Non-steroidal antiinflammatory drugs lead to a significant reduction of the atherosclerosis rate in a model with rabbits [6]. The inhibition of the proinflammatory synthesis of prostanoids in the vascular wall is discussed as a possible anti-atherosclerotic mechanism of NSAID’s [9]. The key enzyme for the synthesis of prostaglandines is cyclooxygenase. Since 1991 a constitutive (COX-1) and an inductive (COX-2) isoenzyme have been distinguished. COX-1 is responsible for the physiological synthesis of prostanoids. COX-2, however, is expressed under influence of cytokines or endotoxin, so it is mainly found in inflammatory tissue [10-12].
The various NSAID’s show differences in their isoenzyme selectivity [6,13]. Newer NSAID’s have a higher COX-2-selectivity [14-18]. Highly selective COX-2- inhibitors like Celecoxib [19] can be of importance in the prophylaxis of atherosclerosis [20] alongside their “classical” indication, the rheumatic diseases, especially where conventional NSAID’s are not suited due to their toxicity (gastric ulcus [17,18,21], gastric perforation, gastrointestinal bleeding) [22]. This possible field of application has been out of focus of the current discussion on cardiovascular risks in highly selective COX-2-inhibitors. According to current research, all NSAID’s bear such a risk. As a common mechanism the NaCl-water-retention is discussed as a typical unwanted effect. In contrast to there are the therapeutically wanted effects of the NSAID’s like the inhibition of inflammatory activity in the atherosclerotic plaques and anti-oxidative effects with consecutive improvement of availability of nitrate monoxide the deficiency of which causes the endothelial dysfunction in atherosclerosis. The objective of the present study was therefore to examine the importance of the COX-2 and the prostanoids generated by this enzyme, as well as the probably COX-independent isoprostanes [23,24] on the development of atherosclerosis.
2. Methods and Materials
2.1. Rabbit Model of Atherosclerosis
The experiment with animals was approved under number 509c-42502-98/91 on 8. august 1998 by “Bezirksregierung Hannover” (Dezernat 504). The rabbits were kept in single cages at specific-pathogen-free-standard. The used protocol for the rabbit model of atherosclerosis is based on the one developed by Bode-Böger et al. [25]. 48 male White New Zealand rabbits bred by Charles River with body weight between 1.7 and 2.7 kg at the beginning of the tests were studied for 12 weeks after a fourteen day period of adaptation with feeding standard pellets (Ssniff KH, company Ssniff Soest) ad libitum. 4 collectives were studied: a healthy control with standard feed and 3 collectives with cholesterol-enriched diet (4 weeks of feeding with 1% cholesterol and the following 8 weeks with 0.5% cholesterol enriched feed ad libitum (Altromin, company Sniff, Soest) (Table 1). The 3 cholesterol-fed collectives were subdivided into a control group, a DHEA-group (oral medication of 100 mg DHEA per day and rabbit, Company Sigma Aldrich) and a Celecoxib-group (oral application of 1/10 capsule Celebrex™, Searle SA per animal) (Figure 1). At the beginning of the study and every 14 days 24-h-urine was collected for the measurement of the isoprostanes, prostanoids [26] and for the measurement of nitrate and nitrite (Table 2). Every 4 weeks the triglycides and cholesterol levels in the plasma were appointed by VetMedLab in Ludwigsburg, Germany. Subsequently the animals were euthanized by intravenous application of ketamin (25 mg/kg, Ketamin 10% WDT) and Eutha 77™ (800 - 1600 mg per animal, Essex). The aortas were extracted, opened longitudinally and conserved in formaline for later plaque measurement [Figure 6].
2.2. Plaque-Planimetry
The aortas of all examined rabbits were opened longitudinally and photographed accordingly. Then the digitali-
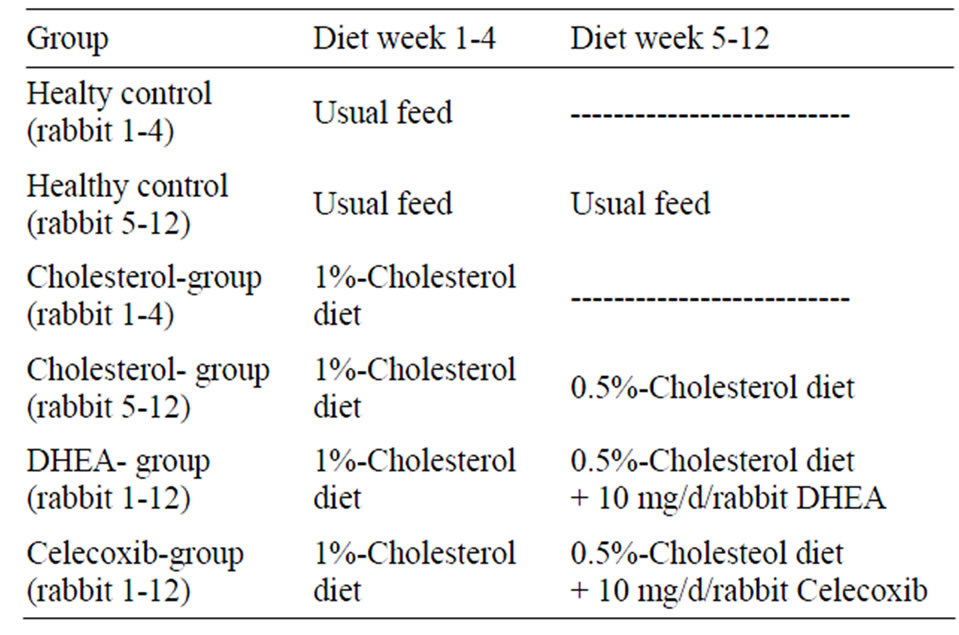
Table 1. Summary of process of study.
sation for the appointment of the plaque part of the total surface of the aortas was performed. The plaque area was illustrated in percentage of total area. The analysis was carried out by using Microsoft Excel™ (Figure 6).
2.3. Appointment of Creatinine in 24-H-Urine
The concentration of creatinine in the 24-h-urine was determined spectrometically by the alkaline reaction of pikrin acid with an automatic analysis equipment (Beckman-Creatinin-Analyzer 2, Beckman, Galway, Ireland).
2.4. Appointment of Prostaglandines and Isoprostanes
The following prostaglandines and isoprostanes were measured in 24-h-urine using gaschromatography/tandemmasss pectrometry: PGE (Figure 2), PGE-MUM (Figure 3) and 8-iso-PGF2α, 2,3-dn-6-keto-PGF1α, 2,3-dn-TXB- 2. The valid method for measurement by Tsikas et al. [27,28] is highly sensitive, very exact but very intensive in laboratory work.
2.5. Analysis of Nitrate and Nitrite in 24-H-Urine According to Tsikas et al. [27]
Neither nitrate nor nitrite levels can be gatherered by gaschromatography directly. Their quantification was therefore performed by using the simultaneous gaschromatographic measurement of the pentafluorobenzyl-derivates of nitrate and nitrite according to the method by Tsikas et al. [29].
2.6. Measurement of Triglycerides and Total Cholesterol Levels in Plasma
These laboratory analyses were performed by the “Institut für klinische Prüfung GmbH, Veterinärmedizinisches Labor (VetMedLab)”, Postfach 1110, 71611 Ludwigsburg, Germany.
The measurement of concentrations was performed in
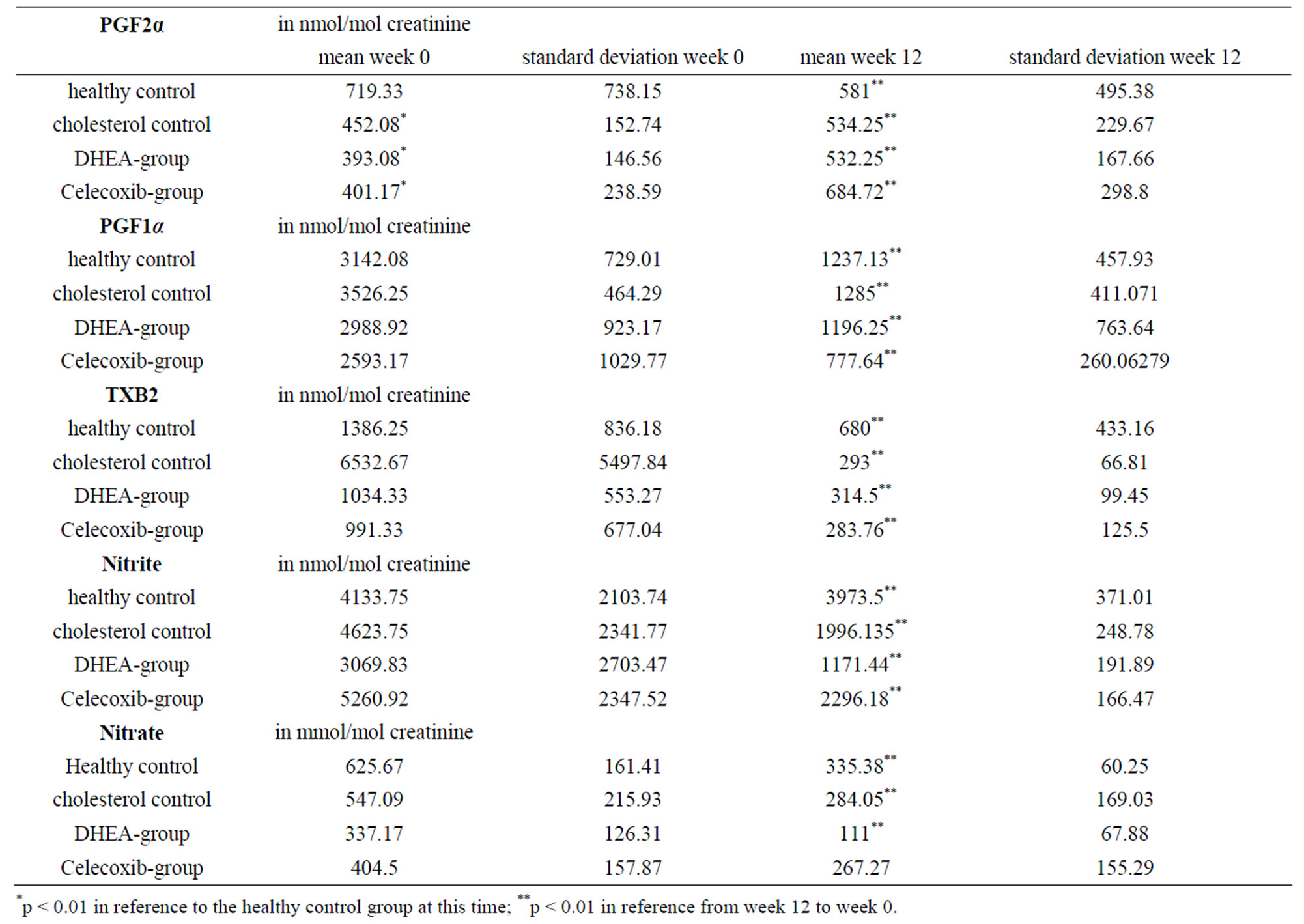
Table 2. Results.
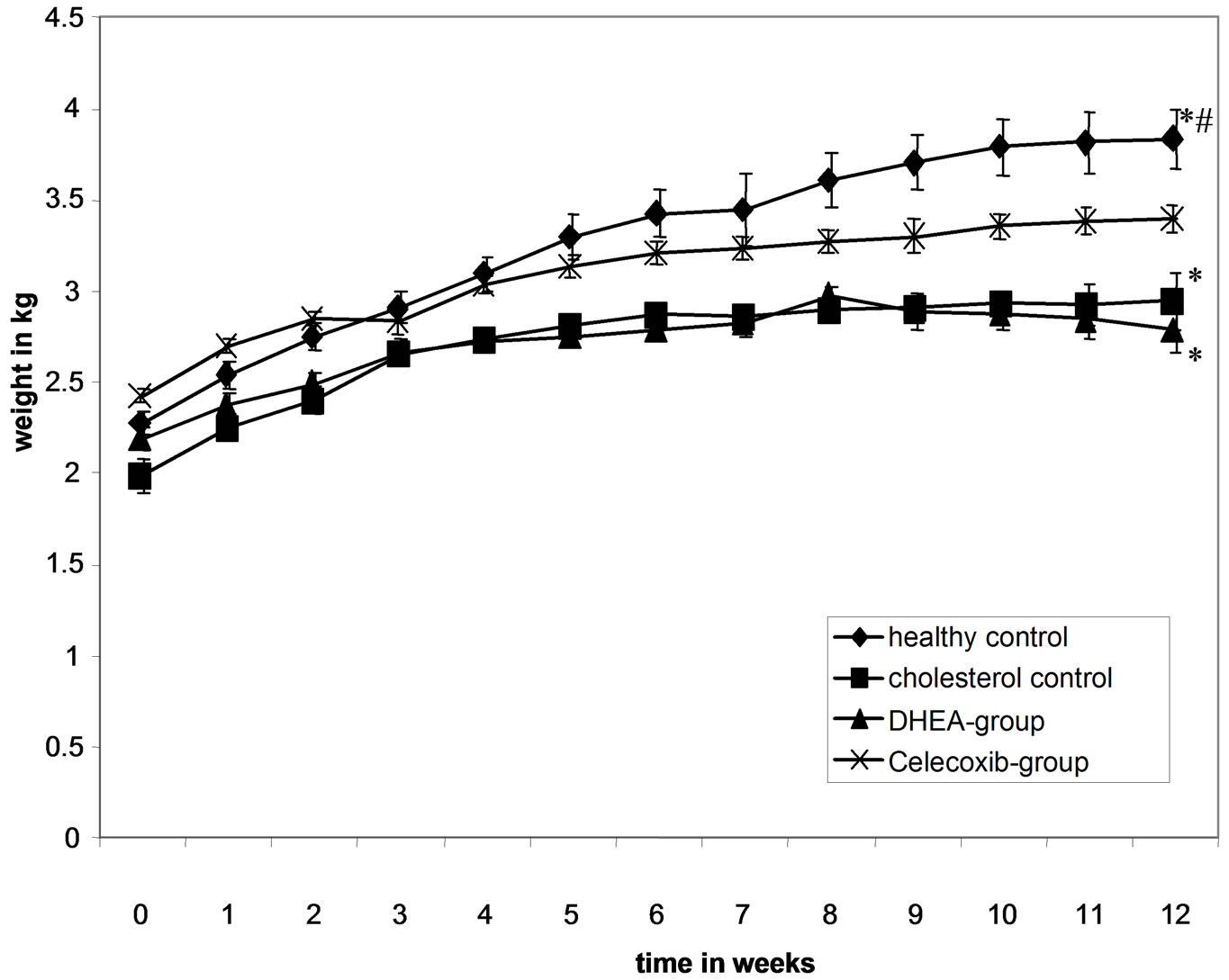
Figure 1. Data for body weight.
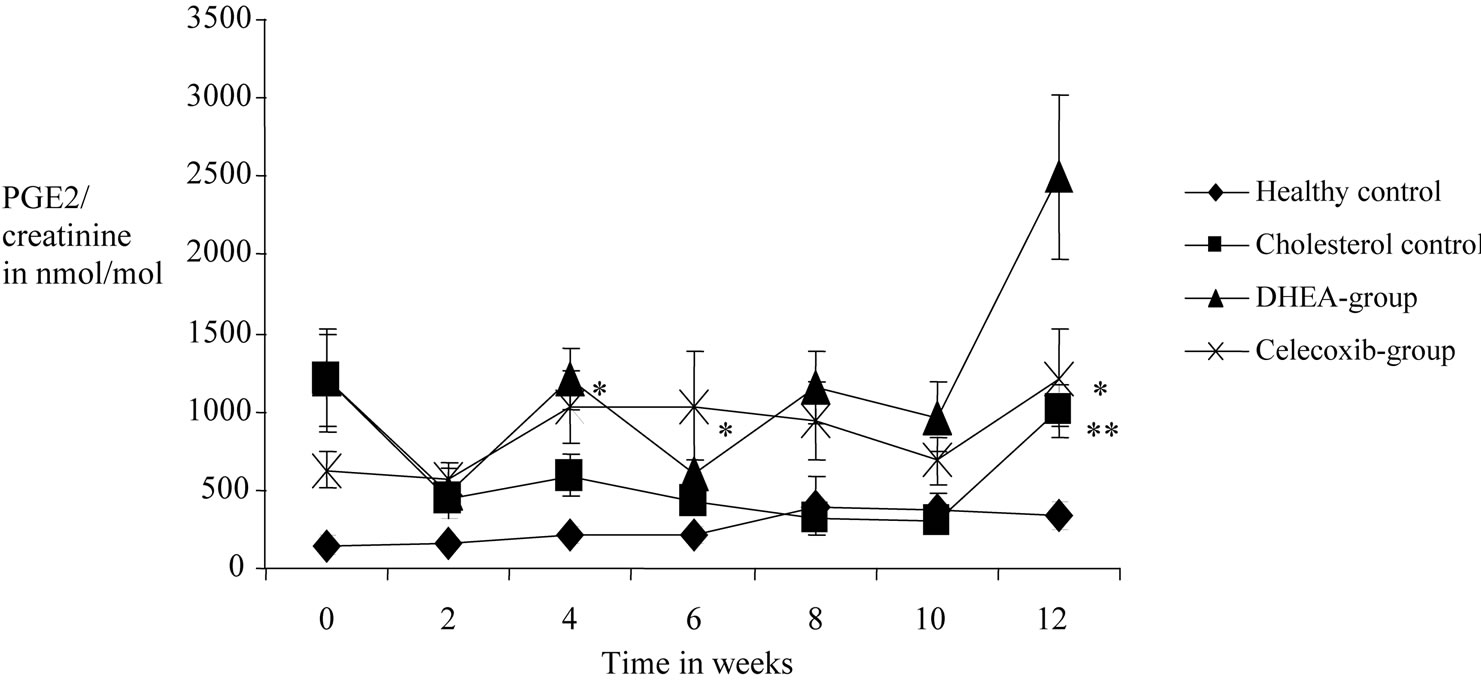
Figure 2. Effects of DHEA and selective COX-2 inhibitor celecoxib on PGE-2 values measured in 24-h-urine of 12 weeks cholesterol-fed rabbits over the time. Samples were taken every second week and analysed by gas chromatography/tandemmass-spectrometry. Each group contained 12 rabbits. One usually fed group and one cholesterol-fed group were tested for control and also shown here. *Highly sinificant difference in reference to the previous point of measurement in the group with p value < 0.01. **Highly significant difference in reference to the healthy control with p value < 0.01.
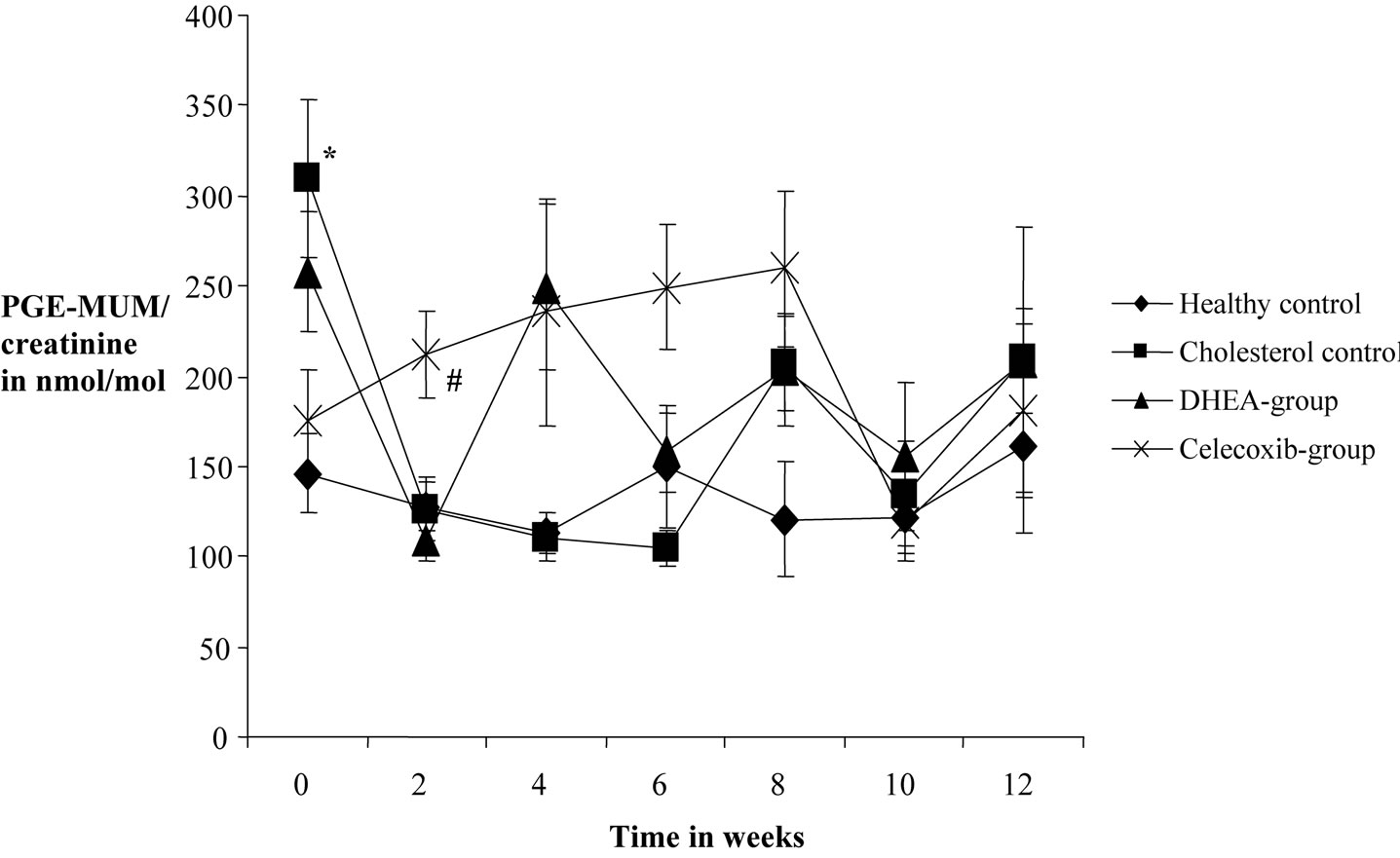
Figure 3. Effects of DHEA and selective COX-2 inhibitor celecoxib on PGE-MUM production measured in 24-h-urine of 12 weeks cholesterol-fed rabbits over the time. Samples were taken every second week and analysed by gas chromatography/ tandem-mass-spectrometry. One usually fed group and one cholesterol-fed group without treatment were tested for control and are also shown here. *Highly significant difference in reference to the healthy control at this time with p value < 0.01. Highly significant difference in reference to cholesterol control group at this time with p value < 0.01. #Highly significant difference in reference to the DHEA-group at this time with p value < 0.01.
mg/dl (Figures 4 and 5).
2.7. Statistical Analysis
The rabbits were randomized in several experimental groups. All performed statistical tests (T-tests) were based on a significance level of p > 0.05. The 2-samplet-test was used for the comparison during the course of the experiment within one experimental collective for dependent samples. In order to compare the different experimental collectives at the same time points in the course of the experiment we used the 2-sample-t-test under assumption of equal variances. The analysis of data was carried out on a Medion™-computer using the software Excel™ by Microsoft, USA.
3. Results
3.1. Physiological Parameters
The animals’ intake of the cholesterol diet was lower, which resulted in significantly (*) less weight gain in the
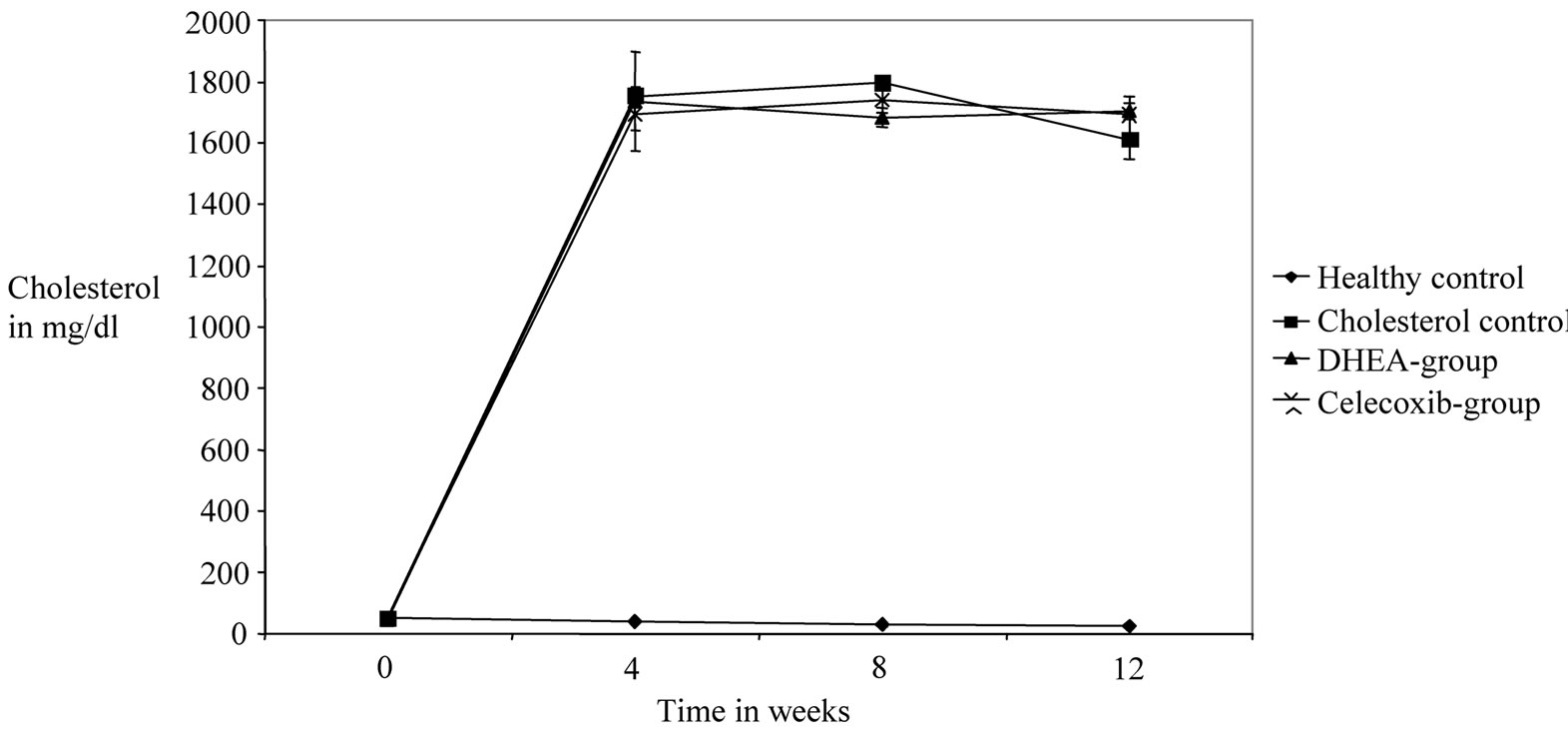
Figure 4. Effects of DHEA and selective COX-2 inhibitor celecoxib on progession of cholesterol values in plasma of 12 week cholesterol-fed rabbits over the time. A usually fed group and a cholesterol-fed control group are also shown. Blood samples were taken every 4 weeks from each rabbit and analysed by VetMEd Labor Ludwigsburg. Each group consists of 12 animals. No significant differences were recognized between the 3 cholesterol-fed groups.
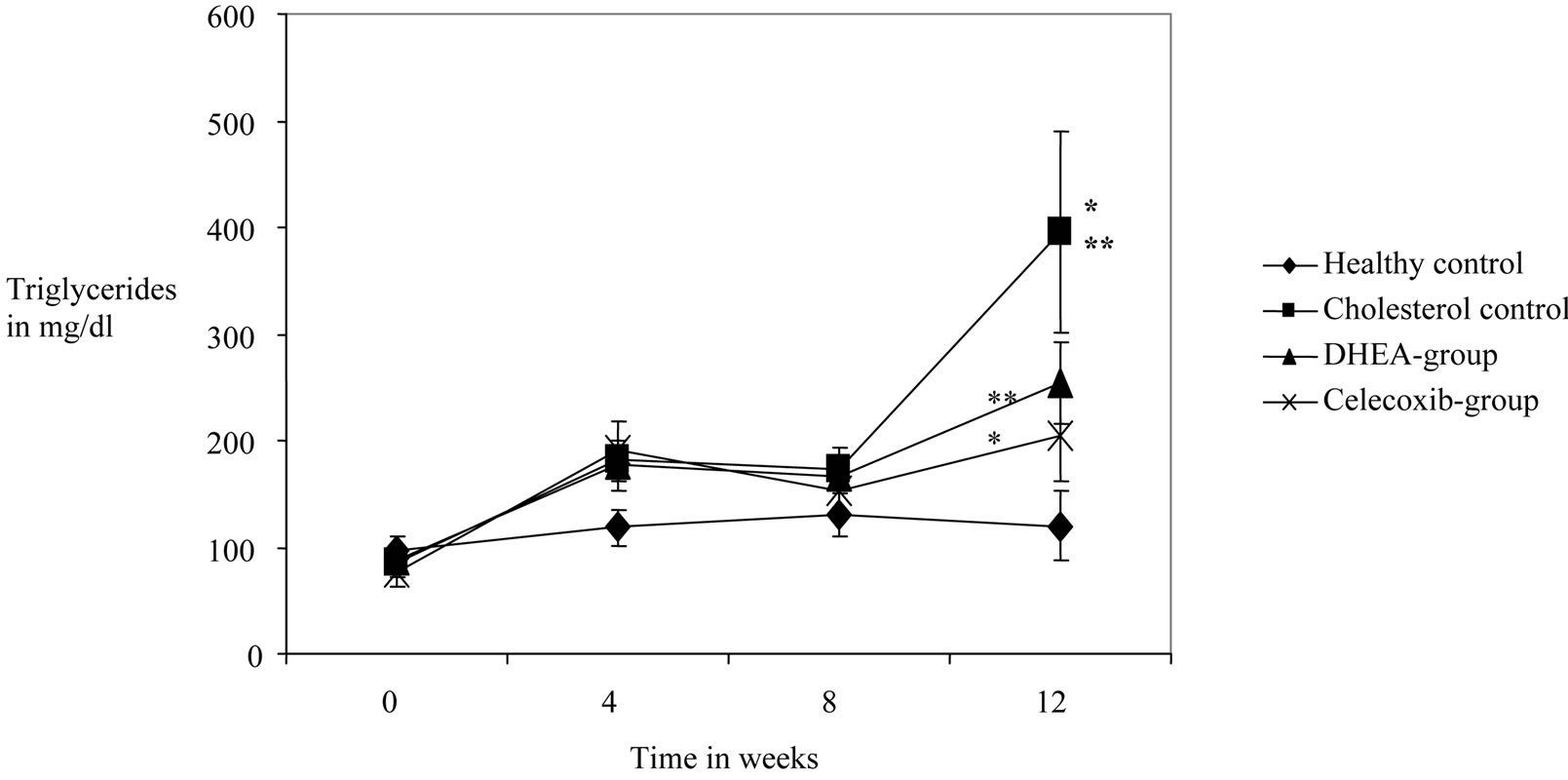
Figure 5. Effects of DHEA an selective COX-2 inhibitor celecoxib on triglycride values measured in plasma of 12 weeks cholesterol-fed rabbits. Every 4 weeks blood samples were taken and triglycerids were measured by VetMedLabor Ludwigsburg. Each group contains 12 rabbits. Control groups with usually fed-rabbits and chlolesterol-fed rabbits are also shown. *Highly significant difference in comparison to the previous point of measurement with p value <0.01. **Highly significant difference in comparison to the healthy control with p value < 0.01.
3 groups fed with cholesterol. The Celecoxib-group, however, exhibited significantly higher (#) gain in body weight than the other 2 cholesterol-fed groups (Figure 1).
3.2. Isoprostanes, Prostanoids, Nitrate Und Nitrite in 24-H-Urine (Table 2)
In conclusion to the determined parameters in the 24-hurine (prostanoids, isoprostanes, nitrate, nitrite) it must be stated that significant differences in the base values, as well as considerable fluctuations in the course of the measurements could be found. Effects caused by varying urine volumes were avoided by relating all values to creatinine levels. Within the 3 isoprostanes (8-iso-PGF2a, 2,3-dn-6-keto-PGF1a, 2,3-dn-TXB2) a significant decrease of all values in the 24-h-urine was shown during the course of experiment from week 0 to wek 12 (p < 0.01**) in all 4 groups. The results for nitrate and nitrite showed considerable fluctuations as well as signifcant differences in base values. The fluctuations of nitrate values proceed in the same direction in all 4 groups. The nitrite values only showed such a parallel progression between the DHEAand the Celecoxib-group.
For PGE and PGE-MUM, value stability was shown within the healhy control group. There were no significant changes during the course of the study. In the other 3 collectives again considerable fluctuations and distributions could be found. As before, there were already significant differences between the groups in the base values (Figures 2 and 3).
3.3. Measurements in Blood and Plasma (Figures 4 and 5)
For the identified parameters in plasma the following results could be shown: There were no significant differences in the cholesterol and triglyceride levels in the base values of the 4 groups. While the cholesterol values in the healthy group were lowered significantly, the levels in the other three groups increased in a highly significant manner (p < 0.001) during the course of the study. Between the 3 cholesterol-fed groups no significant differences could be found. Only a small (non-significant) increase of triglyceride values could be found in the healthy control. The 3 cholesterol-fed groups showed a significant increase in TGL values. The cholesterol control group demonstrated significantly higher values than the DHEA-group and a highly significant increase in values compared to the Celecoxib-group. A significant influence of the cholesterol diet was shown for cholesterol and TGL values in the plasma. Also the medication with DHEA and Celecoxib resulted in a significant, even highly significant decrease in TGL values toward the end of the experiment/study in week 12.
3.4. Plaques-Planimetry (Figure 6)
The healthy control group showed a value of 0% plaque percentage/rate. The cholesterol-fed control group had an average of 81.42%, the DHEA group 80.69% and the Celecoxib group showed an average of 69.39%. Figure 2 illustrates the statistical implications. The 3 cholesterolfed collectives/groups had highly significant higher plaque rates than the healthy control group. The average of the DHEA was numerically lower than the average in the cholesterol control. In comparison to the cholesterol group it was not significantly lower. The Celecoxib group, however, had a highly significant lower plaque rate than the cholesterol controland the DHEA-group. Consequently, a highly significant decrease of plaque formation by medicating with Celecoxib could be determined.
4. Discussion
In our study we were able to prove that a highly significant reduction of atherosclerosis rates (plaque rates) can be achieved by medication with the selective COX-2 inhibitor Celecoxib [Figure 6]. This, however, could not be shown for medication with DHEA, as it has been shown as to be possible in other animal experimental studies [7]. The physiological parameters lead us to the following conclusions: The poorer taste of the cholesterol diet resulted in a decreased intake, which in return resulted in significantly lower weight gain in the 3 cholesterol-fed groups. By medicating with Celecoxib, however, significantly higher weight gains in comparison to the other 2 cholesterol groups could be achieved (Figure 1). The parameters (Isoprostane, nitrate, nitrite, PGE2 und PGE-MUM) measured in the urine showed a normal variance like in other studies [30-32]; there were no differrences between the groups. All of the parameters were placed in relation to creatinine to avoid the effects of varying urine volumes [28,33]. The regularity of the
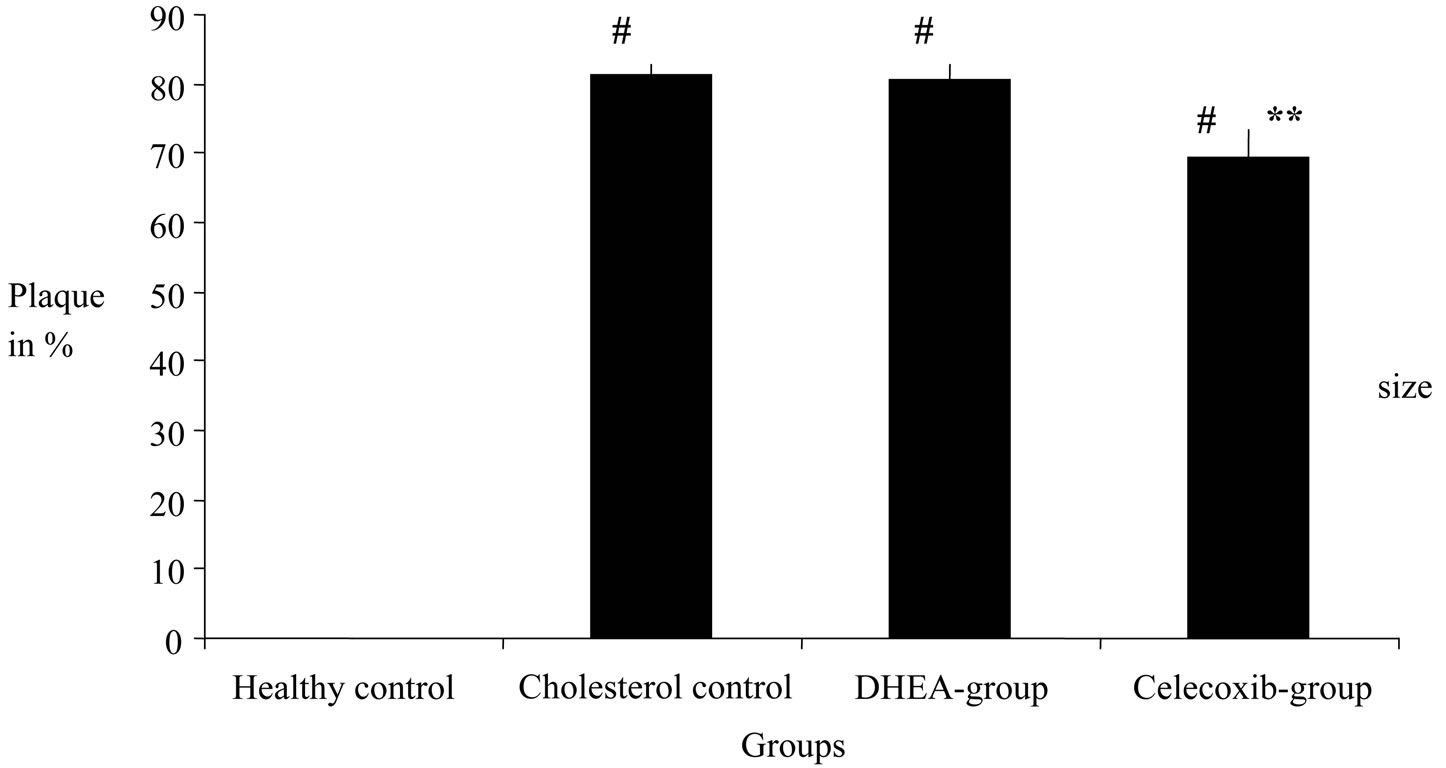
Figure 6. Effects of DHEA and selective COX-2 inhibitor celecoxib on atherosclerotic plaque size by 12 week cholesterol-fed rabbit aorta. Plaques were digitally measured of formalin fixed aortas. A usually-fed group and a cholesterol-fed group without treatment are also shown. Each bar represents the average of 12 rabbits. **Highly significant difference in comparison to the cholestrol control with p value of <0.01. #Highly significant difference in comparison to the healthy control with p value of <0.01.
fluctuations in the nitrate values stand out in all 4 groups as well as for the nitrite values in the DHEA and Celecoxib-group. This phenomenon could not be explained by our study. The constant PGE-2 and PGE-MUM values in the healthy control group and the considerable fluctuation and dispersion of the values in the other 3 groups may enable the finding of a causal influence of a cholesterol diet like other studies were able to [25] (Figures 2 and 3). The fluctuation of the other parameters measured in the urine make this seem unlikely. As only the laboratory parameters triglycerides and total cholesterol determined in the plasma were suitable for a statistical conclusion, we were unable to reconstruct the exact mechanism of the reduction of the atherosclerosis rate by COX-2 inhibition. To further understand this mechanism, we recommend future studies with larger collectives, the use of special metabolic cages and the immediate analysis of the urine samples. As expected the total concentration of cholesterol in the 3 cholesterol-fed collectives/ groups was highly significant higher than in the healthy control like in other studies too [25] (Figure 4). Within the collective of the healthy control a significant reduction in values was shown from week 0 to week 12, which could not be demonstrated in the other study collectives. This only confirms the triggering of atheroscleroris by feeding rabbits with a cholesterol diet [1,25], but does not explain the reduction of atherosclerosis through the COX inhibitor Celecoxib. The triglyceride values lead us to certain indications for the cause of the reduction in atherosclerosis—all 3 study collectives that were fed with cholesterol showed highly significant higher values than the healthy control. The DHEA group showed significantly lower triglyceride values in week 12 than the cholesterol control group, and the Celecoxib groups showed even highly significant lower values than the cholesterol control (Figure 5). Thus it could be demonstrated that a significant reduction of atherosclerosis can be obtained by treatment with selective COX-2 inhibitors (in our case Celecoxib) (Figure 6), which in return reduce the triglyceride values in plaque in a highly significant manner. Other studies showed these conclusions for DHEA [7].
5. Conclusion
In our study we were able to demonstrate that COX-2 plays a considerable role in the mechanisms of atherosclerosis, as a statistically highly significant reduction of the rate of atherosclerosis was achieved through treatment with the COX-2 inhibitor Celecoxib. A significant reduction of the rate of atherosclerosis by treatment with DHEA could not be shown in our study, as has been shown previously in other studies [7] (Figure 6). We also found out that the triglycerid values in the plasma could be significantly lowered by medication with Celecoxib (Figure 5). This may open a new perspective on the influence of the COX-2-inhibitor Celecoxib on atherogenesis, as the reduction of the count of triglycerides in plasma may cause fewer lipids to be bound in atherosclerotic lesions. The relevance of prostanoids and of isoprostanes importance remains unclear in this context.
REFERENCES
- R. A. Cohen, K. M. Zitnay, C. C. Haudenschild and L. D. Cunningham, “Loss of Selective Endothelial Cell Vasoactive Functions Caused by Hypercholesterinemia in Pig Coronary Arteries,” Circulation Research, Vol. 63, No. 5, 1988, pp. 903-910.
- E. Barret-Connor and K. T. Khaw, “A Prospective Study of Dehydroepiandrosterone Sulfate Mortality and Cardiovascular Disease,” New England Journal of Medicine, Vol. 315, No. 24, 1986, pp. 1519-1524.
- E. E. Baulieu, “Dehydroepiandrosterone (DHEA): A Fountain of Youth?” Journal of Clinical Endocrinology & Metabolism, Vol. 81, 1996, pp. 3147-3151. doi:10.1210/jc.81.9.3147
- A. J. Moralea, J. J. Nolan and J. C. Nelson, “YEN Ssc. Effects of Replacement Dose of Dehydroepiandrosterone in Men and Women of Advancing Age,” Journal of Clinical Endocrinology & Metabolism, Vol. 78, 1994, pp. 1360-1367. doi:10.1210/jc.78.6.1360
- K. T. Khaw, “Dehydroepiandrosterone, Dehydroepiandrosterone Sulphate and Cardiovascular Disease,” Journal of Endodontics, Vol. 150, 1996, pp. S149-S153.
- J. K. Gierse, S. D. Hauser, D. P. Creely, C. Koboldt, S. H. Rangwala, P. C. Isakson and K. Seibert, “Expression and Selective Inhibition of the Constitutive and Inducible Forms of Human Cyclooxygenase,” Biochemical Journal, Vol. 305, 1995, pp. 479-484.
- G. B. Gordon, D. Bush, H. F. Weisman, “Reduction of Artherosclerosis by Administration of Dehydroepiandrosterone,” Journal of Clinical Investigation, Vol. 82, No. 2, 1988, pp. 712-720. doi:10.1172/JCI113652
- D. M. Herrington, “Dehydroepiandrosterone and Coronary Atherosclerosis,” Annals of the New York Academy of Sciences, Vol. 774, 1995, pp. 271-280. doi:10.1111/j.1749-6632.1995.tb17387.x-i1
- P. T. Leese, R. C. Hubbard, A. Karim, P. C. Isakson, S. S. Yu and G. S. Geis, “Effects of Celecoxib, a Novel Cyclooxgenase-2-Inhibitor, on Platelet Function in Healthy Adults: A Randomised, Controlled Trial,” Journal of Clinical Pharmacology, Vol. 40, No. 2, 2000, pp. 124- 132. doi:10.1177/00912700022008766
- J. C. Frölich, “Selektive Cyclooxygenasehemmer-Eine neue Generation von Antirheumatika,” Deutsches Ärzteblatt, Vol. 47, No. 23, 1996, pp. 3100-3101.
- P. E. Lipsky and P. C. Isakson, “Outcome of Specific COX Inhibition in Rheumatoid Arthritis,” Journal of Rheumatology, Vol. 24, Suppl. 49, 1997, pp. 9-14.
- K. Seibert, Y. Zhang, K. Leahy, S. Hauser, J. Masferrer, W. Perkins, L. Lee and P. Isakson, “Pharmacological and Biochemical Demonstration of the Role of Cyclooxygenase 2 in Inflammation and Pain,” Proceedings of the National Academy of Sciences USA, Vol. 91, 1994, pp. 12013-12017
- J. C. Frölich, “A Classification of NSAID’s According to the Relative Inhibition of Cyclooxygenase Isoenzymes,” Journal of Pharmacological Sciences, Vol. 18, 1997, pp. 1-35.
- J. C. Frölich, “Prostaglandin Endoperoxide Syntherase Isoenzymes: The Clinical Relevance of Selective Inhibition,” Annals of the Rheumatic Diseases, Vol. 82, 1995, pp. 712-720.
- J. C. Frölich and D. O. Stichtenoth, “Renal Side Effects of NSAID’s: Role of COX-1 and COX-2,” Selective Cox-2 Inhibitors: Pharmacology, Clinical Effects and Therapeutical Potential, Springer, Berlin, 1998, pp. 87- 97.
- D. O. Stichtenoth, H. Zeidler and J. C. Frölich, “Neue Nichtsteroidale Antirheumatika: Selektive Hemmstoffe der Induzierbaren Cyclooxygenase,” Medizinische Klinik Vol. 93, 1998, pp. 407-415.
- D. O. Stichtenoth and J. C. Frölich, “COX-2 and the Kidneys,” Current Pharmaceutical Design, Vol. 6, No. 17, 2000, pp. 1737-1753.
- D. O. Stichtenoth and J. C. Frölich, “Therapie Mit Präferentiellen und Spezifischen COX-2-Inhibitoren,” Internist, Vol. 42, 2001, pp. 421-426.
- R. C. Hubbard, D. L. Mehlisch, D. R. Jasper, M. J. Nugent, S. Yu and P. C. Isakson, “SC-58625, a Highly Selective Inhibitor of COX-2, Is an Effective Analgesic in an Acute Post-Surgial Pain Model,” Journal of Investigative Medicine, Vol. 44, 1996, p. 293A.
- R. Jouve, I. Juhan-Vague, M. Aillaud, M. Serment-Jouve and H. Payan, “Comparison of the Effects of Aspirin and Indomethacin on Aortic Artherogenesis Induced in Rabbits,” Artherosclerosis, Vol. 42, 1982, pp. 319-321. doi:10.1016/0021-9150(82)90159-9
- L. S. Simon, A. Weaver, D. Y. Graham, et al., “AntiInflammatory and Upper Gastrointestinal Effects of Celecoxib in Rheumatoid Arthritis,” JAMA, Vol. 282, No. 20, 1999, pp. 1921-1928. doi:10.1001/jama.282.20.1921
- D. O. Stichtenoth and J. C. Frölich, “The Second Generation of COX-2-Inhibitors,” Drugs, Vol. 63, No. 1, 2003, pp. 33-45. doi:10.2165/00003495-200363010-00003
- J. D. Morrow and L. J. Roberts II, “The Isoprostanes: Unique Bioactive Products of Lipid Peroxidation,” Progress in Lipid Researc, Vol. 36, No. 1, 1997, pp. 1-21. doi:10.1016/S0163-7827(97)00001-5
- J. D. Morrow and L. J. Roberts II, “The Isoprostanes, Current Knowledge and Directions for Future Research,” Biochemical Pharmacology, Vol. 51, No. 1, 1996, pp. 1-9. doi:10.1016/0006-2952(95)02072-1
- S. M. Bode-Böger, R. H. Böger, S. Kienke, M. Böhme, L. Phivtongngam, D. Tsikas and J. C. Frölich, “Chronic Dietary Supplementation with L-Arginine Inhibits Platelet Aggregation and Thromboxane A2 Synthesis in Hypercholesteraemic Rabbits in Vivo,” Cardivascular Research, Vol. 37, 1998, pp. 756-764. doi:10.1016/S0008-6363(97)00295-2
- N. Delanty, M. Reilly, D. Practico, J. A. Lawson, J. F. Mc Carthy, “8-epi PGF2-Generation during Coronary Reperfusion,” Circulation, Vol. 95, 1997, pp. 2492-2499.
- D. O. Stichtenoth and J. C. Frölich, “COX-2 and the Kidneys,” Current Pharmaceutical Design, Vol. 6, No. 17, 2000, pp. 1737-1753.
- D. Tsikas, F.-M. Gutzki, M. Böhme, I. Fuchs and J. C. Frölich, “Solidand Liquid-Phase Extraction for the Gas Chromatographic-Tandem Mass Spectrometric Quantification of 2,3-Dinor-thromboxane B2 and 2,3-Dinor-6- oxo-prostaglandin F1α in Human Urine,” Journal of Chromatography A, Vol. 885, 2000, pp. 351-359. doi:10.1016/S0021-9673(99)00967-X
- D. Tsikas, “Silmultaneus Derivazation and Quantification of the Nitric Oxide Metabolites Nitrite and Nitrate in Biological Fluids by Gas Chromatography/Mass Spectrometry,” Analytical Chemistry, Vol. 72, No. 17, 2000, pp. 4064-4072. doi:10.1021/ac9913255
- D. Practicó, L. Iuliano, L. Spagnioli, J. Maclouf, F. Violi and G. A. FitzGerald, “Monocytes in Human Artherosclerotic Plaque Contain Levels of 8-epi PGF2. An Index of Oxidative Stress in Vivo,” Circulation, Vol. 94, 1996, p. 1611.
- L. J. Roberts II and J. D. Morrow, “The Generation and Actions of Isoprostanes,” Biochimica et Biophysica Acta, Vol. 1345, No. 2, 1997, pp. 121-135.
- E. Schwedhelm, D. Tsikas, T. Durand, F.-M. Gutzki, A. Guy, J.-C. Rossi and J. C. Frölich, “Tandem Mass Spectrometric Quantification of 8-Iso-prostaglandin F2α and Its Metabolite 2,3-Dinor-8-iso-prostaglandin F2α in Human Urine,” Journal of Chromatography B, Vol. 744, 2000, pp. 9-112.
- D. Tsikas, E. Schwedhelm, J. Fauler, F.-M. Gutzki, E. Mayatepek and J. C. Frölich, “Specific and Rapid Quantification of 8-Iso-Prostaglandin F2α in Urine of Healthy Humans and Patients with Zellweger Syndrome by Gas Chromatography-Tandem Mass Spectrometry,” Journal of Chromatography B, Vol. 716, 1998, pp. 7-17. doi:10.1016/S0378-4347(98)00275-8

